Abstract
The interplay between the triel bond and the pnicogen bond in BF3···NCXH2···Y (X = P, As, Sb; Y = H2O, NH3) complexes was studied theoretically. Both bonds exhibited cooperative effects, with shorter binding distances, larger interaction energies, and greater electron densities found for the ternary complexes than for the corresponding binary ones. The cooperative effects between the triel bond and the pnicogen bond were probed by analyzing molecular electrostatic potentials, charge transfer, and orbital interactions. The results showed that the enhancement of the triel bond can mainly be attributed to the electrostatic interaction, while the strengthening of the pnicogen bond can be ascribed chiefly to the electrostatic and orbital interactions. In addition, the origins of both the triel bond and the pnicogen bond were deduced via energy decomposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, the structural chemistry of Lewis acid–base complexes involving BF3 has been the focus of many experimental and theoretical studies [1–12]. This interest stems partly from the fact that the dative linkages appear to be intermediate between van der Waals interactions and fully formed chemical bonds. Therefore, it has been demonstrated that these complexes can exhibit interesting structural chemistry and provide an unusual perspective on the evolution of molecular physical properties between the limits of van der Waals and chemical interactions [13]. Upon crystallization, these structures show dramatic changes in bond lengths and bond angles. For example, in the BF3···NCH complex, the B–N distance and N–B–F angle are 1.638 Å and 105.6°, respectively, in the solid state [3], whereas the corresponding gas-phase values are 2.473 Å and 91.5°, as determined by microwave spectroscopy of a supersonic jet [4]. These structural changes are usually attributed to crystal packing effects, while a cooperative mechanism is responsible for the short B–N distance in the BF3···NCH complex [14]. There have been lots of studies on the reactivity [15], thermodynamics [16], spectroscopy [17], and structures [18] of a wide range of Lewis acid–base complexes involving BF3 since the concept of such complexes was first introduced by G.N. Lewis in 1923 [19]. Recently, Murray et al. [20] and Grabowski [21, 22] have labeled Lewis acid–base interactions involving BF3 as π-hole interactions due to the presence of a π-hole (a region with positive electrostatic potential) on the boron atom. Clark and coauthors [23, 24] considered hydrogen bonding to be a σ-hole interaction and used this to explain the wide range of directionality of hydrogen bonding. Actually, researchers have tried to explain the formation of all intermolecular interaction types with the σ-hole concept, and it has been shown that many intermolecular interactions can indeed be explained as σ-hole interactions [23, 24]. It is now accepted that the existence of a σ-hole on an atom of a group IV–VII element is responsible for the formation of halogen [25–27], chalcogen [28–30], pnicogen [31–34], and tetrel [35–38] bonds.
The pnicogen bond has recently been recognized as a new and important type of intermolecular interaction, based on the results of experimental [39–41] and theoretical [42–50] studies. In view of the role of the σ-hole in pnicogen bonding, the electrostatic interaction appears to be the main contributor to the stability of pnicogen-bonded complexes [49, 51]. As a result, the pnicogen bond becomes stronger with increasing pnicogen atomic mass [49, 51]. On the other hand, Scheiner thought that the stability of pnicogen-bonded complexes can be partly ascribed to charge transfer from the bonding orbital in a Lewis base to an antibonding orbital in the pnicogen donor [44]. Energy decomposition results also support this hypothesis for the origin of pnicogen bonds based on electrostatic potentials and orbital interactions. Although research into pnicogen bonding has only just started, the types of Lewis base that participate in it vary; they include lone pairs on F, N, and O atoms, π electrons in unsaturated hydrocarbons or aromatic compounds [51–53], σ electrons in metal hydrides [49, 54], and single electrons in radicals [55], anions [56], and carbenes [57]. The presence of so many studies of pnicogen bonds in the literature can be attributed to the fact that pnicogen bonding plays important roles in crystal materials [38, 52, 58], chemical reactions [55], and biological systems [59]. The above applications of pnicogen bonding are dependent on its directionality and strength. The introduction of substituents is an effective means of regulating the strength of a pnicogen bond. It has been demonstrated that pnicogen bond strength is reinforced by the presence of an electron-withdrawing substituent on the Lewis acid center and/or an electron-donating one on the Lewis base center [44, 60]. Another method of tuning the strength of a pnicogen bond is through cooperative effects generated by combining the pnicogen bond with other interactions [49, 60–67]. In the clusters (PH2F) n and (PH2Cl) n , n = 2–7, the pnicogen bonds strengthen as the length of the chain increases due to cooperative effects between the pnicogen bonds (caused mainly by the electrostatic interaction) [61]. The pnicogen bond in (PH2F)2 is also strengthened when an F–H···F hydrogen bond is introduced into this cluster [62]. The F–H···F hydrogen bond enhances the pnicogen bond in PH2F–NH2F when it forms at the P–F end, but it weakens the pnicogen bond when it occurs at the N–F end [63]. It has been shown that the pnicogen atom acts as a dual Lewis acid–base [66, 68]. Interestingly, however, in the complexes H3N···FH2X···MCN (X = P, As; M = Cu, Ag, Au), where a pnicogen bond coexists with a coordination interaction, the stronger coordination interaction strengthens the weaker pnicogen bond while the pnicogen bond weakens the coordination interaction [68].
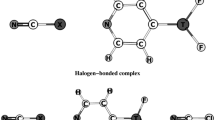
Cooperative effects are an important driving force for the formation of new chemical species, and play a critical role in accomplishing the functions associated with intermolecular interactions. In the work reported in the present paper, we studied the cooperative effects between the pnicogen bond and the triel bond in BF3···NCXH2···H2O and BF3···NCXH2···NH3 (X = P, As, Sb) complexes, as shown in Fig. 1. The changes in the strengths of both of these bonds were estimated by analyzing binding distances, interaction energies, and electron densities. To unravel the mechanism for the cooperativity effects in these systems, natural bond orbital (NBO), molecular electrostatic potential, and energy decomposition investigations were performed for these complexes.
Theoretical methods
All calculations were performed using the Gaussian 09 program [69]. The structures of the complexes and the respective monomers were optimized using the second-order Møller–Plesset perturbation theory (MP2) with the aug-cc-pVTZ basis set for all atoms except antimony (Sb). The aug-cc-pVTZ-PP basis set, which uses small-core relativistic pseudopotentials to describe the inner core orbitals, was used for Sb to account for relativistic effects. Frequency calculations were carried out at the same level to confirm that the optimized structures were local minima on their potential surfaces. Interaction energies were computed with supermolecular methods in which the geometries of BF3 were frozen in the complexes and those of other monomers were optimized. These quantities were corrected for basis set superposition error (BSSE) by the counterpoise procedure suggested by Boys and Bernardi [70].
Molecular electrostatic potentials (MEPs) on the 0.001-au electron density contour were calculated at the MP2/aug-cc-pVTZ level with the Wave Function Analysis–Surface Analysis Suite (WFA-SAS) program [71]. Topological analysis of all complexes was carried out using Bader’s theory of atoms in molecules (AIM) with the help of the AIM2000 software [72]. Natural bond orbital (NBO) analysis [73] was performed at the HF/aug-cc-pVTZ level via procedures included in Gaussian 09 in order to analyze orbital interactions and charge transfer. An energy decomposition analysis of the interaction energy was performed using the GAMESS program [74] with the localized molecular orbital–energy decomposition analysis (LMOEDA) method [75] at the MP2/aug-cc-pVTZ level.
Results and discussion
Pnicogen-bonded and triel-bonded dyads
Due to the electron-deficient nature of the boron atom, this atom is often considered a Lewis acid, i.e., it presents electron depletion (it has a π-hole) [76]. As a result, the boron atom in BF3 forms a triel bond with the N atom of NCXH2 (X = P, As, Sb). It is obvious from Fig. 1 that, upon the formation of the triel bond (i.e., with complexation), the planar BF3 monomer becomes umbrella-structured; this phenomenon has also been observed in many Lewis acid–base complexes of BF3 [1–12]. It has been shown that the structures of BF3 complexes have structures that are between trigonal and tetrahedral [22]. This deformation is expected to be more prominent in the complexes with stronger triel bonds, as evidenced by a larger F–B···N angle (Table 1). As the atomic number of the pnicogen increases, the B···N distance shortens and the interaction energy becomes more negative, indicating that the triel bond strengthens. Grabowski stressed the importance of electrostatic interactions in triel bonds, based on the relationship between the electrostatic potential of the π-hole of the triel atom and the interaction energy [22]. Thus, we attempted to explain changes in the strength of triel bonding by linking them to changes in the negative electrostatic potential on the N atom of NCXH2 (Table 2). It is clear that the electrostatic potential on the N atom of NCXH2 becomes more negative when X is changed from P to Sb, due to the smaller electronegativity of the Sb atom. This is in line with the strength of triel bonding. The triel bond is stronger in BF3···NCXH2 than in BF3···NCH, which has a binding distance of 2.364 Å and an interaction energy of −5.2 kcal/mol at the MP2/aug-cc-pVTZ level [22]. This shows that the group XH2 is an electron donor when it is adjoined with the electron-withdrawing group CN.
The pnicogen bonds in the NCXH2···H2O and NCXH2···NH3 complexes are σ-hole interactions, unlike the triel bond in BF3···NCXH2. The strength of the pnicogen bond is dependent on the nature of atom X and the Lewis base. That is, the pnicogen bond is stronger (the binding distance is shorter and the interaction energy is larger) when X is heavier. This is consistent with the negative electrostatic potential on the X atom (Table 2). As expected, the presence of the stronger Lewis base NH3 leads to a stronger pnicogen bond than the presence of the weaker Lewis base H2O. Clearly, the pnicogen bonds in the NCXH2···H2O and NCXH2···NH3 complexes are much weaker than the triel bond in BF3···NCXH2. In addition, the pnicogen bond in NCPH2···NH3 is weaker than that in FPH2···NH3 [44], even though –CN is a stronger electron-withdrawing group. This means that other interactions besides the electrostatic interaction, such as charge transfer, are also important in the formation of pnicogen bonds [44]. A stronger pnicogen bond corresponds to a better oriented pnicogen bond, i.e., a bigger C–X···N/O angle.
The formation of the triel bond causes a different change in the C≡N bond length and a different shift in its frequency to those caused by the formation of the pnicogen bond. Specifically, the C≡N bond is shortened by the formation of the triel bond and the corresponding stretching vibration displays a blueshift, while the C≡N bond is elongated by the formation of the pnicogen bond and the respective stretching vibration shows a redshift. Moreover, the C≡N bond in the triel bond shows a larger change in bond length and a larger frequency shift than that in the pnicogen bond. The C–X bond is stretched whether it is in the triel bond or in the pnicogen bond.
The existence of the triel bond and the pnicogen bond is demonstrated by the presence of the B···N and X···O/N bond critical points (BCPs) in Fig. 2, respectively. Table 3 shows that the electron densities at these BCPs change in a manner consistent with the strength of the corresponding interaction. This supports a conclusion drawn based on numerous studies of hydrogen bonds—that the electron density at the intermolecular BCP often correlates with the strength of the interaction [77]. The electron density at the B···N BCP exceeds the range 0.002–0.04 au suggested for hydrogen bonds [78]; it corresponds to the (stronger) triel bond. The Laplacian of the electron density at the B···N BCP is positive for triel-bonded complexes, indicating that it cannot be classified as a covalent bond [79]. This also holds true for the pnicogen bonds. However, the energy densities at the B···N and Sb···N BCPs are negative, which is often attributed to a partially covalent interaction [79]. For the remaining interactions, the energy density is positive, corresponding to a pure closed-shell interaction [79].
It was demonstrated that the two important orbital interactions LPN → LP*B and LPN → BD*B–F are present in the triel bond of the BF3···NCH complex [22]. However, these orbital interactions are not found in BF3···NCXH2 because the corresponding triel bond is classified as a covalent bond in the NBO approach. For the pnicogen bond, there is the orbital interaction LPN(O) → BD*C–X, where LPN(O) is the lone pair on the N or O atom in the Lewis base and BD*C–X denotes the C–X antibonding orbital in NCXH2 (X = P, As, Sb). This orbital interaction correlates with the interaction energy of the pnicogen bond, indicating that the orbital interaction is also important for the formation of the pnicogen bond. This orbital interaction is also responsible for the elongation of the C–X bond in the pnicogen bond. It is clear that charge transfer occurs from the Lewis base to the Lewis acid upon complexation. The formation of the triel bond is associated with considerable charge transfer, 0.16~0.21e, consistent with the nature of a covalent interaction. Clearly, the charge transfer in BF3···NCXH2 is much larger than that (0.034e) in BF3···NCH [20]. Less charge is transferred upon the formation of the pnicogen bond, but the charge transfer is again correlated with the interaction strength.
To elucidate the origins of the triel bond and the pnicogen bond, the energies of both interactions were decomposed into five components: electrostatic energy (E ele), exchange energy (E ex), repulsion energy (E rep), polarization energy (E pol), and dispersion energy (E disp). Table 4 shows that the triel bond has a larger exchange energy, which corresponds to a bigger overlap between the molecular orbitals of both molecules, although it also has a larger repulsion energy due to the shorter distance between molecules. For the triel bond, the electrostatic energy is comparable with the polarization energy. The relatively large polarization energy suggests that the shapes of the orbitals change significantly, which is a typical characteristic of the formation of a covalent bond. For the pnicogen bond, the electrostatic interaction is dominant since the electrostatic energy is more negative than the polarization and dispersion energies. Very recently, Politzer and coauthors pointed out that the Hellmann–Feynman theorem can provide a straightforward interpretation of hydrogen-bond and other σ-hole interactions in terms of Coulombic interactions, which encompasses polarization and therefore dispersion [80].
Interplay between the pnicogen bond and the triel bond
The geometric and energetic results computed for the ternary complexes are summarized in Tables 5 and 6, respectively. For the triel and pnicogen bonds, the binding distance is shorter and the interaction energy more negative for the ternary complexes than for the respective binary systems. This indicates that both the triel bond and the pnicogen bond are stronger in the ternary systems than in the binary systems. This conclusion is further supported by the increases in electron density at the B···N and X···O/N BCPs in the ternary complexes as compared to the binary complexes (Table S1 in the “Electronic supplementary material,” ESM). The shortening of the B···N distance varies from 0.057 Å in BF3···NCSbH2···H2O to 0.116 Å in BF3···NCPH2···NH3; the shortening of the B···N distance is thus clearly related to the strength of the pnicogen bond. For a given Lewis base in the pnicogen bond, the B···N distance shortens less as the pnicogen bond interaction strength increases. However, for the same pnicogen atom, the B···N shortens more when the stronger Lewis base (NH3) is used. The shortening of the X···O/N distance ranges from 0.120 to 0.187 Å, which is larger than the shortening of the B···N distance. This result contrasts with that seen for BF3···NCH···NCH [81]; in this case, the B···N distance shortens much more than the H···N distance does. Obviously, the dependence of the shortening of the X···O distance on the strength of the triel bond is the reverse of the dependence of the shortening of the X···N distance on the strength of the triel bond. That is, enhancing the strength of the triel bond leads to greater shortening of the X···O distance but less shortening of the X···N distance. When both the triel bond and the pnicogen bond are strengthened, the F–B···N angle increases a little while the C–X···O/N angle changes only slightly.
The increase in the bond interaction energy as a percentage of the total interaction energy is more prominent for the pnicogen bonds than for the triel bonds. The strengthening of both the triel bond and the pnicogen bond in each ternary complex indicates that these interactions show a synergistic effect. The presence of this synergistic effect is further confirmed by the negative cooperative energy (E coop). The cooperative energy makes the largest contribution to the stability of each ternary complex (17.1–25.6 % of the total interaction energy). This contribution is larger than that of the cooperativity between the hydrogen bond and the pnicogen bond [50, 60]. E coop as a percentage of the total interaction energy correlates with the strength of the triel bond and that of the pnicogen bond. In particular, it increases when the Lewis base is changed from H2O to NH3 but decreases when it is changed from P to Sb. As a result, the largest contribution is obtained for the cooperative energy in BF3···NCPH2···NH3.
The C≡N bond is contracted in each ternary complex (Table S2 in the ESM), and this contraction is larger than that in the corresponding binary system, while the C–X bond is elongated and its elongation is greater than that in the corresponding binary system due to the interplay between the triel bond and the pnicogen bond. The C≡N stretching vibration thus exhibits a blueshift for each ternary complex. The blueshifts for the ternary complexes of H2O are larger than those for the corresponding BF3···NCXH2 complexes but smaller than those for the ternary complexes of NH3.
When NCXH2 forms a pnicogen bond with H2O or NH3, the negative MEP on the N atom of NCXH2 is larger than that on the N atom of the NCXH2 monomer (Table 2). This shows that the N atom of NCXH2 in the NCXH2···H2O and NCXH2···NH3 complexes is a stronger Lewis base when there is a triel bond, so the electrostatic interaction enhances the triel bond in each ternary complex. When NCXH2 forms a triel bond with BF3, the positive MEP on the X atom of NCXH2 is larger than that on the X atom of the NCXH2 monomer (Table 2). Furthermore, the increase in the positive MEP on the X atom of NCXH2 shows a consistent change as the pnicogen bond is strengthened. This indicates that the enhancement of the pnicogen bond can mainly be attributed to the electrostatic interaction.
Table 7 presents the charge transferred in the triel bond and the pnicogen bond as well as the second-order perturbation energy of the pnicogen bond in each ternary complex. It is clear that more charge is transferred in both interactions in the ternary complexes as compared to the corresponding binary complexes. However, this increase in the charge transferred shows a consistent change with increasing interaction energy of the pnicogen bond, but not with increasing interaction energy of the triel bond. The LPN(O) → BD*C–X orbital interaction is stronger in the ternary complexes, and the increase in the associated perturbation energy shows a consistent change with increasing pnicogen bond interaction energy. This means that this orbital interaction also enhances the pnicogen bond.
Conclusions
Ab initio calculations have been performed for the ternary complexes BF3···NCXH2···Y (X = P, As, Sb; Y = H2O, NH3) and their respective binary complexes. The triel bond in BF3···NCXH2 is partially covalent and becomes stronger when X is changed from P to Sb. The pnicogen bond in NCXH2···Y is governed by the electrostatic interaction and becomes stronger as the X atom becomes heavier and the Lewis base Y becomes stronger. When the triel bond and pnicogen bond coexist in the same ternary complex, both interactions are strengthened by considerable cooperative energy. The pnicogen bond is enhanced more than the triel bond. The strengthening of both the triel bond and the pnicogen bond can be explained by the more negative electrostatic potential on the N atom in NCXH2···Y and the more positive electrostatic potential on the X atom in BF3···NCXH2, respectively.
References
Jurgens R, Almlöf J (1991) Chem Phys Lett 176:263–265
Dvorak MA, Ford RS, Suenram RD, Lovas FJ, Leopold KR (1992) J Am Chem Soc 114:108–114
Burns WA, Leopold KR (1993) J Am Chem Soc 115:11622–11623
Reeve SW, Burns WA, Lovas FJ, Suenram RD, Leopold KR (1993) J Phys Chem 97:10630–10637
Jiao HJ, Schleyer PR (1994) J Am Chem Soc 116:7429–7430
Fujiang D, Fowler PW, Legon AC (1995) J Chem Soc Chem Commun 24:113–114
Leopold KR, Canagaratna M, Phillips JA (1997) Acc Chem Res 30:57–64
Wells NP, Phillips JA (2002) J Phys Chem A 106:1518–1523
Giesen DJ, Phillips JA (2003) J Phys Chem A 107:4009–4018
Venter G, Dillen J (2004) J Phys Chem A 108:8378–8384
Phillips JA, Giesen DJ, Wells NP, Halfen JA, Knutson CC, Wrass JP (2005) J Phys Chem A 109:8199–8208
Hase Y (2007) Spectrochim Acta A 68:734–738
Hunt SW, Leopold KR (2001) J Phys Chem A 105:5498–5506
Cabaleiro-Lago EM, Rios MA (1998) Chem Phys Lett 294:272–276
Jensen WB (1980) The Lewis acid–base concepts: an overview. Wiley-Interscience, New York
Pearson RG (1997) Chemical hardness—applications from molecules to solids. Wiley, Weinheim
Mulliken RS, Person WB (1969) Molecular complexes. Wiley, New York
Hargittai M, Hargittai I (1977) The molecular geometries of coordination compounds in the vapor phase. Elsevier, Amsterdam
Lewis GN (1923) Valence and the structure of atoms and molecules. The Chemical Catalog Company, Inc., New York
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) J Mol Model 18:541–548
Grabowski SJ (2015) ChemPhysChem 16:1470–1479
Grabowski SJ (2014) ChemPhysChem 15:2985–2993
Hennemann M, Murray JS, Politzer P, Riley KE, Clark T (2012) J Mol Model 18:2461–2469
Politzer P, Murray JS, Clark T (2013) Phys Chem Chem Phys 15:11178–11189
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Acc Chem Res 38:386–395
Metrangolo P, Meyer F, Pilati T, Proserpio DM, Resnati G (2007) Chem Eur J 13:5765–5772
Cavallo G, Metrangolo P, Pilati T, Resnati G, Sansotera M, Terraneo G (2010) Chem Soc Rev 3:3772–3783
Sanz P, Mó O, Yanez M (2002) J Phys Chem A 106:4661–4668
Wang W, Ji B, Zhang Y (2009) J Phys Chem A 113:8132–8135
Murray JS, Lane P, Clark T, Politzer P (2007) J Mol Model 13:1033–1038
Del Bene JE, Alkorta I, Sanchez-Sanz G, Elguero J (2011) J Phys Chem A 115:13724–13731
Scheiner S (2011) Chem Phys Lett 514:32–35
Grabowski SJ (2013) Chem Eur J 19:14600–14611
Murray JS, Lane P, Politzer P (2007) Int J Quantum Chem 107:2286–2292
Grabowski SJ (2014) Phys Chem Chem Phys 16:1824–1834
Mani D, Arunan E (2013) Phys Chem Chem Phys 15:14377–14383
Bauz A, Mooibroek TJ, Frontera A (2013) Angew Chem Int Ed 52:12317–12321
Murray JS, Lane P, Politzer P (2009) J Mol Model 15:723–729
Zahn S, Frank R, Hey-Hawkins E, Kirchner B (2011) Chem Eur J 17:6034–6038
Joshi PR, Ramanathan N, Sundararajan K, Sankaran K (2015) J Phys Chem A 119:3440–3451
Sarkar S, Pavan MS, Row TNG (2015) Phys Chem Chem Phys 17:2330–2334
Solimannejad M, Gharabaghi M, Scheiner S (2011) J Chem Phys 134:024312
Scheiner S (2011) J Chem Phys 134:094315
Scheiner S (2011) J Phys Chem A 115:11202–11209
Adhikari U, Scheiner S (2012) J Phys Chem A 116:3487–3497
Adhikari U, Scheiner S (2012) Chem Phys Lett 532:31–35
Scheiner S (2011) J Chem Phys 134:164313
Scheiner S, Adhikari U (2011) J Phys Chem A 115:11101–11110
Li QZ, Li R, Liu XF, Li WZ, Cheng JB (2012) J Phys Chem A 116:2547–2553
Li QZ, Li R, Liu XF, Li WZ, Cheng JB (2012) ChemPhysChem 13:1205–1212
An XL, Li R, Li QZ, Liu XF, Li WZ, Cheng JB (2012) J Mol Model 18:4325–4332
Bauzá A, Quiñonero D, Deyá PM, Frontera A (2013) CrystEngComm 15:3137–3144
Xu HY, Wang W, Zou JW (2013) Acta Chim Sin 71:1175–1182
Ma FY, Li AY (2014) Comput Theor Chem 1045:78–85
Alkorta I, Elguero J, Solimannejad M (2014) J Phys Chem A 118:947–953
Del Bene JE, Alkorta I, Elguero J (2014) J Phys Chem A 118:3386–3392
Zhuo HY, Li QZ (2015) Phys Chem Chem Phys 17:9153–9160
Politzer P, Murray JS, Janjić GV, Zarić SD (2014) Crystals 4:12–31
Bauzá A, Quiñonero D, Deyá PM, Frontera A (2012) Phys Chem Chem Phys 14:14061–14066
Del Bene JE, Alkorta I, Elguero J (2014) J Phys Chem A 118:2360–2366
Esrafili MD, Vakili M, Solimannejad M (2014) Chem Phys Lett 609:37–41
Alkorta I, Sánchez-Sanz G, Elguero J, Del Bene JE (2012) J Chem Theory Comput 8:2320–2327
Del Bene JE, Alkorta I, Sánchez-Sanz G, Elguero J (2012) J Phys Chem A 116:9205–9213
Esrafilia MD, Mohammadian-Sabeta F, Solimannejad M (2015) J Mol Graph Model 57:99–105
Solimannejad M, Ramezani V, Trujillo C, Alkorta I, Sánchez-Sanz G, Elguero J (2012) J Phys Chem A 116:5199–5206
Del Bene JE, Alkorta I, Sánchez-Sanz G, Elguero J (2013) J Phys Chem A 117:3133–3141
Zhuo HY, Li QZ, Li WZ, Cheng JB (2014) J Chem Phys 141:244305
Zhuo HY, Li QZ, Li WZ, Cheng JB (2015) New J Chem 39:2067–2074
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Scalmani G, Cossi M, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, KleneMLX KJE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, ZakrzewskiVG DS, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-LahamMA PCY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Gonzalez C, Wong MW, Pittsburgh PA, Pople JA (2009) Gaussian 09, revision A02. Gaussian Inc., Wallingford
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1691
Bader RFW (2000) AIM2000 program, version 2.0. McMaster University, Hamilton
Reed AE, Curtiss LA, Weinhold FA (1988) Chem Rev 88:899–926
Schmidt MW, Baldridge KK, Boalz JA, Elbert ST, Gorden MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Su PF, Li H (2009) J Chem Phys 13:014102
Politzer P, Murray JS, Clark T (2010) Phys Chem Chem Phys 12:7748–7757
Parthasarathi R, Subramanian V, Sathyamurthy N (2006) J Phys Chem A 110:3349–3351
Koch U, Popelier PLA (1995) J Phys Chem A 99:9747–9754
Arnold WD, Oldfield E (2000) J Am Chem Soc 122:12835–12841
Politzer P, Murray JS, Clark T (2015) J Mol Model 21:52
Fiacco DL, Leopold KR (2003) J Phys Chem A 107:2808–2814
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21573188).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, MX., Zhuo, HY., Li, QZ. et al. Theoretical study of the cooperative effects between the triel bond and the pnicogen bond in BF3···NCXH2···Y (X = P, As, Sb; Y = H2O, NH3) complexes. J Mol Model 22, 10 (2016). https://doi.org/10.1007/s00894-015-2882-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2882-z