Abstract
Background
IgG4-related sclerosing cholangitis (IgG4-SC) needs to be differentiated from primary sclerosing cholangitis (PSC). In this study, we performed a retrospective study to reveal cases in which liver needle biopsy was useful for differential diagnosis.
Methods
Nineteen patients with IgG4-SC and 22 patients with PSC were studied. All patients underwent endoscopic retrograde cholangiography and liver needle biopsy. We defined small bile duct involvement of IgG4-SC histologically as damage to the small bile duct associated with infiltration of ≥10 IgG4+ plasma cells per high power field (HPF). Clinicopathological characteristics were compared between IgG4-SC patients with and without small bile duct involvement.
Results
Small bile duct involvement was observed in 5 (26%) of the patients with IgG4-SC. Patients with small bile duct involvement showed a higher incidence of intrahepatic biliary strictures on cholangiography (80 vs. 21%, p = 0.038). Conversely, 4 of 7 (57%) patients with intrahepatic biliary strictures on cholangiography had histologically evident small duct involvement. The number of IgG4+ plasma cells was significantly correlated with the site of the most proximal stricture on cholangiograms (p = 0.021). The number of IgG4+ plasma cells per HPF was significantly higher in IgG4-SC patients with intrahepatic biliary strictures than in those with PSC (13.4 vs. 0.4 cells/HPF, p < 0.001).
Conclusions
Involvement of small bile ducts is more frequent in patients with intrahepatic biliary strictures on cholangiography, and liver needle biopsy is especially useful for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IgG4-related sclerosing cholangitis (IgG4-SC) is a recently recognized disease entity characterized by high serum IgG4 concentrations, a high prevalence in adults, responsiveness to steroid, and histologically evident sclerosing inflammation involving numerous IgG4+ plasma cells [1, 2]. IgG4-SC is considered to be a biliary manifestation of IgG4-related systemic disease [3]. It is usually associated with autoimmune pancreatitis (AIP) and rarely manifests as cholangitis alone [4, 5]. It is important to differentiate IgG4-SC from primary sclerosing cholangitis (PSC), for which only liver transplantation is an effective treatment. Serological examination of IgG4, cholangiography, and the presence of extra-biliary lesions are useful for differential diagnosis, but histological detection of IgG4+ plasma cell infiltration is also recommended [6–9]. Endoscopic biopsy of the Vater ampulla or bile ducts, and liver needle biopsy have been reported as potentially informative procedures, but they cannot reveal IgG4+ plasma cells in all patients [10–13]. Thus, it is important to reveal cases in which needle biopsy was useful for differential diagnosis.
IgG4-SC usually involves the extra- and intra-hepatic large bile ducts [1, 2]. Inflammation containing IgG4+ plasma cells rarely extends to the peripheral portal tracts and tends to involve small bile ducts, for which liver needle biopsy would be useful for detecting IgG4+ plasma cells. IgG4-related small duct lesions in a liver needle biopsy sample can be diagnostic because such lesions have not been reported in other biliary disorders [12, 13]. Cholangiography can also occasionally reveal strictures in the intrahepatic bile ducts as well as the large bile ducts, showing a PSC-like overall appearance [7]. It seems important to know the clinical characteristics of patients with histological small bile duct involvement, because such patients need to be carefully differentiated from PSC, and in this situation liver needle biopsy would be diagnostic.
In the present study, we retrospectively examined liver needle biopsy specimens from patients with IgG4-SC, and investigated the correlation between histologically evident small bile duct involvement and clinical/cholangiogram characteristics. Our goal was to reveal cases in which liver needle biopsy was especially useful for the diagnosis of IgG4-SC.
Patients and methods
Patients
This study involved 19 patients with IgG4-SC diagnosed at Nagoya City University Hospital and affiliated hospitals between 1990 and 2009. The study was approved by the Institutional Human Investigation Committee of Nagoya City University Graduate School of Medical Sciences, and informed consent was obtained from all patients. IgG4-SC was associated with AIP in 18 patients, and was not in 1 patient. The diagnosis of AIP was made on the basis of the revised Japanese diagnostic criteria [14] or Asian diagnostic criteria [15]. The patient without AIP fulfilled the HISORt criteria for IgG4-associated cholangitis [1]. Extra-pancreatobiliary lesions were observed in 11 (58%) patients. Eight patients (44%) showed relapse during the clinical course. Twenty-two patients with PSC who underwent liver biopsy were enrolled as a control group. PSC was diagnosed according to the diagnostic criteria reported by Lindor et al. [16]. The average age of patients with IgG4-SC and PSC was 65 (range 44–73) and 38 (12–67) years, and the male/female ratio was 15/4 and 9/13, respectively.
Laboratory findings
Alanine aminotransferase, aspartate aminotransferase, total bilirubin, γ-glutamyl transpeptidase, and alkaline phosphatase were measured in all patients. IgG levels were measured in all patients except 2 (1 patient each with IgG4-SC and PSC). Serum IgG4 levels were measured in 13 (68%) patients with IgG4-SC, and were found to be elevated in 11 (85%) of them (normal range ≤135 mg/dl; average 628 mg/dl; range 60–2960 mg/dl). Thirteen (59%) patients with PSC also underwent serological examination of IgG4, and all of them were within the normal range (average 33 mg/dl; range 7–110 mg/dl). The laboratory findings of patients with IgG4-SC are summarized in Table 1.
Histology and immunohistochemistry
All patients underwent liver needle biopsy. Liver biopsies were taken by percutaneous sampling of the right lobe with a 14-gauge needle before administration of corticosteroid. The biopsy specimens were fixed in neutral formalin and embedded in paraffin. Serial 3-μm-thick sections were cut from paraffin-embedded tissue blocks, stained with hematoxylin and eosin (H&E) and Azan-Mallory, and immunostained with mouse anti-human IgG4 monoclonal antibody (MC011, dilution 1:100; The Binding Site Ltd., Birmingham, UK) using an automated immunostainer (DISCOVERY HX, Ventana, Ventana Medical Systems, Tucson, AZ). The following 10 histological features were evaluated: (1) portal inflammation (1+, mild; 2+, moderate; 3+ severe); (2) bile duct damage (−, absent; +, present); (3) canalicular cholestasis (−, absent; +, present); (4) bile duct loss (−, absent; +, present); (5) periductal onion-skin (concentric) fibrosis (−, absent; +, present); (6) eosinophil infiltration [−, 0–4 cells/high power field (HPF ×400) in the portal area; +, ≥5 cells/HPF]; (7) plasma cell infiltration (−, 0–9 cells/HPF in the portal area; +, ≥10 cells/HPF); (8) neutrophil infiltration (−, 0–4 cells/HPF in the portal area; +, ≥5 cells/HPF); (9) portal inflammatory nodules [13] (−, absent; +, present); (10) central venulitis (−, absent; +, present); and (11) lobular hepatitis (−, 0–2 spotty necrosis/HPF; +, ≥3 spotty necrosis/HPF). The number of IgG4+ plasma cells was evaluated as the average count of positive plasma cells in 3 HPFs. Mild portal inflammation was defined as the presence of a few inflammatory cells in the portal tracts, and severe portal inflammation meant inflammatory expansion of the portal tracts with occasional lymphoid aggregation. Moderate portal inflammation was considered to be intermediate between them. Bile duct damage was considered to be present when bile ducts showed disturbance of epithelial polarity, narrowing of the bile duct lumen, angulated tubular structure, or eosinophilic cytoplasmic change. Central venulitis was defined as inflammatory cell infiltration into the walls of central veins.
Surgical specimens of IgG4-related disease including IgG4-SC usually show 50–200 IgG4+ plasma cells per HPF, but previous reports suggested that the presence of ≥10 IgG4+ plasma cells in biopsy samples is highly suggestive of IgG4-related disease [17]. In this study, we defined small bile duct involvement of IgG4-SC as the presence of bile duct damage associated with ≥10 IgG4+ plasma cells per HPF. All biopsy specimens were evaluated by an experienced pathologist (YZ) blinded to the other data.
Cholangiography
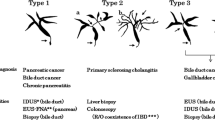
Endoscopic retrograde cholangiography (ERC) was performed in all 19 patients with IgG4-SC. In this study, we defined hilar bile ducts as hepatic ducts (common, right, and left) and their first branches. The bile ducts proximal to the hilar duct were considered intrahepatic bile duct. Common bile ducts were classified into those proximal (extrapancreatic) and distal (intrapancreatic) to the superior border of the pancreas. Cholangiographic findings were classified according to our previous report: type 1, localized stricture only in the distal common bile duct; type 2, presence of stricture involving intrahepatic bile ducts; type 3, localized stricture in both hilar and distal common bile ducts; type 4, stricture in only hilar ducts [18]. The cholangiograms were analyzed by two experts (IN, TN) who were unaware of the other clinical data.
Statistical analysis
Clinical data for the patients are expressed as mean (SD). The chi-square test and Fisher’s exact test for categorical comparisons of data were used for comparison of histological features as appropriate. Mann-Whitney U test or Kruskal-Wallis was used for comparison of the numbers of IgG4-positive plasma cells. Differences at p < 0.05 were considered significant.
Results
Cholangiography
The cholangiograms of the 19 patients with IgG4-SC were classified into 4 types: 4 (21%) cases of type 1, 7 (37%) of type 2, 5 (26%) of type 3, and 3 (16%) of type 4 (Fig. 1). The stricture was observed in the intrahepatic bile ducts in 7 (37%) patients, hilar ducts in 10 (53%), proximal common bile duct in 1 (5%), and distal common bile duct in 11 (58%). Eight (42%) patients had multifocal biliary strictures. All PSC patients showed type 2 cholangiograms, comprising 19 (90%) patients with both intra- and extrahepatic strictures and 3 (10%) with intrahepatic strictures only.
Histological findings of liver biopsy
Histological findings of liver needle biopsies are summarized in Table 2. Small bile duct involvement was observed in 5 (26%) patients with IgG4-SC (Fig. 2a), in whom the number of IgG4+ plasma cells per HPF ranged from 14 to 25 (average 21) (Fig. 2b), and was significantly higher than that in 14 patients without small bile duct involvement (number of IgG4+ cells/HPF, range 0–6, average 2) (p < 0.001). Comparison of other histological findings between those two groups revealed that patients with small duct involvement more frequently showed moderate to severe portal inflammation (p = 0.002), bile duct damage (p = 0.017), plasma cell infiltration (≥10 cells/HPF, p < 0.001), and neutrophil infiltration (≥5 cells/HPF, p = 0.037) (Table 2; Figs. 2, 3). Central venulitis and portal inflammatory nodules were observed in only one patient each, both with small bile duct involvement (Fig. 2c, d). Onion-skin periductal fibrosis, a characteristic finding of PSC, was observed in 2 IgG4-SC patients without small duct involvement (Fig. 3b). Histological diagnosis was possible for patients with small bile duct involvement, but patients without this feature could not be differentiated from PSC by histology alone.
Liver needle biopsy findings of patients with small bile duct involvement. a The portal tract is enlarged with moderate inflammatory cell infiltration and bile duct damage. b Immunostaining of IgG4 reveals IgG4+ plasma cell infiltration. c Portal inflammatory nodule consisting of spindle-shaped stromal cells and inflammatory cells is identified (arrows). d Inflammatory cells infiltrate into the wall of central vein (arrows). a H&E ×200, b IgG4 immunostaining ×400, c H&E ×200, d H&E ×200
A more significant difference was identified between PSC and IgG4-SC with small duct involvement than between PSC and IgG4-SC without small duct involvement (Table 2). Patients with small duct involvement more frequently showed moderate to severe portal inflammation (p = 0.007) and plasma cell infiltration (≥10 cells/HPF, p < 0.001) than those with PSC. In contrast, canalicular cholestasis was more commonly observed in patients without small duct involvement than in patients with PSC (p = 0.016) (Fig. 3c).
Clinical characteristics of patients with small duct involvement
Table 1 compares the clinical features between patients with and without small bile duct involvement. Patients with small bile duct involvement more commonly showed type 2 cholangiograms. That is, cholangiography revealed intrahepatic biliary stricture in 4 of 5 (80%) patients, whereas 3 of 14 (21%) patients without histological small duct lesions showed abnormalities of the intrahepatic bile ducts (p = 0.038). The levels of serum IgG and IgG4 were slightly higher in the group with small duct involvement, but the difference was not significant (p = 0.092 and p = 0.094, respectively). There were no significant differences in terms of laboratory data, incidence of extrapancreatobiliary lesions, and relapse.
Comparison between findings of liver biopsy and cholangiography
The most proximal area of stricture was the intrahepatic bile duct in patients with type 2 cholangiogram (n = 7), the hilar bile duct in types 3 and 4 (n = 8), and the intrapancreatic bile duct in type 1 (n = 4). As shown in Fig. 4, the number of IgG4+ plasma cells was significantly correlated with the site of the most proximal stricture. Significant IgG4+ cell infiltration (≥10 cells/HPF) was observed in 4 (57%) of the 7 type 2 cases, whereas only 1 (8%) of the 12 cases with a non-type 2 cholangiogram had this histological diagnostic feature. Interestingly, all patients with a type 1 cholangiogram showed only mild to minimal portal inflammation without any IgG4+ plasma cells.
Discussion
The present study revealed that small bile duct involvement, defined as the presence of bile duct damage associated with ≥10 IgG4+ plasma cells per HPF, was evident in 5 (26%) patients with IgG4-SC. Patients with small bile duct involvement more commonly showed strictures of the intrahepatic bile ducts on cholangiography (80 vs. 21%, p = 0.038). Thus, liver needle biopsy is especially useful for patients with intrahepatic biliary strictures on cholangiography.
It is a critical issue to differentiate IgG4-SC and PSC, because the therapeutic approaches for each are completely different from the other. IgG4-SC is effectively treatable with corticosteroids, whereas only liver transplantation is an effective therapy for PSC. In the clinical field, this differential diagnosis is made on the basis of clinical features, serological examination, and radiological findings [6–8]. We have reported previously that characteristic cholangiographic features are useful for discriminating IgG4-SC from PSC [7]. There is no doubt that the serum IgG4 level is the most useful parameter for differential diagnosis [1]. Needle biopsy samples from the liver or pancreas, and endoscopic biopsy samples from the Vater ampulla or common bile duct, are suggested to be potentially informative. However, none of them alone is unequivocally diagnostic, and more importantly the usefulness of each biopsy in individual cases cannot be predicted.
We have reported that endoscopic transpapillary bile duct biopsy was unsatisfactory for the diagnosis of IgG4-SC, with a sensitivity of only 18% (3/17) [11]. In contrast, a recent study by Zhang et al. [19] revealed that 23 (23%) of 98 liver explants with PSC showed periductal infiltration with abundant IgG4+ plasma cells (>10/HPF) in the hilar area, suggesting that IgG4+ plasma cells may be detectable by bile duct biopsy in PSC patients. However, their study showed that no increased IgG4+ plasma cells were observed in portal tracts and liver parenchyma adjacent to large bile duct. These results suggested that liver needle biopsy might be more useful than bile duct biopsy in differentiating IgG4-SC from PSC. The present study suggested that liver needle biopsy is especially useful for patients with evident intrahepatic biliary stricture on cholangiograms. This seems important, because IgG4-SC becomes more difficult to differentiate from PSC when intrahepatic bile duct strictures are radiologically identified.
There have been two previous studies of liver needle biopsy samples taken from patients with IgG4-SC [12, 13]. Umemura et al. [12] clearly showed the usefulness of IgG4 immunostaining of liver needle biopsy samples for diagnosis of IgG4-SC and its differential diagnosis from PSC. More importantly, they demonstrated a variety of intrahepatic histological changes in patients with AIP or IgG4-SC under the concept of IgG4 hepatopathy. Deshpande et al. [13] reported detailed histological differences in liver pathology between IgG4-SC and PSC. They concluded that IgG4-SC showed more significant inflammatory changes in both the portal tracts and liver parenchyma, and also revealed that portal inflammatory nodules are a histological finding characteristic of IgG4-SC. In the present study, one patient showed phlebitis in a relatively large central vein (central venulitis), which might be another specific feature of IgG4-SC, because this has not been reported in PSC. Taken together, the data suggest that only three histological features of liver biopsy specimens are specific for IgG4-SC: (1) the presence of many infiltrating IgG4+ plasma cells, (2) portal inflammatory nodules, and (3) central venulitis. In contrast, the only specific feature for PSC is bile duct loss, which was not identified in any of the IgG4-SC patients in the present and two previous studies. Periductal onion-skin fibrosis has been considered characteristic for PSC, although it can also be observed in IgG4-SC. We should always consider the possibility of false-negative results by liver needle biopsy specimens because the distribution of small bile duct involvement is patchy.
Another important issue is how many IgG4+ plasma cells are sufficient for diagnosis. In the HISORt criteria, involvement of other organs by AIP is diagnosed by biopsy based on a threshold of ≥10 IgG4+ plasma cells per HPF [17]. We agree to apply this idea for liver needle biopsy, because other hepatobiliary disorders rarely exhibit occasional positive cells (<10 positive cells/HPF) in peripheral portal tracts, and the presence of ≥10 IgG4+ plasma cells per HPF is extremely rare in our experience. In the present study, the sensitivity and specificity of this threshold for the diagnosis of IgG4-SC were 26 and 100%, respectively. If this threshold had been applied to the previous two studies by Umemura et al. [12] and Deshpande et al. [13], the sensitivities would have been 21 and 60%, respectively. Although this diagnostic sensitivity is insufficient, it would have increased to 57% if restricted to patients with intrahepatic biliary stricture on cholangiograms. In the present study, there were no correlations between serum IgG4 concentrations and the number of IgG4+ plasma cells. Umemura et al. [12] reported that a highly significant association was found between serum IgG4 concentrations and the number of IgG4+ plasma cells. Their results were thus different from ours. Various results concerning correlations between serum IgG4 concentrations and the number of IgG4+ plasma cells have been reported in other organ specimens with AIP patients.
Before starting this study, we expected that some patients with IgG4-SC might show small duct involvement despite having a normal intrahepatic/hilar cholangiogram, as is the case for small-duct PSC. However, all the patients with small duct involvement showed cholangiographic abnormalities in either the hilar or intrahepatic bile ducts. The common bile duct, especially its distal part, is commonly affected by AIP. It should be noted that patients with only intrapancreatic stricture on cholangiograms did not show IgG4+ plasma cell infiltration into the peripheral portal tracts, suggesting that liver needle biopsy is less useful for such patients. Another point of interest was that patients without small bile duct involvement more frequently showed canalicular cholestasis presumably induced by severe stricture of the large bile ducts, in comparison with PSC. Canalicular cholestasis may be one of the features suggestive of IgG4-SC in this situation. However, it should be noted that IgG4-SC patients with localized large duct strictures must be differentiated more carefully from pancreatobiliary cancer rather than from PSC. We consider that canalicular cholestasis is not useful for such differential diagnosis.
In conclusion, small bile duct involvement of IgG4-SC is observed more frequently in patients with intrahepatic biliary strictures on cholangiography, and liver needle biopsy is especially useful for the diagnosis of IgG4-SC in such patients.
References
Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–15.
Nakazawa T, Ohara H, Ando T, Hayashi K, Naitoh I, Okumura F, et al. Clinical course and indications for steroid therapy of sclerosing cholangitis associated with autoimmune pancreatitis. Hepatogastroenterology. 2009;56:584–8.
Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–4.
Hamano H, Kawa S, Uehara T, Ochi Y, Takayama M, Komatsu K, et al. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis? Gastrointest Endosc. 2005;62:152–7.
Hayashi K, Nakazawa T, Ohara H, Ando T, Takada H, Tanaka H, et al. Autoimmune sclerosing cholangiopancreatitis with little pancreatic involvements by imaging findings. Hepatogastroenterology. 2007;54:2146–51.
Nakazawa T, Ohara H, Sano H, Ando T, Aoki S, Kobayashi S, et al. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005;30:20–5.
Nakazawa T, Ohara H, Sano H, Aoki S, Kobayashi S, Okamoto T, et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc. 2004;60:937–44.
Nishino T, Oyama H, Hashimoto E, Toki S, Oi I, Kobayashi M, et al. Clinicopathological differentiation between sclerosing cholangitis with autoimmune pancreatitis and primary sclerosing cholangitis. J Gastroenterol. 2007;42:550–9.
Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–203.
Kubota K, Kato S, Akiyama T, Yoneda M, Fujita K, Ogawa M, et al. Differentiating sclerosing cholangitis caused by autoimmune pancreatitis and primary sclerosing cholangitis according to endoscopic duodenal papillary features. Gastrointest Endosc. 2008;68:1204–8.
Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, et al. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147–55.
Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463–71.
Deshpande V, Sainani NI, Chung RT, Pratt DS, Mentha G, Rubbia-Brandt L, et al. IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod Pathol. 2009;22:1287–95.
Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–31.
Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan–Korea symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–8.
Lindor KD, LaRusso NF. Primary sclerosing cholangitis. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Disease of the liver. 9th ed. Philadelphia: JB Lippincott; 2003. p. 673–84.
Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–6.
Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32:229.
Zhang L, Lewis JT, Abraham SC, Smyrk TC, Leung S, Chari ST, et al. IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am J Surg Pathol. 2010;34:88–94.
Acknowledgments
This study was supported by the Pancreas Research Foundation of Japan and by Health and Labor Sciences research grants (Research on Specific Diseases, Intractable Diseases of the Pancreas, Ministry of Health Labor and Welfare, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naitoh, I., Zen, Y., Nakazawa, T. et al. Small bile duct involvement in IgG4-related sclerosing cholangitis: liver biopsy and cholangiography correlation. J Gastroenterol 46, 269–276 (2011). https://doi.org/10.1007/s00535-010-0319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0319-0