Abstract
We present a theoretical study on the structure, stability, spectra and electronic properties of imidazole (Im) adsorbed on gold nanoclusters (Aun, n = 2, 4, 6, 8, 10, and 20). These computations were performed using various density functional theories with and without inclusion of Grimme’s (D3) dispersion correction. For small clusters, we also carried out wavefunction-based ab initio (MP2 and SCS-MP2) computations for comparison. Vibrational, atoms in molecules (AIM) and natural bond orbital (NBO) analyses clearly reveal the occurrence of charge transfer (CT) through covalent (N1–Au) and noncovalent interactions that play important roles in the stability of the Im@Aun complexes with anchor assisted H-bonds (Cα–H · Au). Therefore, gold clusters can act as H-bond acceptors with biomolecules for development of new materials and applications. Our study establishes also the ability and reliability of PBE0 and M05-2X functionals compared to B3LYP and PBE for an accurate description of covalent and noncovalent interactions between Im and gold clusters since they lead to close agreement with MP2. Finally, we show that the Au8 cluster may be viewed as large enough to mimic the 3D gold surface.

Imidazole@gold nanoclusters
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gold bulk surfaces have relatively high inertness in any chemical environment. [1] Therefore, adsorption of molecules on such surfaces is difficult, whereas reactivity as well as adsorption capacity on Aun clusters vary and depend strongly on clusters size, i.e., n. This may be connected to the possible different Au–Au coordination numbers in Aun clusters. Particularly, inner gold Au–Au bonds are always shorter and stronger than the outer bonds [2–6]. Specifically, investigations into the interaction of biomolecules with gold nanoparticles (Aun, n = 1–20/40 called also nanoclusters) represent a very active research topic in biological, chemical and material sciences [7–23]. These features can play important roles in various fields of application of gold complexes, such as sensors, biosensors, drug-delivery, molecular electronic devices and energy materials [7, 8, 9].
Numerous experimental and theoretical studies have been performed on the complexes formed between various sizes of selected Aun nanoparticles and biomolecules such as DNA bases, proteins, peptides and nitrogen-based bases for biochemical applications [10–23]. For instance, nitrogen based biomolecules such as guanine (G) [10, 11], cytosine (C) [12, 13], adenine (A) [14], thymine (T) [15], uracil (U) [16], histidine (HIS) [17], cysteamine [18] and DNA base pairs (AT and GC) [19–23], with gold clusters and surfaces have attracted widespread attention. For gold clusters binding with nucleic acid base pairs (GC@Aun and AT@Aun, where n = 4 and 8), DFT-B3LYP calculations by Kumar et al. [14] revealed that neutral gold complexes are more stable than the corresponding anions. Using also B3LYP method, Leszczynski and co-workers [21] studied the interaction of purine base G, and the Watson-Crick GC base pair with gold nanoclusters. They reported vertical ionization energies, electron affinities and charge transfer characteristics for these complexes. These studies clearly revealed that A, G, and C adsorb at Au electrodes whereas base unit T does not, which is in line with the experimental studies by Tao et al. [24] of nucleic acid bases in interaction with the Au (111) surface. Accordingly, strong binding of the N-atom on gold surface/nanoclusters requires an unprotonated nitrogen part of the aromatic ring.
Another important issue relative to the investigation of molecules–gold nanoclusters concerns the quantification with high accuracy of various types of covalent and noncovalent interactions that may take place during chemisorption and physisorption of molecules on gold surfaces. The strength and nature of the interactions between organic adsorbate and metal substrate can be probed experimentally through, for instance, temperature-programmed desorption (TPD) and microcalorimetry measurements [25, 26]. Alternatively, theoretical approaches can be used to predict the structure, stability, bonding, binding sites and properties of these nanomaterials. In 2006, Aikens and Schatz [27] pointed out a chemical surface-enhanced Raman scattering (SERS) enhancement for pyridine linked to Au20 cluster (i.e., pyridine@Au20) using time dependent-density functional theory (TD-DFT) and SERS spectroscopy; however, the origins of such enhancement were not definitively established. In 2008, Iori et al. [17] treated the interaction between the HIS (Im model) side chain and the Au (111) surface using DFT-PBE. They established the existence of an unconventional H-bond (namely a Cα–H⋯Au bond) that is uncertain in DNA base pairs and a HIS model with Au (111) surface [17]. Moreover, they found that the unprotonated N1 atom of the Im moiety interacts directly with the top site of the fcc (111) hexagonal lattice [17]. In 2012, Cao and co-workers [28] found an unconventional N–H⋯Au H-bond in nucleobase–gold complexes through anion photoelectron spectroscopy and DFT calculations. Last year, experimental and theoretical reports on various phases of benzenediamine molecules with gold surface using X-ray resonant photoemission spectroscopy (RPES) revealed that ultrafast charge transfer (CT) across the metal–organic interface does not require solely covalent bonds but may occur also through noncovalent interactions [29].
Generally, gold interacts with N-based biomolecules through CT, van der Waals (vdWs), and hydrogen (H)-bonded interactions such as N–H⋯Au, C2–H⋯Au, and C5–H⋯Au types of interaction (see Fig. 1 for atom numbering). These unconventional types of H-bonds can be calculated by modern experimental and theoretical techniques [28]. Theoretically, quantification of CT through covalent and noncovalent interactions within organic-metal clusters can be evaluated from selection of a suitable functional. With this purpose in mind, Urban and co-workers characterized the type of metal–ligand interaction, bonding and CT properties of coinage metals and lone-pair ligands using different DFTs and CCSD (T) methods in connection with relativistic basis sets [30], and showed that the PBE0 functional is well suited to treating these kinds of complexes. At present, we use a set of exchange correlation density functionals (i.e., B3LYP, PBE, PBE0 and M05-2X) in connection with large basis sets to deduce the equilibrium structures, energetics, spectroscopy and chemical bond types (covalent, CT, vdWs) in Im@Aun complexes, where n = 2, 4, 6, 8, 10, and 20. We first check the capability of various DFT functionals for deriving reliable data for Im@Aun through comparison to MP2 geometries and energetics for smaller Im@Aun (n = 2 and 4) complexes. We also considered dispersion effects via inclusion of Grimme’s corrections. Based on the evaluation, we select PBE0 and M05-2X functionals for the treatment of larger sized clusters (n = 6–20). Mainly, we show that Im@Aun properties (both structural parameters and binding energies (BEs) converge to those of Im adsorbed on Au bulk for n ∼8 and that Im@Aun complexes are stabilized by a strong nitrogen–gold bond together with weak anchor assisted H-bond (AAHB) interactions. Finally, we discuss the possible applications of the present findings.
Computational details
Difficulties in studying organic-metal cluster complexes reside in: (1) a balanced description of metal–metal and metal–hetero atoms within the complexes, (2) the correct accounting for electron correlation and dispersion that plays a crucial role in their bonding and structure, (3) the accurate description of several types of interactions that are a priori in action (e.g., covalent bonding within Im, Au–Au bond and covalent and/or non-covalent Au–Im bondings). Our approach overcomes all these difficulties, leading to accurate predictions for Im@Aun complexes.
For the description of H, C, N atoms, we used either 6–31 + G** or aug-cc-pVTZ basis sets [31–33]. Gold atoms were described using the Los Alamos effective core potential (ECP) Lanl2DZ [34] and the associated 6-31 + G**ULanl2DZ basis set (denoted hereafter as BS1) or aug-cc-pVTZULanl2DZ basis set (denoted as BS2).
For geometry optimizations, we used GAUSSIAN 09 [35]. We considered closed-shell configuration for Im@Aun (n = 2, 4, 6, 8, 10, and 20) complexes, which are taken in their ground singlet states. Following the investigations of Tao et al. [24], we looked for nanoclusters linking to the unprotonated nitrogen of Im since the other sites would lead to less stable isomers of Im@Aun. The initial structures of these complexes were inspired from those of the corresponding isolated Aun clusters as established elsewhere [36–39]. Most of them are detailed in a review by Häkkinen [5] and references therein. The Im@Aun equilibrium geometries were then freely optimized using DFT-B3LYP, PBE, and PBE0 methods. In addition, we used the M05-2X functional. Recent reports on this kind of molecular systems proved that the M05-2X method is a priori well suited for accurate description of vdWs and dispersion interactions [40–44]. Indeed, close agreement was noted between M05-2X results and those derived using CCSD(T)/CBS on hydride ion–water clusters [45]. All optimizations were performed with Opt = tight and Int = Ultrafine keywords of GAUSSIAN 09. For some complexes, additional SCF = QC or XQC keywords were used to force convergence. Further Møller-Plesset (MP2) [46] and spin-component scaled MP2 (SCS-MP2) [47] computations were carried out for Im@Au2 and Im@Au4 to benchmark suitable DFT methods for the treatment of the larger Im@Aun complexes. These computations were performed using the MOLPRO (version 2012.1) package [48]. Recent reports on H-bonded and stacked dimers have clearly established that SCS-MP2 is well suited for stacking whereas MP2 describes H-bonded dimers well [49]. To address long-range interactions, such as H-bonding and vdWs, we performed additional computations where we used Grimme’s latest version of empirical correction term (DFT-D3) [50–52]. These computations consist of single-point energy correction for geometries optimized using PBE0 and M05-2X methods.
To quantify the nature of intermolecular interactions of CT between Im and gold clusters and of the strength of AAHBs within Im@Aun clusters, we calculated the vibrational frequencies of these clusters and performed atoms-in-molecules (AIM) analysis as implemented in the AIM 2000 package [53]. AIM is a useful tool to probe covalent and possible weakly bonded systems including vdWs complexes [54–59]. The wave function was generated from M05-2X/BS1 calculation using optimized geometry at the same level. In addition, we analyzed the CT properties between gold clusters and Im using an NBO [60] approach. NBO analysis was carried out at PBE0/BS1 level of theory. Details of the corresponding analysis and the full set of results are given in the electronic supplementary material (ESM).
Results and discussion
Figures 1, 2, 3, 4, 5 and 6 display the optimized structures of Im@Aun (n = 2, 4, 6, 8, 10, and 20). All represent equilibrium structures, all with positive frequencies. The main geometrical parameters computed at DFT-B3LYP, PBE, PBE0 and M05-2X are given, in connection with the BS1 basis set for comparison. The calculated binding energies (BEs) are listed in Tables 1 and 2. For the Im moiety adsorbed on Aun clusters, similar equilibrium geometries were found. For Im@Aun (n = 2, 4), we present also those deduced using the larger BS2 basis set. Moreover, further computations at the MP2/BS1 and MP2/BS2 levels were also carried out for these two complexes. The respective MP2 and SCS-MP2 BEs are provided in Table 1.
Equilibrium geometries of Im@Aun clusters and bonding
Im@Au2
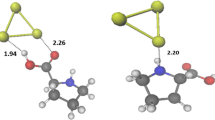
At all levels of theory, we found a planar Im@Au2 stable complex (Fig. 1). This is due to the non-disturbing environment from the gold side (i.e., linear) with perfect orbital interaction between N1 and Au atoms (Fig. S1 in ESM). DFT Au-N1 equilibrium distances are 2.149 (2.117), 2.110 (2.077), 2.116 (2.0847) and 2.146 (2.124) Å and the Au–Au equilibrium distances are 2.568 (2.565), 2.550 (2.545), 2.541 (2.538) and 2.550 (2.546) Å, using the BS1 (BS2) basis set (Fig. 1). Therefore, BS2 leads to slightly shorter equilibrium distances (differences are less than 0.03 Å). MP2 predicts equilibrium distances within the same range as DFT methods. Earlier reports on G@Au2 complex optimized at B3LYP/BS1 derived Au–N and Au–Au distances of 2.162 Å and 2.569 Å, respectively [10]. These results are in close agreement with ours at the same level of theory. For both G@Au2 and Im@Au2 complexes, the Au–Au distance is slightly longer than its value in the isolated Au2 dimer. For CH and NH distances in Im, all electronic structure methods lead to sensitively the same equilibrium distances (Fig. 1). Generally, M05-2X predicts shorter distances than the other DFTs but close to MP2 data and hence predicts stronger interactions between N1⋯Au in Im@Aun complexes.
Im@Au4
For Au4, a Y-shape, a rhombic and a trans-chain structure are known (see for instance Ref. [5]). For nanoclusters of Au4 with Im, we found three different stable planar conformations: Im@Au4a, Im@Au4b, and Im@Au4c (Fig. 2). Im@Au4c is obtained by linking Im to rhombic Au4. Im@Au4a and Im@Au4b result from attaching Im to the Au4 Y-structure, which is no longer symmetrical due to the unsymmetrical nature of Im and, in particular, because of the establishment of the H-bonded interaction (C–H⋯Au) with the neighboring gold atoms (dashed lines in Fig. 2). Similarly, the more stable symmetrical tetrahedral (Td) shape of isolated Au4 within the Im@Au4 cluster converts into Im@Au4a during the optimizations (all methods). The creation of this H-bonded interaction may be related to the relatively acidic nature of the C2-carbon atom in Im. Such H-bonds were already noted for the G@Au2 complex and are known as anchor assisted H-bonds (AAHB) [10]. These H-bonds and weakly bound interactions were confirmed by AIM topography analysis. For illustration, Fig. 7 presents the ρ(rc) and ∇2ρ(rc) values, with the bond critical points (BCP), the ring critical points (RCP) and the cage critical points (CCP) pointed out. The corresponding NBO analyses and discussions can be found in the ESM.
Molecular graphs of Im@Aun complexes at M05-2X/BS1 geometries. Black ρ(rc) values, red ∇2ρ(rc) values. These values are shown near to the bond paths corresponding to Au⋯N, C2–H⋯Au, and C5–H⋯Au interactions. Red dots Bond critical points (BCP), yellow dots ring critical points (RCP), green dots cage critical points (CCP)
Similar to Im@Au2, the N1 atom of the Im moiety coordinates directly with an Au atom with a computed Au–N1 distance of ∼2.1 using either DFT/BS1 or DFT/BS2. The intra Im distances are similar to those given in Fig. 1 for Im@Au2. All DFT methods predict Au–Au distances ranging from ∼2.6 to 2.7 Å, which are slightly longer than those found for Im@Au2. We compute AAHB distances in the order of 3.6 and 4.2 Å using B3LYP that reduce by 0.2–0.6 Å using either PBE0 or M05-2X. Figure 2 shows that AAHB distances are distinctly shorter in Im@Au4a than in Im@Au4b,4c. This is in line with the larger BE computed for Im@Au4a (Table 1). Generally, the M05-2X functional provides shorter C2–H Au H-bonds than the other DFTs. M05-2X and PBE0 account for weak interactions at both BS1 and BS2 levels while B3LYP does not. The reliability of these functionals may affect the energetics of these complexes (see next section). Small changes are observed only in the Au–N1 distance with PBE and PBE0 methods. Moreover, M05-2X and PBE0 always predict shorter NH–Au distances (at AAHB) and hence stronger interactions within both moieties of the Im@Au4 complexes. Moreover, close agreement is found with MP2 results (Table 1). Accordingly, PBE0/BS1 and M05-2X/BS1 levels are suited to the treatment of larger n complexes.
Im@Au6
DFT and ab initio [MP2/CCSD(T)] computations [61] showed that the Au6 hexamer presents three stable forms of D3h, C5v and D4h symmetries. These were used as starting points to link with Im. Figure 3 displays the optimized geometries of Im@Au6 isomers using DFT and BS1. Three isomers were found (Im@Au6a, Im@Au6b and Im@Au6c) where Im forms a complex with the Au6 cluster with an unprotonated nitrogen. Im@Au6a consists of a planar Au6 cluster bonding to Im, which is located in the perpendicular plane of the Au6 plane, whereas both Au6 and Im belong to the same plane in Im@Au6b and Im@Au6c isomers. Figure S1 in the ESM shows that there is a favorable interaction between the orbitals of N1 and the Au adatom for these configurations. A further charge transfer stabilization through covalent and noncovalent bonds may take place also. Indeed, Im@Au6a and Im@Au6b present an adatom type of interaction that is characterized by a unique Au–N1 bond, whereas the Im@Au6c isomer form is stabilized by three different types of interactions consisting of an Au⋯N1 bond and two H-bonded (C–H⋯Au) weak noncovalent interactions as those described above for Im@Au4. The Au–N1 distance was computed to be ∼2.2 to 2.3 Å. Even though other possible conformations were searched, optimizations led solely to Im@Au6a, Im@Au6b and Im@Au6c clusters forms. This is due to orbital overlapping and additional types of noncovalent interactions within these clusters that favor these three conformations. Note that Im@Au6b converts into Im@Au6a at PBE0/BS1 and M05-2X/BS1 levels.
Im@Au8
The three most stable forms of Au8 are of D4h, C2v and Td symmetries [62]. When Im interacts with Au8, six isomers are found: Im@Au8a, Im@Au8b, Im@Au8c, Im@Au8d, Im@Au8e and Im@Au8f (Fig. 4). In Im@Au8a,8b,8e,8f, the gold octamer is a planar star-/wheel-like molecule with Im either in the gold atom plane or orthogonal to it. In Im@Au8c and Im@Au8d, Im is connected to 3D Au8 either as an adatom or to the surface of the cluster. For the Au8 cluster, Hansen et al. [62] recently predicted a similar non-planar structure, which has the lowest energy isomer using CCSD(T). Im@Au8a, Im@Au8b, Im@Au8c and Im@Au8e clusters present relatively short Au⋯N distances (of ∼2.2 Å) whereas a slightly longer Au⋯N distance (of ∼2.3 Å) is computed for Im@Au8d and Im@Au8f. The latter can be considered as models for Im adsorbed on an Au(111) surface [63]. It is interesting to discuss the Im@Au8f geometry obtained from the M05-2X method. For instance, this cluster shows a structure that is tilted slightly towards gold surface whereas other methods (B3LYP, PBE and PBE0) predict Im exactly perpendicular to the surface. Based on our geometrical data, hybrid meta functionals are well suited to the accurate description of covalent and dispersive interactions. No obvious H-bonded interaction (C–H⋯Au) is visible for Im@Au8. Surprisingly, the PBE method leads to a relatively shorter Au⋯N distance (2.257 Å) compared to the other DFT methods.
Im@Au10
Optimized geometries of Im@Au10 complexes are presented in Fig. 5. In total, five structures were computed (Im@Au10a–e). Im@Au10a–c clusters are planar whereas 3D structures were found for Im@Au10d–e. Interestingly, Im@Au10 clusters show a transition from 2D to 3D structures as already noted for isolated Au10 [64]. For Im@Au10a,b,c,e clusters, Im links to the Au10 cluster via a unique Au–N bond in the N1 atom, whereas the N1 atom of Im forms two coordination covalent bonds with two gold atoms with equal distances (of 2.261 Å) in Im@Au10d. For Im@Au10a, B3LYP, PBE, and PBE0 predict a stable structure, whereas this isomer shows different geometry and converts into Im@Au10d using the M05-2X method because of the unequal arrangement of gold atoms at the Im interaction site. Here, the excess exchange correlation functional favors geometry reorganization. The other complexes have a double ring (hexagon model) type for gold clusters (i.e., planar) but Im forms two different types of interactions. Im@Au10b has a unique Au and N1 bond. Im@Au10e presents a Au⋯N bond and an H-bonded interaction (C–H⋯Au). No H-bonds were found for Im@Au10a and Im@Au10c. For Im@Au10d, a C–H⋯Au bond was characterized in addition to the two Au⋯N bonds already noted above, leading to a further stabilization of this isomer. Our results agree with those found for cyclometalated 6-benzylpyridines interacting with gold (III) conformers, where a short Au–N distance ranging from 2.098 to 2.105 Å was predicted using B3LYP/def2-TZVP [65]. In contrast, the calculated Au–N1 bond distances were shorter than in the Im@Au(111) surface (i.e., ≈2.3 Å) [63].
Im@Au20
Through analysis of combined photoelectron spectroscopy and density functional results, Li et al. [2] proposed a highly symmetrical tetrahedral structure for Au20 gold clusters. Aikens and Schatz [27] suggested two different types of cluster model: (1) a tetrahedral gold cluster similar to that proposed by Li et al. [2] and (2) a vertex model with an adatom structure. The Au20 tetrahedral
Binding energies of Im@Aun clusters
In addition to geometries, BEs of complexes are very important in validating suitable DFT methods for the accurate description of noncovalent interactions [45]. The BEs of all complexes were calculated within the supermolecule approach and corrected for basis set superposition error (BSSE) using the procedure suggested by Boys and Bernardi [72]. The counterpoise (CP) method implemented in the Gaussian package was used to carry out BSSE correction. The BEs of various complexes were calculated using the following energy expression:
where E AB is the total energy of the Im@Aun complex at equilibrium, E A is the energy of the Aun gold nanocluster and E B is the energy of Im.
In benchmark computations, we calculated the BEs of Im@Au2 and Im@Au4 using either BS1 or BS2 and DFT approaches. Therefore, their BSSE-corrected BEs calculated at the B3LYP, PBE, PBE0, M05-2X and MP2 (SCS-MP2) methods using BS1 and BS2 [single point (SP)] basis sets are listed in Table 1. BE enhancement was observed when the size of the basis set increases (from BS1 to BS2). Also, using B3LYP, computed BEs at BS1 and BS2(SP) energies were almost equal except for Im@Au2. As expected, the B3LYP/BS1 method was not sufficient to predict the geometry and energies of Im and gold clusters. The same BEs at B3LYP and PBE/BS2 levels were in very good agreement with SCS-MP2 rather than MP2 values. Moreover, differences between MP2/BS2 with DFT methods were B3LYP (−19), PBE(−19), PBE0(−27) and M05-2X (−28) (all values are in kJ mol−1). As established in the literature, B3LYP and PBE methods are very suitable for the description of covalent interactions. To scrutinize the ability of these functionals to describe noncovalent types of interaction, we treated Im@Au4 clusters, which are stabilized by both (Au⋯N and C–H⋯Au) interactions. M05-2X, PBE and PBE0 methods predict BEs relatively higher than the corresponding B3LYP methods. Values using M05-2X and PBE0 are particularly close to SCS-MP2 values. From this, we conclude that use of PBE and B3LYP methods with BS2 is appropriate for covalently bonded interactions whereas noncovalent interactions need an additional exchange correlation functional (i.e., PBE0 or M05-2X). Finally, SP energies and BEs at optimized structures using either BS1 or BS2 were found to be close. Based on these benchmarks, we calculated the BEs of larger size gold clusters (n ≥6) using the BS2 basis set, where SP computations were performed at the respective optimized structure at the corresponding DFT/BS1 level.
At the M05-2X/BS2 level, we computed BEs of ∼ −126, ∼ −168, ∼ −160 and ∼ −145 (in kJ mol−1) for Im@Au2, Im@Au4a, Im@Au4b, Im@Au4c, respectively. Such large BEs can be explained by the strong Au–N bond created upon complexation. Moreover, isomer Im@Au4a is more stable than the other isomers. This is due to the formation of relatively stronger unconventional (C–H⋯Au) H-bonds between gold and Im. Im@Au4b possesses an analogous type of interaction but different C–H groups are involved in the AAHB, resulting in a reduction in BE(Im@Au4b) reduction by ∼8 kJ mol−1. Indeed, it can be found from our calculations that the acidic nature of the C2–H Au interaction within Im@Au4a is relatively stronger than the C5–H⋯Au H-bond in Im@Au4b.
Table 2 presents the BSSE-corrected BEs computed using PBE0 and M05-2X for Im@Aun (n = 6, 8, 10 and 20). The corresponding values computed using B3LYP and PBE are listed in Table S1 of the ESM. Close examination of Table 2 reveals that adatom model isomers are more stable than the other weakly bonded models, except for Im@Au10 where the 3D isomers (Im@Au10d and Im@Au10e) exhibit outstanding stability (BEs of ∼ −200 kJ mol−1) due to the strong chemical bonds occurring there. These two Au10 clusters together with Im@Au6c, Im@Au8d, Im@Au8f and Im@Au20b may mimic the Au surface model. The calculated dipole moment value of these clusters is close to zero, hence confirming that these clusters may mimic the surface environment. This is largely corroborated by a smaller Au–N distance (i.e., ≤2.3 Å) for adatom type complexes whereas Au–N distances of ∼2.3 Å are computed for surface type models.
At the M05-2X/BS2 level, the calculated BEs for Im@Au6c, Im@Au8d, Im@Au8f and Im@Au20b complexes are −57.5, −60.3, −72.9, and −53.0 kJ mol−1, respectively. In particular, Au8 surface models (Au8d and Au8f) have larger BEs than the other surface mimicking models. This is due to the additional AAHB (C2–H⋯Au) interaction within Im@Au8d at the bridge site, and to dispersion interactions within the Im@Au8f complex. Note that the calculated M05-2X/BS2 BEs (∼ −50 kJ mol−1) for surface model clusters are in close agreement with the recent PBE treatment of Im adsorbed on a gold (111) surface {Im@Au(111), BE = ∼ −42 kJ/ mol−1 [63]} and an earlier PBE study [17] of HIS adsorbed on a gold (111) surface) (HIS@Au(111), BE = −45.6 mol−1).
Tables 1 and 2 also present the D3 corrected PBE0 and M05-2X BEs. For Im@Au2 and Im@Au4, Table 1 shows that an enhancement of BEs is observed for PBE0 + D3 (ranging from −7 to–13 kJ mol−1) whereas M05-2X and M05-2X + D3 lead to similar BEs (differences of ∼ −2 kJ mol−1). Hence, M05-2X represents better predictions for these complexes than PBE0. These BEs are also in close agreement with the SCS-MP2 method. Similar remarks can be drawn for larger sized gold clusters interacting with Im (shown Table 2), where D3 dispersion accounts for −9 to −31 with PBE0 and −2 to −8 kJ mol−1 with M05-2X, respectively. The largest deviations are for Im@Au6c, Im@Au8d, Im@Au8f and Im@Au20b, which are “surface mimicking models”. More generally, our work reveals that dispersions play an important role in the stability of Im@Aun complexes.
AIM analysis
Bader’s AIM theory has been employed to quantify the nature of bonding between two molecules or surfaces [53–58]. Several studies have reported characterization of the nature of bonding between organic molecules and metal clusters using AIM parameters [22, 73–76]. AIM can also be used to characterize the agostic type of interaction since it provides more precise quantitative measures with which to describe them. The electron density [ρ(rc)] and Laplacian [∇2ρ(rc)] at the bond critical points (BCPs) of the Au N1, C2-H Au, and C5-H · Au interactions of molecular graphs are shown in Fig. 7. The red, yellow and green dots indicate BCP, RCP (ring) and CCP (cage), respectively. In addition to ρ(rc) and its ∇2ρ(rc), other significant parameters such as the kinetic (Gc), potential (Vc), and total (Hc = Gc + Vc) energy density values are provided in Table 3.
The ∇2ρ(rc) values indicate the nature of bonding; in particular, positive and negative values of ∇2ρ(rc) dictate noncovalent and covalent character, respectively [55]. The electronic energy density also provides the valuable information about the nature of bonding. Gc and Vc are always positive and negative, respectively, whereas Hc value depends on the relative magnitude of Gc and Vc. If the Gc value is larger than the absolute value of Vc, the Hc value is positive, thus a purely closed shell interaction is expected. Otherwise, the negative Hc indicates that the interactions correspond to some degree of covalent character. It can be found from our calculated negative values of Hc that all our Au · N1 (Im) complexes are covalent in nature.
Figure 7 shows that the ρ(rc) values of Im@Aun clusters range from 0.0608 to 0.0988 a.u. The strongest interaction was found for Au4a,b complexes [ρ(rc) values of ∼0.0966 a.u.) and the interaction at surface models such as Au6c, Au8d and Au20b [ρ(rc) values range from 0.0608 to 0.0658 a.u.]. ∇2ρ(rc) values exhibited similar trends. The calculated values of ρ(rc) and ∇2ρ(rc) at the H-bonds (designated as HBCP) for all the clusters ranged from 0.0051 to 0.0092 and 0.0036 to 0.0084 a.u., respectively. These values clearly indicate the involvement of weak H-bonding interactions in the stabilization of these clusters. Close scrutiny of our AIM analysis (see Fig. 7) shows that the C2–H Au interaction is present only in Im@Au4a rather than the Im@Au4b complex (here C5–H⋯Au) hence rationalizing the observed major stability of Im@Au4a vs. Im@Au4b. Similarly, the large BE found for Im@Au10d (∼ −233 kJ mol−1) due to the formation of double Au⋯N1 (Im) bonds (Fig. 5) is also consistent with our ρ(rc) and ∇2ρ(rc) values at BCPs for this complex. Generally, the calculated electron density at the BCPs correlates roughly with the strength of interaction reported by several authors [77–81].
Vibrational spectroscopy
Table 4 lists the M05-2X/6-31 + G** calculated vibrational stretching frequencies, intensities and red/blue shifts. The shifts were calculated from the asymmetric (νas) and symmetric (νss) stretching frequencies of N–H and C–H of isolated Im. Upon formation of Im@Aun complexes, the free N–H group in Im always red shifts whereas νCH frequencies are blue-shifted except Im@Au4a. Similarly, all free N–H and H-bond N–H have red shifts. It can be observed that the red/blue shifts and intensities of C–H⋯Au (νC2H) are significantly higher than that of C–H⋯Au (νC4H) interactions. For instance, the calculated νNH, νC2H and νC3H frequency shifts for Im@Au4a are −10, −43, and 23 cm−1, respectively. The νNH shifts range from −50 to −5 cm−1and the νC2H and νC4H shifts are in the ranges ±43 to 38 cm−1 respectively. The largest effect is observed for the Im@Au4a complex (−43 cm−1) νC2H frequency. This is due to the strong AAHB interaction between C2–H and the gold surface. Moreover, we observed a strong enhancement of the intensity of the N–H stretching bands on the complexes compared to free Im. The effects on C–H stretching band intensities were less pronounced. Complexation-induced shifts and changes in the intensity of the corresponding bands can be checked by means of vibrational spectroscopy as performed by Gruene et al. [71] for isolated small gold clusters.
UV–vis spectra
Recent benchmark calculations showed that the PBE0 method is accurate enough for the prediction of the optoelectronic properties [82]. Therefore, UV–vis adsorption spectra of Im@Aun (n = 2–20) complexes along with those of the respective monomers (depicted in Fig. 8) were computed from TDDFT calculations using the PBE0 functional. It can be found from this figure that isolated Au2 and Im exhibit bands below 250 nm, whereas Im@Aun complexes exhibit intense peaks from 400 to 700 nm. It is interesting to note that small-sized clusters with Im have bands in both ranges with moderate intensities. Comparison of the spectra in Fig. 8 reveals a significant intensity enhancement as the gold clusters size increases. This is due to the increased efficiency of ligand-to-metal charge transfer in accordance with NBO analysis (cf. ESM)
Systematic changes observed in the absorption spectrum and intensity with cluster size clearly reveals that Im@Aun complexes have potential applications. Recent experimental and theoretical studies on adenine with gold in solution showed similar trends [83]. They also pointed out that, after complex formation, there is an immediate reduction in the intensity of surface plasmon absorbance and a significant red shift in the wavelength (from 525 to 717 nm). Last year, experimental studies on thiol derivatives adsorbed on gold clusters and embedded in polymers [84] showed significant visible fluorescence activity when the thiols were substituted with Im, which is in line with the present findings.
General trends
Figure 9 displays the evolution of BEs and Au–N distances versus n for both adatom and surface model clusters at M05-2X/BS2 level. We also give the corresponding values for Im adsorbed on Au(111) surface [Im@Au(111)] [63]. This figure shows that the calculated |BEs| decrease gradually from Au2 to the Au20-mer except for Im@Au4 clusters because of the additional unconventional H-bonds (i.e., AAHB) that stabilize such complexes further. For surface model clusters, |BEs| converge smoothly to the corresponding value for Im@Au(111). Im@Au8, Im@Au10 and Im@Au20 clusters also present some common characteristics with Im adsorbed on a Au (111) surface [Im@Au(111)]. Therefore, these clusters can be considered as suitable models for investigating organic molecules interacting with the Au (111) surface at low computational cost. Moreover, the nature of the interaction at AAHB can be quantified from AIM analysis. This clearly reveals that gold can act as a potential H-bond acceptor from different environments. These important findings were confirmed by NBO and AIM analysis (see ESM) and vibrational spectra for these complexes. Recently, Aiswaryalakshmi et al. [74] studied the hydrogen bonded complexes formed between the square pyramidal Fe(CO)5 with HX (X = F, Cl, Br). They showed that Fe can act as a strong H-bond and halogen bond acceptor [74]. Similarly, Sun and Felice [22] found that gold can act as H-bond acceptor with biomolecules (N–H⋯Au and N/O⋯Au). In addition, very recent investigations on Aun clusters with halogen molecules revealed the covalent nature of the interaction between gold and halogen(s) within such clusters [75], whereas further stabilization is ensured by the occurrence of noncovalent interactions between gold and their complexes as in the present case.
Evolution of the Au–N distances and BEs of Im@Aun complexes vs n at M05-2X/BS2 level compared with the Im@Au(111) surface [63]
Both BEs and structures of Im@Aun clusters clearly show that various types of noncovalent interaction stabilize these complexes. AIM analysis indicates the involvement of weak H-bonding interactions in the stabilization of these clusters in addition to the Au–N bond for which a donor-acceptor type interaction was confirmed by NBO analysis (see ESM for further details). Indeed, the donor-acceptor interactions in these clusters involve the interaction of the lone pairs of the N1 nitrogen atom [LP(1)N1] of Im and Au clusters antibonding orbitals (cf. Table S2). For Im@Au2, the charge transfer from lone pair of N1 to the antibonding orbital of gold [BD*(1)Au –Au] is higher than the LP(1)N to LP*(1)Au interactions. Therefore, the nature of N1 and the Au1 bond is covalent. Similarly, charge transfer in Im@Au6c and Im@Au20b (surface models) exhibits such LP(1)N to LP*(1) Au interactions. An analogous type of charge transfer interaction is also observed in adatom models (Im@ Au6a, Au8a, Au8b, Au10a and Au20a). Back bonding effects of gold to Im and intra molecular charge transfer in Im play crucial roles in Im@Aun complexes. Especially, significant space through charge transfer from the LP of gold [LP*(6)Au1) to C2 and C5 atoms of Im (RY*(2)C2(5)] was observed from our surface model (Im@Au20b). In addition, charge transfer characterization features of these complexes have also been observed from their shape in frontier molecular orbital analysis. For illustration, Figures S1 and S2 display the isosurface density (0.02 a.u) plots for the Im@Aun complex calculated at PBE0/BS1. Both surface models Im@Au6c, and Im@Au20b complex HOMO orbitals are localized on the N atom of Im and the corresponding LUMO orbitals are localized mostly at gold clusters.
Conclusions
We treated Im@Aun (n = 2, 4, 6, 8, 10, and 20) complexes using various DFT functionals and basis sets. We found that electron correlation plays a crucial role in the description of these organo-metal complexes and at interfaces. Our work establishes the capability of GGA-PBE0 and M05-2X functionals for providing accurate results for the chemisorption of small molecules on metal clusters and for physisorption, which is dominated mostly by vdWs interactions.
Upon complexation of Im with Aun clusters, strong Au⋯N bonds occur between Au and the deprotonated nitrogen of Im as well as weak C–H⋯Au H-bond interactions [unconventional type of AAHB (C2–H⋯Au)]. Vibrational, AIM and NBO analyses revealed the role of various types of noncovalent interactions in the stability of these complexes. On the whole, Im molecular arrangements at gold clusters depend on both covalent and dispersive interactions. Principally, surface mimicking models (such as Im@Au6c, Im@Au8d, Im@Au8f and Im@Au20b) have more of a dominant dispersion effect than the other, adatom-type, complexes. Finally, we characterized a strong bond between Im and Aun species that may serve for self-assembled monolayers (SAMs) of organic molecules on gold instead of the well-known gold–sulfur ones for further applications of SAMs [85].
References
Boyen HG, Kastle G, Weigl F, Koslowski B, Dietrich C, Ziemann P, Spatz JP, Riethmuller S, Hartmann C, Moller M, Schmid G, Garnier MG, Oelhafen P (2002) Science 297:1533–1536
Li J, Li X, Zhai HJ, Wang LS (2003) Science 299:864–867
Pyykko P (2004) Angew Chem Int Ed 43:4412–4456
Pyykko P (2008) Chem Soc Rev 37:1967–1997
Hakkinen H (2008) Chem Soc Rev 37:1847–1859
Yang Z, Leon I, Wang LS (2013) J Chem Phys 139:021106
Wang J (2000) Nucleic Acids Res 28:3011–3016
Lynch I, Salvati A, Dawson KA (2009) Nat Nano 4:546–547
Sindhu K, Rajaram A, Sreeram KJ, Rajaram R (2014) RSC Adv 4:1808–1818
Zhang L, Ren T, Yang X, Zhou L, Li X (2013) Int J Quantum Chem 113:2234–2242
Zhang X, Sun CQ, Hirao H (2013) Phys Chem Chem Phys 15:19284–19292
Kryachko ES, Remacle F (2005) J Phys Chem B 109:22746–22757
Rosa M, Corni S, Felice RD (2012) J Phys Chem C112:21366–21373
Kumar A, Mishra PC, Suhai S (2006) J Phys Chem A 110:7719–7727
Lv G, Wei F, Jiang H, Zhou Y, Wang X (2009) J Mol Struct (THEOCHEM) 915:98–104
Martinez A (2010) J Phys Chem C 114:21240–21246
Iori F, Corni S, Felice RD (2008) J Phys Chem C 112:13540–13545
Cossaro A, DellAngela M, Verdini A, Puppin M, Kladnik G, Coreno M, Simone M, Kivimaki A, Cvetko D, Canepa M, Floreano L (2010) J Phys Chem C 114:5011–15014
Storhoff JJ, Elghanian R, Mirkin CA, Letsinger RL (2002) Langmuir 18:6666–6670
Martinez A (2009) J Phys Chem A 113:1134–1140
Shukla MK, Dubey M, Zakar E, Leszczynski J (2009) J Phys Chem C 113:3960–3966
Sun W, Felice RD (2012) J Phys Chem C 116:24954–24961
Jena NK, Chandrakumar KRS, Ghosh SK (2012) J Phys Chem C 116:17063–17069
Tao NJ, de Rose JA, Lindsay SM (1993) J Phys Chem 97:910–919
Jalkanen J, Zerbetto F (2006) J Phys Chem B 110:5595–5601
Ihm H, Ajo H, Gottfried J, Bera P, Campbell C (2004) J Phys Chem B 108:14627–14633
Aikens CM, Schatz GC (2006) J Phys Chem A 110:13317–13324
Cao GJ, Xu HG, Li RZ, Zheng W (2012) J Chem Phys 136:014305
Kladnik G, Cvetko D, Batra A, Dell’Angela M, Cossaro A, Kamenetska M, Venkataraman L, Morgante A (2013) J Phys Chem C 117:16477–16482
Pasteka L, Rajsky T, Urban M (2013) J Phys Chem A 117:4472–4485
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Dupuis M, Rys J, King HF (1976) J Chem Phys 65:111–116
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96:6796–6806
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Frisch MJ et al. (2009) Gaussian 09 Revision, A. 1, Gaussian Inc, Wallingford, CT
Furche F, Ahlrichs R, Weis P, Jacob C, Gilb S, Bierweiler T, Kappes MM (2002) J Chem Phys 117:6982
Xiao L, Tollberg B, Hu X, Wang L (2006) J Chem Phys 124:114309
Assadollahzadeh B, Schwerdtfeger P (2009) J Chem Phys 131:064306
David J, Guerra D, Restrepo A (2009) Chem Phys Lett 539:64–69
Staroverov VN, Scuseria GE, Tao J, Perdew JP (2003) J Chem Phys 119:12129
Grimme S (2004) J Comput Chem 25:1463–1473
Zhao Y, Truhlar DG (2005) Phys Chem Chem Phys 7:2701–2705
Zhao Y, Lynch BJ, Truhlar DG (2005) Phys Chem Chem Phys 7:43–52
Schultz NE, Zhao Y, Truhlar DG (2005) J Phys Chem A 109:4388–4403
Prakash M, Gopalsamy K, Subramanian V (2011) J Chem Phys 135:214308
Curtiss LA, Redfern PC, Raghavachari K, Rassolov V, Pople JA (1999) J Chem Phys 110:4703
Grimme S (2003) J Chem Phys 118:9095
Werner HJ et al. (2012) Molpro 2012.1 a package of ab initio programs (http://www.molpro.net.)
Bachorz RA, Bischoff FA, Hofener S, Klopper W, Ottiger P, Leist R, Frey JA, Leutwyler S (2008) Phys Chem Chem Phys 10:2758–2766
Grimme S (2006) J Comput Chem 27:1787–1799
Grimme S (2006) J Chem Phys 124:034108
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Biegler-Konig F, Schonbohm J, Derdau R, Bayles D, Bader RFW (2000) AIM version 1; Bielefeld, Germany
Bader RFW (1990) Atoms in molecules. a quantum theory. Oxford University Press, New York
Grabowski SJ, Sokalski WA, Dyguda E, Leszczynski J (2006) J Phys Chem B 110:6444–6446
Parthasarathi R, Subramanian V, Sathyamurthy N (2007) J Phys Chem A 111:13287–13290
Prakash M, Gopalsamy K, Subramanian V (2009) J Phys Chem A 113:13845–13852
Kumar RM, Elango M, Subramanian V (2010) J Phys Chem A 114:4313–4324
Prakash M, Subramanian V (2011) Phys Chem Chem Phys 13:21479–21486
Glendening ED, Reed AE, Carpenter JA, Weinhold F, NBO Version 31
Han Y-K (2006) J Chem Phys 124:024316
Hansen JA, Piecuch P, Levine BG (2013) J Chem Phys 139:091101
Prakash M, Mathivon K, Benoit DM, Chambaud G, Hochlaf M (2014) Phys Chem Chem Phys 16:12503–12509
Götz DA, Schäfer R, Schwerdtfeger P (2013) J Comp Chem 34:1975–1981
Schmidbaur KFH, Al-juaid SS (2013) Inorg Chem 19:9669–9674
Zhao HY, Ning H, Wang J, Su XJ, Guo XG, Liu Y (2010) Phys Letters A 374:1033
Ford MJ, Soulé de Bas B, Cortie MB (2007) Mat Sci Eng B 140:177
Kryachko ES, Remacle F (2007) Int J Quant Chem 107:2922–2934
Zhao LX, Lei YM, Zhang M, Feng XJ, Luo YH (2009) Physica B 404:1705
Yang A, Fa W, Dong J (2010) Phys Lett A 374:4506
Gruene P, Rayner DM, Redlich B, van der Meer AFG, Lyon JT, Meijer G, Fielicke A (2008) Science 321:674–676
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Jamshidi Z, Farhangian H, Tehrani AZ (2013) Int J Quan Chem 113:1062–1070
Aiswaryalakshmi P, Mani D, Arunan E (2013) Inorg Chem 52:9153–9161
Zhao Q (2014) J Mol Model 20:2133–2138
Shankar R, Kolandaivel P, Senthilkumar L (2010) J Phys Org Chem 24:553–567
Scheiner S, Grabowski SJ, Kar T (2001) J Phys Chem A 105:10607–10612
Grabowski SJ (2001) J Phys Chem A 105:10739–10746
Parthasarathi R, Subramanian V, Sathyamurthy N (2006) J Phys Chem A 110:3349–3351
Parthasarathi R, Raman SS, Subramanian V, Ramasami T (2007) J Phys Chem A 111:7141–7148
Mandal A, Prakash M, Kumar RM, Parthasarathi R, Subramanian V (2010) J Phys Chem A 114:2250–2258
Solomon RV, Bella AP, Vedha SA, Venuvanalingam P (2012) Phys Chem Chem Phys 14:14229–14547
Arikrishnan J, Sheerin SKT, Murugavelu M, Karthikeyan B (2011) Indian J Chem 50A:46–50
Carotenuto G, Nicola D, Nicolais L (2013) Adv Mater Sci Eng 54:8284
Häkkinen H (2012) Nat Chem 4:443–455
Merrick JP, Moran D, Radom L (2007) J Phys Chem A 111:11683–11700
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the research through the Research Group project No. RGP-VPP-333. We are also grateful for a Marie Curie International Research Staff Exchange Scheme Fellowship within the 7th European Community Framework Programme under Grant No. IRSES-GA-2012-31754, the COST Action CM1405 MOLIM. M.P. thanks LABEX Modélisation & Expérimentation pour la Construction Durable (MMCD, U. Paris-Est) for financial support and Prof. V. Subramanian (CSIR-CLRI, Chennai, India) for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2486 kb)
Rights and permissions
About this article
Cite this article
Prakash, M., Chambaud, G., Al-Mogren, M.M. et al. Role of size and shape selectivity in interaction between gold nanoclusters and imidazole: a theoretical study. J Mol Model 20, 2534 (2014). https://doi.org/10.1007/s00894-014-2534-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2534-8