Abstract
Density functional theory (DFT) was used to investigate palladium(0)-catalyzed cycloaddition of alkynyl aryl ethers and alkynes to generate 2-methylidene-2H-chromenes. Calculations indicated that the cycloaddition had five possible reaction pathways: I, II, III, IV, and V. In the palladium(0)-alkynyl aryl ether complex IM1, the oxidative addition of the Caryl–H bond occurred prior to the dissociation of a ligand PMe3. The dissociation of a ligand PMe3 from the five-coordinated complex IM2 was much easier to achieve than the hydrogen transfer reaction and the substitution reaction of alkynes. In the palladium(0)-hydride complex IM4, the hydrogen migration of H1 from palladium to carbon C1 was much easier to achieve than migration to carbon C2. In the four-coordinated aryl-palladium-alkyne complexes IM6a and IM6b, the alkyne insertion reaction into the Pd–Caryl bond occurred prior to that into the Pd–Calkenyl bond. The reaction channel IM1 → TS1 → IM2 → IM4 → TS3a → IM5a → IM6a → TS4a1 → IM7a1 → TS5a1 → IM8a was the most favorable among the catalytic reaction pathways of the cycloaddition of alkynyl aryl ethers and 2-butynes catalyzed by the palladium(0)/PMe3 complex. Moreover, hydrogen migration was the rate-determining step for this channel. The dominant product was 2-methylidene-2H-chromenes P1, which is in agreement with experimental studies.

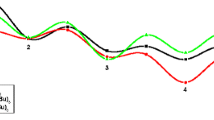
Energy surface of the most favorable channel in the palladium-catalyzedcycloaddition of alkynyl aryl ethers with alkynes
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metal-catalyzed functionalization of C–H bonds is a rapidly growing field in synthetic organic chemistry and organometallic chemistry that enables efficient access to a wide variety of building blocks [1–5]. Much effort has naturally been devoted to developing more convenient and efficient strategies for catalytic C–H functionalization reactions. During the last three decades, many successful applications of catalytic C–H functionalization reactions directed toward the construction of C–C or C–N bonds have been reported in synthetic communities. Many transition metals, such as manganese [6], iron [7], cobalt [8], nickel [9], ruthenium [10], rhodium [11, 12], palladium [9, 13–17], iridium [18], and platinum [19] have been found to catalyze C–H functionalization reactions.
Recently, Minami et al. [13] reported palladium-catalyzed cycloaddition of alkynyl aryl ethers with alkynes via ortho C–H activation to give the product 2-methylidene-2H-chromenes in 93 % yield, as illustrated in Scheme 1. They showed that the active catalyst was the palladium(0) complex, and suggested a likely mechanism with two possible reaction pathways: C–H bond activation and cleavage, alkyne insertion, and reductive elimination; C–H bond activation, hydrogen migration, and alkyne insertion.
In order to understand the reaction mechanism of palladium(0)-catalyzed cycloaddition of alkynyl aryl ethers and alkynes to generate 2-methylidene-2H-chromenes, the cycloaddition of alkynyl aryl ethers and 2-butynes catalyzed by the palladium(0)/PMe3 complex was studied in the present work. Specifically, this study focused on: (1) the energetics of the overall catalytic reaction pathways in palladium(0)-catalyzed cycloaddition, (2) the structural features of intermediates and transition states, (3) the most favorable reaction channel, (4) the possible reaction channels of hydrogen migration, and (5) the possible pathways of alkyne insertion in this reaction mechanism. The possible reaction pathways of palladium(0)-catalyzed cycloaddition of alkynyl aryl ethers and 2-butynes are outlined in Scheme 2.
Computational details
All calculations were carried out with the Gaussian 09 programs [20]. The geometries of all the species were fully optimized using the density functional theory (DFT) [21] M06 method [22]. The 6-31G(d,p) basis set was used for carbon, oxygen, silicon, phosphorus, and hydrogen atoms, and the LANL2DZ basis set was used for palladium, adding one set of f-polarization function with an exponent of 1.472 [23]. Frequency calculations at the same computational level were performed to confirm each stationary point to be either a minimum (IM) or a transition structure (TS). The intermediates were characterized by all real frequencies. The TSs were verified by intrinsic reaction coordinate (IRC) [24] calculations and by animating the negative eigenvector coordinates with a visualization program (Molekel 4.3) [25, 26]. To obtain the starting structures for TS optimizations, the relaxed potential energy surface (PES) scan was performed to find an IRC of a maximum on the approximate elementary reaction channel. In addition, the bonding characteristics were analyzed by natural bond orbital (NBO) theory [27–30]. NBO analysis was performed by utilizing NBO5.0 code [31] at the same computational level. Furthermore, the electron densities, ρ, at the bond critical points (BCPs) or the ring critical points (RCPs) for some species were calculated with the AIM 2000 program package [32, 33].
Results and discussion
All the optimized structures in this reaction mechanism are illustrated in Figures S1–6 in the Supporting Information. The relative free energies ΔG, enthalpies ΔH, and ZPE corrected electronic energies ΔE are provided in Tables S1–2 in the Supporting Information. Unless otherwise noted, the discussed energies in the following are relative free energies ΔG.
Caryl–H bond functionalization: oxidative addition of the Caryl–H bond
As illustrated in Scheme 2, the oxidative addition of the Caryl–H bond has two possible reaction pathways: the palladium(0)-alkynyl aryl ether complex IM1 went through the oxidative addition to give five-coordinated palladium(0)-hydride complex IM2 via a transition state TS1 with a free energy of 135.6 kJ mol−1, and then the dissociation of ligand PMe3 from IM2 generated the active palladium(0)-hydride complex IM4; the dissociation of ligand PMe3 from IM1 formed an active intermediate IM3, which would undergo the oxidative addition through TS2 with a free energy of 166.0 kJ mol−1, leading to IM4. Hence, the first pathway was more dominant than the second.
NBO analysis of intermediate IM1 showed there was a back-donation π bond between palladium and the πC1–C2 bond of alkynyl: the occupied π bonding orbital (πC1–C2) acted on the empty hybridized orbital of palladium leading to a σ coordinate bond; on the other hand, the occupied d orbital (d xy, d xz, d yz) of palladium acted on the empty π* antibonding orbital (π*C1–C2) leading to a π back-donation bond. In the oxidative addition of the Caryl–H bond, the distance between C4 and H1, d (C4–H1), was 1.720, while d (Pd–C4) and d (Pd–H1) distances are 2.091, 1.608 Å, indicating significant interaction between Pd and H1, C4 occurred; conversely, the C4–H1 bond was weakened considerably, as demonstrated by analyzing the changes in bond orders P ij and electron densities ρ at the BCPs (e.g., Pd–H1 bond, P ij, IM1: 0.000 → TS1: 0.299 → IM2: 0.379; ρ, IM1: 0.000 → TS1: 0.125 → IM2: 0.134 eÅ−3). Transition state TS1 involved a Pd–C2–O1–C3–C4 five-membered ring, and the electron density of the RCP was 0.014 e Å−3. The Pd-H1 and Pd-C4 bonds of IM2 were σ bond, and the orbital energies were −767.6 and −894.6 kJ mol−1, respectively. NBO analysis of IM4 also showed the Pd-H1 and Pd-C4 bonds were σ bond, and the orbital energies were −829.8 and −922.6 kJ mol−1, which were lower than those of IM2. The formation of the back-donation π bond between palladium and the πC1–C2 bond of alkynyl in IM4 weakened and activated the C1–C2 bond, which would result in hydrogen migration.
The most favorable reaction channel: path I
Figure 1 shows the most favorable channel leading to 2-methylidene-2H-chromenes in the palladium(0)-catalyzed cycloaddition of alkynyl aryl ethers and 2-butynes. The hydrogen migration in IM4 had two possible reaction modes: the migration of H1 from Pd to C1 is denoted as “a”, while that of H1 from Pd to C2 was denoted as “b”. Intermediate IM4 went through a hydrogen transfer reaction via TS3a with a free energy of 165.9 kJ mol−1, leading to complex IM5a. Next, the complexation of 2-butyne to IM5a generated complex IM6a. Intermediate IM6a then underwent the alkyne insertion reaction to form complex IM7a1, via the TS4a1 with a free energy of 163.2 kJ mol−1. Finally, intermediate IM7a1 underwent the reductive elimination reaction via TS TS5a1 with a free energy of 12.0 kJ mol−1 to generate the complex IM8a giving the product 2-methylidene-2H-chromene. Hence, hydrogen migration was the rate-determining step for this pathway.
The high stabilization energies of 157.4 and 206.8 kJ mol−1 for the πC1–C2 → σ*Pd–H1 and πC1–C2 → (5 s)Pd in TS3a revealed the strong interaction between πC1–C2 and σ*Pd–H1 and (5 s)Pd orbital and the electron migration tendency from πC1–C2 to σ*Pd–H1 and (5 s)Pd. NBO analysis of IM5a showed that both the Pd–C2 and Pd–C4 bonds were σ bonds, and their orbital energies were −985.5 and −977.1 kJ mol−1, respectively. There was a back-donation π bond between palladium and the πC6–C7 bond in IM6a, and NBO analysis also showed that the Pd–C2 and Pd–C4 bonds were σ bonds, and the orbital energies were −993.2 and −945.9 kJ mol−1. Relative to IM5a, the Pd–C4 bond was weakened and activated because of the formation of the back-donation π bond, which indicated that the alkyne insertion reaction was promoted. Therefore, the alkyne insertion reaction into the Pd–C4 bond occurred prior to that into the Pd–C2 bond. The high stabilization energies for the πC6–C7 → σ*Pd–C4 and (2p)C2 → σ*Pd-C4 in TS4a1 were 370.9 and 314.0 kJ mol−1, which displayed the strong interaction between πC6–C7 or (2p)C2 and σ*Pd–C4 and the electron migration tendency from πC6–C7 or (2p)C2 to σ*Pd–C4. The Pd–C2 and Pd–C7 bonds of IM7a1 showed the strong single-bond character.
The alkyne insertion in IM6a had two possible reaction modes: C6 attacking C4 was denoted as “a1”, while C7 attacking C2 was denoted as “a2”. Another reaction channel had also been studied: the free energies of the corresponding transition states TS4a2 and TS5a2 were 250.1 and 22.5 kJ mol−1, which indicated that this channel was energetically prohibited. Therefore, in IM6a, the alkyne insertion reaction into the Pd–Caryl bond occurred prior to that into the Pd–Calkenyl bond.
The reaction channels: path II
The reaction pathways (path II) are illustrated in Scheme 2: in IM4, the hydrogen migration of H1 from Pd to C2 is denoted as “b”. The intermediates and transition states in these pathways were similar to those discussed above (path I). The free energies of the transition states TS3b, TS4b1, and TS5b1 were 264.7, 249.3, and 38.5 kJ mol−1, respectively. Evidently, hydrogen migration was the rate-determining step for this pathway. Because the free energy of TS3b was higher than that of TS3a by 98.8 kJ mol−1, the reaction pathways (path I) were more dominant than path II. As illustrated in Fig. 2, the HOMO of TS3a was lower than that of TS3b by 33.6 kJ mol−1, while the LUMO of TS3a was higher than that of TS3b by 133.3 kJ mol−1, so TS3a had better stability than TS3b, which agreed with those discussed above.
The other reaction channel was also studied: in IM6b, C6 attacking C4 was denoted as “b1”, while C7 attacking C1 was denoted as “b2”. The free energies of the transition states TS4b2 and TS5b2 were 263.9 and 43.3 kJ mol−1, respectively, which indicated that this channel was also energetically prohibited. Hence, in IM6b, the alkyne insertion reaction into the Pd–Caryl bond occurred prior to that into the Pd–Calkenyl bond, which is in agreement with those discussed above (path I).
The reaction channels: path III
As illustrated in Fig. 3, the intermediate IM2 underwent the hydrogen migration to deliver the complexes IM9a and IM9b via the corresponding transition states TS6a and TS6b. The complexation of IM9a, IM9b and 2-butynes resulted in complexes IM6a, IM6b, respectively. The free energies of TS6a and TS6b were 187.4 and 198.3 kJ mol−1, respectively, which were higher than TS3a by 21.5 and 32.4 kJ mol−1, so reaction pathway path I was more dominant than path III. In the five-coordinated intermediate IM2, the dissociation of a ligand PMe3 occurred prior to the hydrogen transfer reaction.
The reaction channels: path IV
Figure 4 shows the energy profiles of the reaction pathways (path IV). The intermediate IM2 coordinated to 2-butyne and formed a five-coordinated palladium-hydride complex IM10, and then IM10 went through hydrogen migration to complexes IM6a and IM6b via transition states TS7a and TS7b with free energies of 281.3 and 310.0 kJ mol−1, respectively. The free energy of TS3a was much lower than those of TS7a and TS7b (by 115.4 and 144.1 kJ mol−1, respectively) so the pathways (path IV) were energetically prohibited.
The reaction channels: path V
As illustrated in Fig. 5, the five-coordinated palladium-hydride complex IM10 underwent the alkyne insertion reaction through the transition state TS8 with a free energy of 278.9 kJ mol−1 to give complex IM11. Intermediate IM11 went through the cyclization reaction via the TS9a and TS9b with free energies of 122.5 and 115.4 kJ mol−1, respectively, resulting in complexes IM12a and IM12b. Intermediates IM12a and IM12b underwent hydrogen migration to deliver complexes IM8a and IM8b, respectively, via transition states TS10a and TS10b with free energies of −5.8 and 0.9 kJ mol−1, respectively. Therefore, the alkyne insertion reaction was the rate-determining step for the pathway (path V), because the free energy of TS8 was the highest. The free energy of TS8 was much higher (by 113.0 kJ mol−1) than that of TS3a, so the pathways (path V) were energetically prohibited.
Conclusions
The reaction mechanisms of palladium(0)-catalyzed cycloaddition of alkynyl aryl ethers and alkynes to give 2-methylidene-2H-chromenes were explored computationally using DFT methods [at M06/6-31G(d,p) level, LANL2DZ(f) for Pd]. The results indicated that the cycloaddition had five possible reaction pathways (I, II, III, IV, and V). In the palladium(0)-alkynyl aryl ether complex IM1, the oxidative addition of the Caryl–H bond occurred prior to the dissociation of the ligand PMe3. The dissociation of ligand PMe3 from the five-coordinated complex IM2 was much easier to achieve than the hydrogen transfer reaction and the substitution reaction of alkynes. In the palladium(0)-hydride complex IM4, hydrogen migration of H1 from palladium to the carbon C1 was much easier to achieve than to carbon C2. In the four-coordinated aryl-palladium-alkyne complexes IM6a and IM6b, the alkyne insertion reaction into the Pd–Caryl bond occurred prior to that into the Pd–Calkenyl bond.
The reaction channel IM1 → TS1 → IM2 → IM4 → TS3a → IM5a → IM6a → TS4a1 → IM7a1 → TS5a1 → IM8a was the most favorable among all catalytic reaction pathways of the cycloaddition of alkynyl aryl ethers and 2-butynes catalyzed by the palladium(0)/PMe3 complex. Hydrogen migration was the rate-determining step for this channel. The theoretically predicted dominant product was 2-methylidene-2H-chromenes P1, which was in well agreement with the experimental studies [13].
References
Chen X, Engle KM, Wang DH, Yu JQ (2009) Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew Chem Int Ed 48:5094–5115
Johnson KRD, Hayes PG (2013) Cyclometalative C-H bond activation in rare earth and actinide metal complexes. Chem Soc Rev 42:1947–1960
Musaev DG, Figg TM, Kaledin AL (2014) Versatile reactivity of Pd-catalysts: mechanistic features of the mono-N-protected amino acid ligand and cesium-halide base in Pd-catalyzed C-H bond functionalization. Chem Soc Rev 43:5009–5031. doi:10.1039/C3CS60447K
Baillie RA, Legzdins P (2014) Distinctive activation and functionalization of hydrocarbon C-H bonds initiated by Cp*W(NO)(η3-allyl)(CH2CMe3) complexes. Acc Chem Res 47:330–340
Zhang XS, Chen K, Shi ZJ (2014) Transition metal-catalyzed direct nucleophilic addition of C–H bonds to carbon–heteroatom double bonds. Chem Sci 5:2146–2159. doi:10.1039/C3SC53115E
He R, Huang ZT, Zheng QY, Wang C (2014) Manganese-catalyzed dehydrogenative [4 + 2] annulation of N-H imines and alkynes by C-H/N-H activation. Angew Chem Int Ed. doi:10.1002/anie.201402575
Sirois JJ, Davis R, Deboef B (2014) Iron-catalyzed arylation of heterocycles via directed C-H bond activation. Org Lett 16:868–871
Semproni SP, Atienza CCH, Chirik PJ (2014) Oxidative addition and C–H activation chemistry with a PNP pincer-ligated cobalt complex. Chem Sci 5:1956–1960
Iaroshenko VO, Gevorgyan A, Davydova O, Villinger A, Langer P (2014) Regioselective and guided C-H activation of 4-nitropyrazoles. J Org Chem 79:2906–2915
Park Y, Jeon I, Shin S, Min J, Lee PH (2013) Ruthenium-catalyzed C-H activation/ cyclization for the synthesis of phosphaisocoumarins. J Org Chem 78:10209–10220
Huckins JR, Bercot EA, Thiel OR, Hwang TL, Bio MM (2013) Rh(III)-catalyzed C–H activation and double directing group strategy for the regioselective synthesis of naphthyridinones. J Am Chem Soc 135:14492–14495
Hu F, Xia Y, Ye F, Liu Z, Ma C, Zhang Y, Wang J (2014) Rhodium(III)-catalyzed ortho alkenylation of N-phenoxyacetamides with N-tosylhydrazones or diazoesters through C-H activation. Angew Chem Int Ed 53:1364–1367
Minami Y, Shiraishi Y, Yamada K, Hiyama T (2012) Palladium-catalyzed cycloaddition of alkynyl aryl ethers with internal alkynes via selective ortho C-H activation. J Am Chem Soc 134:6124–6127
Maleckis A, Kampf JW, Sanford MS (2013) A detailed study of acetate-assisted C-H activation at palladium(IV) centers. J Am Chem Soc 135:6618–6625
Takemoto S, Grushin VV (2013) Nucleophile-catalyzed, facile, and highly selective C–H activation of fluoroform with Pd(II). J Am Chem Soc 135:16837–16840
Zhang C, Ji J, Sun P (2014) Palladium-catalyzed alkenylation via sp2 C–H bond activation using phenolic hydroxyl as the directing group. J Org Chem 79:3200–3205
Mahindra A, Bagra N, Jain R (2013) Palladium-catalyzed regioselective C-5 arylation of protected l-histidine: microwave-assisted C-H activation adjacent to donor arm. J Org Chem 78:10954–10959
Choi G, Tsurugi H, Mashima K (2013) Hemilabile N-xylyl-N’-methylperimidine carbene iridium complexes as catalysts for C-H activation and dehydrogenative silylation: dual role of N-xylyl moiety for ortho-C-H bond activation and reductive bond cleavage. J Am Chem Soc 135:13149–13161
Bowring MA, Bergman RG, Tilley TD (2013) Pt-catalyzed C–C activation induced by C-H activation. J Am Chem Soc 135:13121–13128
Frisch, MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JAJr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Ehlers AW, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler KF, Stegmann R, Veldkamp A, Frenking G (1993) A set of f-polarization functions for pseudo-potential basis sets of the transition metals Sc–Cu, Y–Ag and La–Au. Chem Phys Lett 208:111–114
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Flükiger P, Lüthi HP, Portmann S, Weber J (2000–2002) MOLEKEL 4.3 Swiss Center for Scientific Computing, Manno. Switzerland
Portmann S, Lüthi HP (2000) An interactive molecular graphics tool. Chimia 54:766–770
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct (THEOCHEM) 169:41–50
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Bader RFW (1990) Atoms in molecules, a quantum theory; international series of monographs in chemistry, vol 22. Oxford University Press, Oxford
Biegler-König F, Schönbohm J, Derdau R, Bayles D, Bader RFW (2002) AIM 2000. Version 2.0, McMaster University.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 31270723, 31370686), Technology Development Program of Shandong Province, China (No. 2013GZX20109), and Youth Science and Technology Innovation Foundation of Shandong Agricultural University (No. 23824).
Calculations reported in the paper were performed on the Gaussian 09 programs by the College of Chemistry and Chemical Engineering, Southwest University. The authors extend our heartfelt gratitude to the distinguished Professor Ming Li in Southwest University for current discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Computational details of the energies and detailed structures of the stationary points in the reactions are given in the supporting information. (DOC 1050 kb)
Rights and permissions
About this article
Cite this article
Meng, Q., Wang, F. Theoretical studies of palladium-catalyzed cycloaddition of alkynyl aryl ethers and alkynes. J Mol Model 20, 2514 (2014). https://doi.org/10.1007/s00894-014-2514-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2514-z