Abstract
Density functional theory (DFT) calculations have been used to investigate the structural properties, dipole moments, polarizabilities, Gibbs energies, hardness, electronegativity, HOMO/LUMO energies, and chemical potentials of trans and cis configurations of eight para-substituted azobenzene derivatives. All properties have been obtained using the B3LYP functional and 6-31++G(d,p) basis set. The planar structures have been obtained for all optimized trans configurations. The energy difference between trans and cis configurations for considered derivatives was found to be between 64.2–73.1 kJ/mole. It has been obtained that the p-aminodiazo-benzene (ADAB) has the difference in the dipole moments between trans and cis forms higher than for trans and cis azobenzene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the year 1937, when Harley published his work [1] about cis isomerization of azobenzene, this photochemical phenomena became widely studied. The idea behind it is photoisomerization of azobenzene under irradiation when non-polar trans azobenzene can be photoisomerized into the polar cis-azobenzene. Due to relatively simple molecular structure and unique characteristics, azobenzene and its derivatives were investigated in different studies as photoswitchable substances [2–4]. Moreover, azobenzene derivatives have vivid colors, which caused them to be used as dyes. Due to the formation of polar cis-isomer, the contact angle can be significantly decreased. This feature can be used to control wettability of surface by photoisomerization [5]. Furthermore, changes in dipole moment cause changes in the surface potential, which can be used to create a surface with the controlled motion and net mass transport [2]. Thus, it would be highly profitable to have an ability to design a specific molecule for certain applications.
Therefore, the correct characterization of both trans and cis forms of azobenzene derivatives is required. From the experimental point of view, synthesis and determination of the properties of interest can be expensive and difficult. In this case, molecular modeling could help to find structures with desirable properties in a relatively short time. In the present study, the effect of structural diversity on electronic properties will be analyzed. A set of molecules has been chosen:
-
1.
Azobenzene as a parent compound;
-
2.
Azobenzene’s derivatives of the type of aminoazobenzene, i.e., azobenzene substituted with an electron-donating groups NH 2, SO 2-NH 2, N-(CH 3) 2;
-
3.
Azobenzene’s derivatives substituted with an electron acceptor group OH, NO 2, CH 2-CH 2-OH;
-
4.
Azobenzene’s derivatives of the pseudostilbene type, i.e., substituted with an electron acceptor at the para-position of a phenyl ring and an electron-donating group at the other para-position of another phenyl ring. This type is also known as push-pull azobenzene due to charge transfer within the molecule.
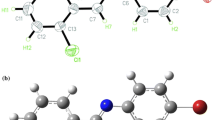
All considered molecules are listed and are presented in the Table 1, the Fig. 1.
The aim of this research is to investigate the effect of substituents of a group of eight azobenzene derivatives in trans and cis forms on structural and electronic properties by DFT method, which was widely and successfully used in studies concerning structural and electronic properties of azobenzene derivatives [6–8].
Methods
Calculations were carried out using the Gaussian03 [9] program with the DFT method and the B3LYP [10–12] functional. The energies of the structures were calculated with a self-consistent field (SCF) convergence of 10−8 a.u. for the density matrix. All structures were optimized using the Berny algorithm [13] with the criteria for convergence being a maximum force less than 45×10−5 and an rms force of less than 3×10−4. We used 6-31++G(d,p) basis set, which is the most appropriate in terms of time, accuracy, and cost of computing resources for the determination of electronic properties [8]. Geometry parameters for each structure were free of constraints and allowed to relax during the optimization process.
The chemical potential μ, absolute hardness η, and electronegativity χ were evaluated by the finite-difference approximation from the ionization potential (I) and electron affinity (A). The last occupied Kohn-Sham orbital energy can be equated with the energy of the highest occupied molecular orbital (HOMO) associated to –I. Moreover, the first unoccupied Kohn–Sham orbital energy is often [13, 14] used as the value for the energy of the lowest unoccupied molecular orbital (LUMO) and so can be equated with –A even through it is crude approximation since the DFT is a ground state theory.
For each considered molecule we have examined trans and cis forms and all possible configurations concerning positions of functional groups in the molecule. The stationary configurations were confirmed by vibrational frequencies analysis: only configurations with real frequencies were used for calculations. In the case of more than one possible configuration, we used Boltzmann statistics to find the probability for each configuration and then to evaluate the average value for desirable property.
Results and discussion
In the present chapter, results of DFT calculations on considered azobenzenes will be analyzed.
Optimized structures
The characteristic geometric parameters of optimized trans and cis configurations are given in the Table 2. It should be noted that planar structures were obtained for all optimized trans configurations.
From Table 2 it was found that upon substitution of the parent trans-AB by an electron donor (NH 2 and N-(CH 3) 2 groups in AAB, SO 2 NH 2AB, MY, ADAB), electron acceptor (HO, CH 2 CH 2HO groups in HOAB and CH 2 CH 2HOAB), and electron donor-acceptor (NH 2 and NO 2 groups in DO 3), the N 1 N 2 bond distance increases in the following order: AB = CH 2 CH 2HOAB < HOAB < AAB < MY = SO 2 NH 2AB < ADAB = DO 3 i.e., for the parent azobenzene, electron acceptor, electron-donating and electron donating-acceptor azobenzenes, respectively. Thus, the electronic effect of substitution causes the change of N 1 N 2 distance: electron-donating groups elongate the N 1 N 2 distance, the electron acceptor groups have no or little effect on this bond and the electron donating-acceptor groups elongate this bond.
As for the cis forms, the following order was found: MY < AB < CH 2 CH 2HOAB < HOAB < DO 3 = AAB = SO 2 NH 2AB < ADAB. The biggest distance was found for ADAB molecule.
It was obtained that the C\(_{1} \prime \textit {N}_{1}\textit {N}_{2} \textit {C}_{1} \) torsion angles for cis configuration are in the following order: CH 2 CH 2HOAB < AB < HOAB < MY < AAB < SO 2 NH 2AB < DO 3 < ADAB. The DO 3 and ADAB molecules present the biggest dihedral angles. The first of them is a push-pull molecule and the second one is an electron-donor-type molecule with bulky substitutes on both sides, whereas molecules with electron-acceptor groups show small angles.
The shortest C 5 C 6 distance in trans-form was obtained for DO 3 being the push-pull molecule, the distance equal to 9.09 Å was obtained for AB, HOAB, and SO 2 NH 2AB molecules. The C 5 C 6 distance equal to 9.12 Å was found for CH 2 CH 2HOAB and AAB, and the biggest distance was found for MY and ADAB. As for the cis-forms, the distance increases in the same order as the dihedral angle.
Electronic properties
Energies E and equilibrium constants K of both the most stable trans and cis configurations are given in Table 3. The equilibrium constants were obtained using formula:
where the △E is difference in the Gibbs energies of cis and trans configurations, T is temperature, R is gas constant.
As expected, trans configurations for each molecule were found to be more stable than cis. From our calculations, the difference between trans and cis configuration energies is 64.2–73.1 kJ/mole, where the smallest difference was found for AB, and the biggest one for ADAB.
It is not so important to consider all configurations in case of energy because we have not obtained a big discrepancy between the average value and energy of the most stable configuration.
Components of polarizability matrix can be determined spectroscopically, but this data is poor because of a hard experimental procedure, especially for molecules with low or no symmetry. This is why molecular modeling calculations can help in this purpose. In Table 4 dipole moments, mean and anisotropic polarizabilities are presented for eight considered azobenzene derivatives.
For most of the molecules, dipole moment of cis-form was found to be greater than that of trans-form. However, for CH 2 CH 2HOAB and DO 3 the situation is opposite. This could be due to more unequal distribution in the trans-isomer. In the [7] negative cis-trans dipole moment was found for molecules with the NO 2 group at the para-position.
The biggest dipole moment in the trans-form was found for push-pull DO 3 molecule. Comparatively big values were obtained also for CH 2 CH 2HOAB and SO 2NH 2AB molecules with bulky functional groups at the para-positions. In the cis-form: SO 2 NH 2AB, DO 3 and ADAB have big dipole moments. Since the ADAB molecule has small dipole moment in trans-form and big in the cis-form, the difference in dipole moments is the biggest among considered molecules. This difference is equal to 4.7 and is greater than that for AB by 1.5 Debye.
The dipole moment of molecule
where p 0 is a dipole moment without an electric field, α is a polarizability second-order tensor, β is the first in an infinite series of hyperpolarizabilities. The polarizability tensor was firstly diagonalized and mean and anisotropic polarizabilities have been obtained using following formulas:
where a x x , a y y , a z z are diagonal elements of polarizability matrix. The biggest mean and anisotropic polarizabilities have been obtained for the ADAB molecule.
Molecular properties
The frontier orbitals of the chemical compounds are very important parameters in identifying their reactivity [15, 16]. A molecule with a high value of HOMO can give electrons to appropriate acceptor molecules with low energy and empty molecular orbitals. Calculated HOMO and LUMO energies for eight azobenzene derivatives are presented in the Table 5. Both trans and cis configurations of ADAB molecule have the highest HOMO value.
In order to investigate the correlation between chemical quantities and molecular properties, calculated hardness (η), electronegativity (χ), and chemical potential (μ) are presented in Table 5 for both configurations of considered molecules. These parameters were obtained using HOMO and LUMO energies according to [17].
The hardness is η=(E L U M O −E H O M O )/2 [18]. This parameter shows the reactivity of the system: a system with a larger η should be less reactive than a system having smaller η [18]. The electronegativity defined by Mulliken [19] as χ=−(E H O M O +E L U M O )/2 as hardness is used to predict chemical behavior. The chemical potential is opposite to electronegativity [20] μ=(E H O M O +E L U M O )/2.
From our calculations it follows that the hardness of presented systems increases in the following order: DO 3 < ADAB < MY < SO 2 NH 2AB < AAB < HOAB < CH 2 CH 2HOAB < AB. So, it means that AB is the least reactive, DO 3 and ADAB are the most reactive molecules among considered. As was expected, trans configurations have smaller chemical potentials than cis, and this means that molecules tend to move from cis to trans configurations.
Conclusions
In the present study, results of DFT calculations on eight azobenzene derivatives have been presented. Concerning the structural properties of considered azobenzene derivatives, the planar trans structure has been obtained for all considered molecules. On both configurations, an asymmetry in structural parameters was obtained for all molecules. The trans configurations were found to be more stable than cis. The relative difference in the dipole moment between the trans and cis configurations was found to be lower than for azobenzene for all considered molecules except for ADAB, for which the difference was obtained equal to 4.7 Debye. For this molecule, the largest mean and anisotropic polarizabilities have been obtained. Concerning molecular properties, the highest reactivities were found for DO 3 and ADAB molecules.
References
Hartley G (1937) Nature 140:281
Ichimura K, Oh SK, Nakagawa M (2000) Science 288(5471):1624
Katsonis N, Lubomska M, Pollard MM, Feringa BL, Rudolf P (2007). Prog Surf Sci 82(7):407
Jiang W, Wang G, He Y, Wang X, An Y, Song Y, Jiang L (2005) Chem Commun 28:3550
El Halabieh RH, Mermut O, Barrett CJ, et al. (2004) Pure Appl Chem 76(7):1445
Hinchliffe A, Nikolaidi B, Soscún Machado HJ (2004) Int J Mol Sci 5(8):224
Wazzan NA, Richardson PR, Jones AC (2010) Photochem Photobiol Sci 9(7):968
Minisini B, Fayet G, Tsobnang F, Bardeau JF (2007) J Mol Model 13(12):1227
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Montgomery Jr J, Vreven T, Kudin K, Burant J et al (2004) Wallingford, vol 26. CT
Becke AD (1993) J Chem Phys 98(7):5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Schlegel HB (1982) J Comput Chem 3 (2):214
Ghanty TK, Ghosh SK (1996) J Phys Chem 100(30):12295
Torrent-Sucarrat M, Luis JM, Duran M, Sola M (2001) J Am Chem Soc 123(32):7951
Fukui K, Yonezawa T, Shingu H (1952) J Chem Phys 20:722
Mendoza-Huizar LH, Rios-Reyes CH (2011) J Mex Chem Soc 55(3):142
Zhan CG, Nichols JA, Dixon DA (2003) J Phys Chem A 107(20):4184
Parr RG, Pearson RG (1983) J Am Chem Soc 105(26):7512
Mulliken RS (2004) J Chem Phys 2(11):782
Pauling L (1960) The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry, vol 18. Cornell University Press
Acknowledgments
This work was performed according to the Russian Government Program of Competitive Growth of Kazan Federal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piyanzina, I., Minisini, B., Tayurskii, D. et al. Density functional theory calculations on azobenzene derivatives: a comparative study of functional group effect. J Mol Model 21, 34 (2015). https://doi.org/10.1007/s00894-014-2540-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2540-x