Abstract
Picoeukaryotes constitute an important component of the living biomass of oceanic communities and play major roles in biogeochemical cycles. There are very few studies on picoeukaryotes found in the Chukchi Sea. This work shows the relationship between community distribution and composition of picoeukaryotes residing in water masses and physicochemical factors in the southern Chukchi Sea studied in both midsummer (July) and early autumn (September), 2012. Illumina 18S V4 rDNA metabarcoding were used as the main tool. In July, Mamiellophyceae, Dinophyceae, and Trebouxiophyceae were the main microbial classes, with Micromonas, Prasinoderma, Telonema, Amoebophrya, Bathycoccus, Picomonas, and Bolidomonas representing the main genera. In September, Trebouxiophyceae surpassed Dinophyceae and was the second main microbial class, with Micromonas, Prasinoderma, Bathycoccus, Bolidomonas, Telonema, Choricystis, and Diaphanoeca representing the main genera. Water mass was the primary factor determining the community composition and diversity of picoeukaryotes. Abundance of Bathycoccus was found to be highly correlated with Alaskan Coastal Water and that of Prasinoderma, Bolidomonas, and Diaphanoeca with Bering Seawater. Nitrate and phosphate content of water in midsummer and dissolved oxygen (DO) and temperature in early autumn were the main factors that shaped the abundance of the picoeukaryote community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Chukchi Sea is a shallow, wide marginal region of the Arctic Ocean. The average depth of the Chukchi Sea is 50 m, and its length is approximately 1000 km. The Chukchi Sea lies north of the Bering Sea and connects to it via the Bering Strait (Grebmeier et al. 2006). Water from the Pacific Ocean flows into the Arctic Ocean by means of the southern Chukchi Sea, delivering freshwater, nutrients, and Pacific biota into the Arctic Ocean (Woodgate and Aagaard 2005; Woodgate et al. 2005; Grebmeier et al. 2006; Pisareva et al. 2015; Linders et al. 2017). The following four main types of water masses are found in the Chukchi Sea: Alaskan Coastal Water (ACW), Summer Bering Sea Water (BSW), Siberian Coastal Water, and remnant Pacific Winter Water (Pisareva et al. 2015). The southeastern Chukchi Sea is mainly influenced by BSW and ACW. The former is cold (3–6 °C) with high salinity (> 32), and the latter is warmer with low salinity (Coachman et al. 1975; Woodgate et al. 2005). The Chukchi Sea is one of the most productive oceanic areas in the world (Grebmeier 2012). It also acts as an important carbon sink, especially upon the formation of sea-ice-associated phytoplankton blooms (Lee et al. 2007; Arrigo et al. 2012; Lowry et al. 2014, 2015). The Chukchi Sea is also a hotspot for studying zooplankton (Springer et al. 1989; Questel et al. 2013; Sigler et al. 2017), benthic fauna, pelagic-benthic coupling (Feder et al. 2005; Grebmeier et al. 2006; Piepenburg et al. 2011; Blanchard and Feder 2014), and seabirds and marine mammals (Moore and Laidre 2006; Aerts et al. 2013; Clarke et al. 2013; Gall et al. 2013; Kuletz et al. 2015). Scientists have also examined the structure and biomass of microbial communities in both seawater (Zhang et al. 2012; Thaler 2014; Yun et al. 2014; Pedrós-Alió et al. 2015) and sea ice (Eddie et al. 2010; Poulin et al. 2011; Majaneva et al 2017; Belevich et al. 2017). However, to the best of our knowledge, very few studies (Zhang et al. 2012; Thaler 2014; Pedrós-Alió et al. 2015) have examined picoeukaryotes (< 3 µm) in the Chukchi Sea, and none of them have compared picoeukaryote community compositions in different water masses during different seasons.
Picoeukaryotes are vital to polar marine ecosystems because they are the most abundant photosynthetic plankton found for the greater part of the year (Lovejoy et al. 2007). They are estimated to thrive with increasing temperatures in the Arctic Ocean (Li et al. 2009). Autotrophic and heterotrophic organisms play important roles in the microbial loop, which is particularly important in polar oceans (Whitman et al. 1998). Along with other regions of the Arctic Ocean, the Chukchi Sea is undergoing increase in temperatures and freshwater and a reduction in volumes of sea ice (Steele et al. 2008; Polyakov et al. 2010). These changes drive shifts in the composition of marine species and carbon cycling and affect the structure of the marine ecosystem in the Chukchi Sea (Grebmeier et al. 2010; Grebmeier 2012; Häder et al. 2014). Picoeukaryotes are significantly associated with the circulation of global oceanic waters and are sensitive to changes in both physicochemical factors and water mass. Some species have been found only in certain water masses and can be used as bioindicators of water mass (Hamilton et al. 2008; Zhang et al. 2012, 2016). Hence, it is essential to record the composition of the picoeukaryote community and their diversity in the Chukchi Sea during different seasons. It is also important to understand how picoeukaryote communities are associated with water masses and physicochemical factors (Hamilton et al. 2008; Zhang et al. 2019). These are the main objectives of the present study, with data presented from midsummer and early autumn of 2012.
Materials and methods
Study area, sample collection and analysis of environmental factors
Samples were collected from seven stations in the southern Chukchi Sea (Fig. 1) aboard the R/V “Xuelong”during the fifth Chinese National Arctic Expedition in both the summer (18 and 19 July) and early autumn (8 September) of 2012. Three stations overlapped in both seasons. Five depths were selected at each station (Table 1), and seawater was collected from each depth using Niskin bottles attached to an SBE911 plus a CTD rosette system (Sea Bird Inc., USA). Water temperatures and salinities were recorded directly using the CTD; nutrients at each depth, including phosphate (PO43−), nitrate (NO3−), nitrite (NO2−), ammonia (NH4+) and silicate (Si), were immediately measured onboard the ship with a SKALAR SAN++ nutrient automatic analyzer (Netherlands). Dissolved oxygen (DO) was measured using the Winkler titration method (Grasshoff et al. 1983). Five-hundred-milliliter water samples were collected from each depth. Each sample was passed through 20-μm, 3-μm and Whatman GF/F glass filters (0.47-mm pore size, 47-mm diameter). Each filter was inserted into a clean glass tube for Chlorophyll a (Chl a) measurement. Chl a was extracted using 10 mL of 90% acetone for 24 h in a − 20 °C freezer and measured with a Turner Designs 10 fluorometer (Parsons et al. 1984).
Sampling sites in the southern Chukchi Sea of 2012: the sampling stations of R1 to R3 were in both July and September, and R4 was only July. Created by Ocean Data View (ODV 4.5, http://odv.awi.de), and
Sampling and molecular detection of picoeukaryote community abundance and composition
One hundred milliliters of water were collected from each depth at the seven stations and prefiltered through a 50-μm-pore-size mesh to analyze the eukaryotic picophytoplankton. Three milliliters of the filtrate from each sample was directly used for measuring the abundance of picophytoplankton by a BD FACSCalibur Flow Cytometer. This analysis process is described in Zhang et al. (2016). Two-liter water samples were collected from each depth at each station. Next, each sample was passed through 20-μm, 3-μm and 0.2-μm filters. The pico-fractions (0.2–3 μm) were collected for analysis of the picoeukaryote biodiversity and community composition. The analysis methods, including DNA extraction and PCR amplification of rRNA genes, are described in Zhang et al. (2019). The V4 region of the eukaryotic SSU rRNA gene was amplified using the universal forward primer 3NDf (5′-GGCAAGTCTGGTGCCAG-3′) and the reverse V4_euk_R2 primer (5′-ACGGTATCTRATCRTCTTCG-3′) (Bråte et al. 2010). These fused primers each included an Illumina adapter, the sequencing primer and an eight-nucleotide barcode inserted between the Illumina adapter and the sequencing primer.

Barcodes were used to sort multiple samples. First, samples were individually amplified for the eukaryotic SSU rRNA V4 region. PCRs were performed in a 20 μL reaction volume containing 2 μL DNA template, 250 μM dNTPs, 0.25 μM of each primer, 2 μL 10 × PCR buffer, and 2.5 U Pfu polymerase (MBI, Fermentas, USA). The PCR conditions consisted of denaturation at 95 °C for 2 min, 25 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension cycle at 72 °C for 10 min. Subsequently, using a limited-cycle PCR on 5 µL of each PCR gel-recycled product, Illumina sequencing adapters and dual-index barcodes were added to each amplicon. Aliquots of PCR products (3 μL) were checked on a 2% agarose gel, purified using a DNA gel extraction kit (Axygen, China), and quantified using a TBS-380 Mini-Fluorometer (Turner BioSystems). Following quantification, products from the different samples were mixed in equal molar ratios for sequencing on a MiSeq platform using a 2 × 300 cycle V3 kit following standard Illumina sequencing protocols.
The raw fastq files were demultiplexed based on the barcode. PE reads for all samples were run through Trimmomatic (version 0.35) to to remove low-quality base pairs using these parameters (SLIDINGWINDOW: 50:20 MINLEN: 50). Trimmed reads were then further merged using FLASH program (version 1.2.11) with default parameters. The low quality contigs were removed based on screen.seqs command using the following filtering parameters, maxambig = 0, minlength = 200, maxlength = 580, maxhomop = 8. The 18 s sequences were analyzed using a combination of software mothur (version 1.33.3), UPARSE (usearch version v8.1.1756, http://www.drive5.com/uparse/), and R (version 3.2.3). The demultiplexed reads were clustered at 98% sequence identity into operational taxonomic units (OTUs) using the UPARSE pipeline (http://www.drive5.com/usearch/manual/uparsecmds.html). The OTU representative sequences were an assignment for taxonomy against Silva 128 database with a confidence score ≥ 0.6 by the classify.seqs command in mothur. Then, the attributions of each sequence at different levels (from phylum to genus) were added according to the NCBI database. The OTUs with relative DNA abundance larger than 1% were blasted in the NCBI database to make sure the existence of the main species. All singletons and sequences belonging to Metazoa and other traditional non-picoeukaryotes, including most diatoms, dinoflagellate, ciliates and cercozoa, and some cryptophytes and chrysophytes, were removed, and R (version 3.4.1) was used to construct an alpha-diversity index (Shannon) from the left sequences. Variations in the alpha-diversity and the corresponding phylotypes between groups of samples were estimated by one-way ANOVA. Venn diagram was used to show the sharing of OTUs among different groups. Similarity of the beta-diversity among different samples were analyzed by Bray–curtis method and ploted by pheatmap. Both MRPP and Anosim was used to analyze the significant of the difference of OTUs between different groups. All the analyses above were done in R (version 3.4.1). The sequence data were submitted to the National Center for Biotechnology Information Sequence Read Archives (SRA) under BioProject ID PRJNA340039.
Statistical analysis of microbial and environmental factors
Two statistical approaches were used to analyze the relationships among microbial communities and environmental factors. The relationships between the biological group, including both the main classes and all the present OTUs, and their corresponding environmental group (environmental factors, including temperature, salinity, nutrients and chl a) were analysed (Canoco for Windows 4.5 software).
The relationships between both biological groups (picoeukaryotic community structure with all the present OTUs) at R and SR stations and their corresponding environmental groups (physicochemical factors, including temperature, salinity, nutrients, DO and chl a) were analyzed using redundant analysis (RDA) (Canoco for Windows 4.5 software). A Detrended correspondence analysis was used for the selection of RDAs of both relationships, as their relative largest axial lengths were 3.17 (R section) and 2.92 (SR section) (< 4, Leps and Smilauer 2003).
Results
Hydrology and water masses in the southern Chukchi Sea
The temperature of water in July ranged from − 0.47 to 6.73 °C (Fig. 2a) and decreased with increasing depth of water. Salinity ranging from 31.05 to 32.77 (Fig. 2a). The profiles showed that the waters were mixed; and only weakly stratified at stion R04. The water mass mainly belonged to the BSW as the whole salinity was > 32, except that at R04-0m (Coachman et al. 1975; Woodgate et al. 2005), comprising Bering Shelf Water and Andre Water (Grebmeier et al. 2006). The temperature of water in September ranged from − 0.64 °C to 7.21 °C (Fig. 2b) and decreased with increasing depth of water. Salinity profiles also showed that the stratification of water ranged from 27.46 to 33.17 (Fig. 2b). They mainly belonged to ACW and BSW (Coachman et al. 1975; Woodgate et al. 2005). ACW, 0–10 m deep, consists of a mixture of the Alaskan Coastal Current and Bering Shelf Water (Grebmeier et al. 2006; Pisareva et al. 2015). ACW showed a lower silicate concentration (< 17 µM) than that seen in BSW (> 23 µM), which received nutrient supplements from Andre Water (Pisareva et al. 2015).
Nutrient-supplemented, upwelled water was detected at station R02 in the months of both July and September (Le et al. 2014). However, nutrient levels here (Supplementary material 1) were lower, and pH, DO, and Chl a were higher at depths of 10–30 m in July (R section) than those found in September (SR section) (average values in July: silicate = 0.12 µM, phosphate = 1.38 µM, ammonia = 1.76 µM, nitrite = 0.11 µM, nitrate = 8.63 µM, pH = 7.83, DO = 12.65 mg L−1, Chl a = 11.86 µg L−1; average values in September: silicate = 27.20 µM, phosphate = 1.81 µM, ammonia = 6.81 µM, nitrite = 0.30 µM, nitrate = 8.63 µM, pH = 7.62, DO = 9.49 mg L−1, Chl a = 1.52 µg L−1). These values suggest that a diatom bloom probably occurred in July, exhausting nutrients, especially the silicate content at station R02 (Perrette et al. 2011; Laney and Sosik 2014; Le et al. 2014). During the bloom, the pico-fraction only accounted for 14% of the total amounts of Chl a. Comparatively, the proportion of Chl a increased up to 56% in September. These findings are in accordance with the results of Le et al. (2014) and Danielson et al. (2017).
Community abundance, diversity, and composition of picoeukaryotes in the southern Chukchi Sea
The community abundance of eukaryotic picophytoplankton in the southern Chukchi Sea (Fig. 3) was found to be 0.47–6.42 × 106 cells L−1 in July, which was approximately one-fourth of their abundance (2.31–23.70 × 106 cells L−1) recorded in September. Average abundance increased fourfold, from 2.09 to 8.76 × 106 cells L−1. Distribution of data for community abundance showed some peaks indicating regional abundance for both surface and bottom water in July as well as September. Comparatively, the distribution of data showed two tongues for the R02/SR02 station corresponding to upwelling.
A total of 849,572 sequences (reads) and 7940 OTUs (at 98% similarity) were identified in our study. The sequence number of each sample ranged from 1239 to 64,905, of which 113–339 OTUs were recognized with 98% similarity (Table 1). The Good’s coverage estimator of the OTUs for all samples was higher than 99%, except for sample R02-30m, which had relatively low values (1239 reads, 113 OTUs and coverage of 97.82%). There were significant differences in both sequence numbers (F = 14.21, p = 0.001) and OTUs (F = 8.46, p = 0.007) between July and September; however, no discernible differences in the Shannon index were found (Table 1). Although specimens belonged to the same water mass, they showed significant differences in the OTUs (F = 10.116, p = 0.001) among different stations in July; however, no clear differences were seen in the sequence numbers and in the Shannon index (p > 0.5). Comparatively, no discernible differences were found between all the three parameters among different stations in September (p > 0.5).
There were significant differences in sequence numbers/reads (p = 0.0003), OTUs (p = 0.00021), and Shannon index values (p = 0.00462) among the three water masses, i.e., ACW-S (ACW in September), BSW-S (BSW in September), and BSW-J (BSW in July). Significant differences were also found between ACW-S and BSW-S (Freads = 27.139, preads = 0.0002, FOTUs = 23.602, pOTUs = 0.0004, FShannon = 33.095, pShannon = 0.00009) (Table 1). Significant differences were found only between OTUs of BSW-J and BSW-S (F = 19.720, p = 0.0002), sequence numbers (F = 26.41, p = 0.00003), and Shannon index values (F = 6.091, p = 0.021) of ACW-S and BSW-S. Generally, ACW-S had the fewest OTUs (821) and BSW-S had the highest number of OTUs (956). The three water masses shared 373 OTUs: BSW-J and BSW-S shared 117 OTUs; BSW-S and ACW-S shared 42 OTUs; and BSW-J and ACW-S shared 25 OTUs (Fig. 4). Figure 5 shows that the picoeukaryote community had distinct regional distributions. The picoeukaryotes community belonged to similar depths of water and closely situated latitudes, and the same water masses probably had similar structures. The community structure at closer depths and latitudes may be more similar than the structure of communities belonging to the same water masses but located at distant sites. The community structure found in September was similar to that seen at higher latitudes and/or deeper waters in July, i.e. SR03-10m had a similar community structure to that of R03-30m and SR04-0m.
The picoeukaryotes identified both in the months of July and September mainly belonged to nine divisions, twelve classes, seven genera, and one species (Table 2). Picoeukaryotes found in the month of July were classified into ten orders and seven families with a proportion (relative 18S rDNA read abundance) greater than 0.5% among all reads, whereas those found in the month of September were classified into eight orders and six families. Phytoplankton, all of which were mixotrophs, were found to have the highest contribution in sequencing libraries of the picoeukaryotic community in the months of July and September, respectively, accounting for 70.7% and 83.8% of the total number of reads. Comparatively, heterotrophs including Choanozoa, Picozoa, Telonemia, and Ciliophora acounted for 5.4% and 7%, respectively, in July and September. Chlorophyta was found to be the most common division, accounting for 56.8% and 69.9% of the total number of reads in the months of July and September, respectively, with Mamiellophyceae, Trebouxiophyceae, and Dinophyceae identified as the first three-domain classes. The contribution of Chlorophyta, Dinoflagellata, Choanozoa, and Chrysophyta to the total picoeukaryotic sequence library increased in September, whereas that of Ochrophyta, Picozoa, Telonemia, and Ciliophora was found to be decreased. Syndiniales was found to be the main order identified in Dinoflagellata, and its increased contribution to the picoeukaryotic library was accompanied by a predominant transfer (from Syndiniales Group II to Syndiniales Group I).
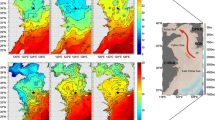
We found eight and seven classes with proportions larger than 1%, respectively, in the months of July and September (Table 1). These classes were distributed differentially at various stations, depths of water, in varying seasons and different water masses (Fig. 6). Mamiellophyceae was found to contribute markedly in all samples, with proportions of 7.9–98.0% and 17.3–89.8% in the months of July and September respectively. The contribution of Mamiellophyceae did not always decrease or increase along with increasing depths of water in both July and September (Fig. 6a, b). This class showed a relatively higher contribution in the ACW than in the BSW (Fig. 6c). Tragin and Vaulot (2019) show that the genus Micromonas is divided into 9 clades corresponding to four species: M. commoda, M. bravo, M. polaris, M. pusilla) and some clades/candidate species. M. pusilla was found mostly in temperate locations while M. polaris and Micromonas clade B3 dominate in arctic and subarctic waters, respectively. In our study, M. pusilla only accounted for 3.1% of the Micromonas and most species cannot be identified.
Proportions of the identified picoeukaryotes at class level in July (a), September (b) and different water masses (c), and at genus level in different water masses (d) of the southern Chukchi Sea in 2012: assemblages with average proportions (relative 18S rDNA read abundances) of larger than 0.5% are shown
In September, Trebouxiophyceae surpassed Dinophyceae were found to be the second most predominant class (Table 2). Prasinophyceae and Choanoflagellatea were two other classes with relatively higher contribution to picoeukaryotic libraries in September than in July. Among the main classes, only Dictyochophyceae (p = 0.0049), Spirotrichea (p = 0.009), and Bolidophyceae (p = 0.0399) showed significant differences in contribution between July and September. Like Mamiellophyceae, Trebouxiophyceae also showed relatively higher contribution in ACW than in BSW (Fig. 6c). However, Dinophyceae, Prasinophyceae, Choanoflagellatea (p = 0.0016), Chrysophyceae (p = 0.0213), and Bolidophyceae (p = 0.0113) showed higher contribution in BSW-S. Telonemea, Dictyochophyceae (p = 0.0301), and Spirotrichea showed higher contribution in BSW-J. As to the main genera with relative DNA contribution greater than 0.5% (Table 1), Amoebophrya, Cryptocaryon, Parauronema, and Picomonas were not found in either of the water masses in September. Comparatively, Diaphanoeca was only found in BSW-S, while Choricystis was found in both ASW-S and BSW-S. Usually, Cryptocaryon (WoRMS, http://www.marinespecies.org/) and Parauronema (Soldo et al. 1978; Pan et al. 2011) were thought as nonpico eukaryotes. However, we did find some OTUs belonging to both genera were identified as picoeukaryotes or picoplankton. Consequently, new candidate species of these genera that are possibly belonging to pico sized protists.
Compared with other stations, station R02 showed the presence of distinct picoeukaryote communities, with relatively high biodiversity (Table 1) however, a low contribution from the eight predominant classes, especially Mamiellophyceae (Fig. 6a). This is in accordance with the algal bloom, during which the blooming species, probably a type of diatom, inhibited the growth of picoeukaryotes.
Environmental correlations of the microbial community
Relationships between water masses, environmental factors and picoeukaryote assemblages were different between July and September, as revealed by RDA (Fig. 7). In July, canonical eigenvalues explain 70.2% of the total relationships, and the sum of the first two axes explains 66.0%. The contribution of environmental factors (C) to microbial distribution, from highest to lowest was as follows: nitrogen (C = 15.73%, p = 0.389) > phosphate (C = 13.33%, p = 0.156) > Chl a (C = 10.94%, p = 0.847) > salinity (C = 12.33%, p = 0.001) > pH (C = 9.33%, p = 0.005) > DO (C = 6.76%, p = 0.329) > temperature (C = 1.22%, p = 0.284) > silicate (C = 0.54%, p = 0.637). In September, canonical eigenvalues explain 89.5% of the total relationships, and the sum of the first two axes explains 83.4%. The influence of environmental factors on community composition is given here. The contribution of environmental factors (C) to microbial distribution, from highest to lowest was as follows: DO (C = 27.67%, p = 0.030) > temperature (C = 26.94%, p = 0.001) > Chl a (C = 12.12%, p = 0.109) > silicate (C = 6.93%, p = 0.637) > salinity (C = 5.41%, p = 0.002) > nitrogen (C = 5.02%, p = 0.275) > phosphate (C = 3.57%, p = 0.366) > pH (C = 1.21%, p = 0.253). Their interactions had different correlation with community structure at different sampling sites. Nitrogen and phosphate were the primary environmental factors influencing the community at BSW-J. Comparatively, silicate and salinity were the primary environmental factors influencing the community at BSW-S. However, DO and temperature were the primary environmental factors influencing the community at ACW-S. DO and temperature were important environmental factors in surface waters in both months. The increase in members of Mamiellophyceae and Trebouxiophyceae was seen mainly in ACW-S with lower nutrients and more fresh water than in BSW-S. However, the numbers of Prasinophyceae, Choanoflagellatea, Chrysophyceae, and Bolidophyceae mainly increased in BSW-S with nutrient supplements (Fig. 6c).
Relationships of picoeukaryote community structure at different sampling sites with physiochemical factors in an ordination diagram with the first two axes of the RDA in July (a) and September (b). Red arrows with different lengths denote relative correlations of different independent variables with the biological factors. T temperature, S salinity, N NO3 + NO2 + NH4+, DO dissolved oxygen, Chl a Chlorophyll a
Discussion
As an important part of the Pacific Arctic Gateway, the Chukchi Sea has a strong influence on the Arctic Ocean through the transport of freshwater, heat, nutrients, and plankton from the Subarctic to the Arctic (Roach et al. 1995). This region is characterized by varying gradients in species composition, diversity, and abundance of fish and invertebrates (Stevenson and Lauth 2012; Mueter et al. 2013). Types of water masses with different physicochemical factors were found to change from midsummer to early autumn in the southern Chukchi Sea (Danielson et al. 2017). Water stratification was seen in both seasons. Both temperature and salinity were higher during midsummer. All macronutrients were supplemented during early autumn, especially silicates, which were exhausted during the diatom bloom (Springer and McRoy 1989; Sakshaug 2004; Laney and Sosik 2014; Le et al. 2014). The nutrient supplement was most discernible at the R02 (SR02) station, which was a typical bloom area, with extremely low picophytoplankton abundance (Fig. 3) and low DNA contribution to picoeukaryotes in July (Fig. 6a). We found that there was a competition between large taxa and smaller ones (Zhang et al. 2016), the predominant inhibited the growth of others (Zhang et al. 2019). However, the diversity of the picoeukaryote community was not affected by the bloom (Shannon Index > 3.80).
We know that the fractionated filtration does not ensure a complete separation of pico-sized forms from nano- and microorganisms (Vaulot et al. 2002; Nielsen et al. 2007; Charvet et al. 2012; Belevich et al. 2017). The picoeukaryotes in our study still contain some OTUs identified as real picoplankton by the SILVA database, although they may belong to classes and domains that are traditionally thought as non-pico ones. Some species of diatoms can be < 3 μm in one dimension and hence are capable of passing through a 3 μm filter. Vaulot et al. (2008) also reported central diatoms as potentially “true”picoplankton. Both Lovejoy et al. (2006) and Kilias et al. (2014) reported the presence of the arctic diatoms Fragilariopsis in picoplankton libraries. Of course, sloppy feeding or cell breakage also bought some non-pico fractions (Vaulot et al. 2002; Nielsen et al. 2007; Charvet et al. 2012; Belevich et al. 2017). We have tried our best to wipe these sequences. The proportion of the increase in the contribution of picophytoplankton to that of total sequencing libraries of the picoeukaryotic community was (70.7%: 83.8%), that of increase in the pico-fraction to the total Chl a was found to be (38%: 53%). The abundance of picophytoplankton (2.09: 8.76 cells L−1), along with a decrease in the levels of total Chl a (3.06:1.08 µg L−1) and the levels of nutrient supplements in water masses in September, do not substantiate the classical assumption that larger phytoplankton would be associated with higher nutrient levels and higher biomass (Springer and McRoy 1989; Danielson et al. 2017).
The Chukchi Sea is one of the most N-limiting area amongst global oceans and is severely N-limited during the season of phytoplankton growth (Brown et al. 2015). During midsummer, nitrogen and phosphate levels were the primary factors affecting the community structure of picoeukaryotes. Diatom blooming exhausted silicates (Supplement Material 1), whereas, relatively high nitrogen and phosphate levels were still detected. These nutrients could nevertheless support the growth of other picophytoplankton except that of Mamiellophyceae, i.e., Trebouxiophyceae and Chrysophyceae. Diatom blooming inhibited the growth of Mamiellophyceae, which would be more abundant in post-bloom conditions, i.e., in the whole water column at station R01 and at a depth of 0–10 m at R03, where the silicate had been exhausted. Interestingly, the bloom was mainly found in the 10–30 m region, with insufficient light (Martini et al. 2016). NO3− reduction and O2 supersaturation in surface waters indicate the growth of phytoplankton. Comparatively, DO and temperature became the primary factors affecting picoeukaryote growth in September when phytoplankton of the picoeukaryote community increased in abundance. Although levels of nutrient supplements did not stimulate primary productivity and biomass of larger phytoplankton, the contribution of autotrophs was higher and some heterotrophs of the picoeukaryote community appeared to have perished. Water stratification with lower nutrient levels, especially N-limiting (N/P = 9) is responsible for such phenomena. Picophytoplanton are predicted to thrive in a warmer more stratified Arctic Ocean (Li et al. 2009; Zhang et al. 2016), because small cells are more effective in acquiring nutrients and less susceptible to gravitational settling (Ardyna et al. 2011). In accordance with Danielson et al. (2017) the Chukchi Sea shows a predominance of smaller phytoflagellates which suggests the possibility of a more important microbial loop in early autumn. Lower levels of DO and reduced pH indicated considerable respiratory activity of heterotrophs in the upwelling, where some mixotrophs and heterotrophs dominated the picoeukaryote community (Fig. 6). The mixotrophs can use light and nutrients to synthesize carbon and can also swallow other microbes to obtain carbon. Most phytoflagellates were mixotrophs, including members of Mamiellophyceae, Chrysophyceae, and Dictyochophyceae (Lovejoy et al. 2002; Rozanska et al. 2008; Lovejoy 2013). They are commonly known to be predominant in the arctic seas (Lovejoy et al. 2006; Terrado et al. 2009; Lovejoy and Potvin 2011). Micromonas and Bathycoccus are abundant in marine coastal waters (Kilias et al. 2014). Amoebophyra is a most commonly recovered clade of Syndiniales Group II, which has been reported to be found at all depths and in all seasons in the Arctic (Terrado 2011). The Syndiniales are either parasitoids, parasitic, or commensally dependent on a host and have complex stages in their life cycles. Diversity of these protists suggests that they evolve rapidly and many varieties may be restricted to a single host (Guillou et al. 2008). Consequently, seasonal changes in composition Syndiniales indicated variations in their hosts in the southern Chukchi Sea. Generally, the composition of the picoeukaryote community in the southern Chukchi Sea is different from that in both, the Central Arctic Ocean and in the European polar Seas (Lovejoy et al. 2006; Zhang et al. 2015). Micromonas are pan-Arctic-dominant (Lovejoy et al. 2007; Zhang et al. 2015). M. polaris and Micromonas clade B3 dominate in arctic and subarctic waters respectively (Tragin and Vaulot 2019). Micromonas with many species or clades were also found to be predominant in the southern Chukchi Sea. M. pusilla, which was found mostly in temperate locations only account for a very small fraction (1.4% of the total reads and 3.1% of the Micromonas reads). This may indicate a complex water environment (mixed of water masses). The abundance of picophytoplankton in the southern Chukchi Sea in 2012 was slightly higher than that in 2008 (July: 1.00 × 106 cells L−1, Zhang et al. 2012) and was comparable to that in the Northern Bering Sea in 2008 (July: 3.48 × 106 cells L−1) and to that in the central Arctic Ocean in 2010 (August: 4.97 × 106 cells L−1, Zhang et al. 2015, 2016).
As in other oceanic waters, the picoeukaryotic community has a distinct composition and diversity in different water masses (Hamilton et al. 2008; Winter et al. 2008; Lovejoy et al. 2011; Zhang et al. 2019), as their movements are primarily determined by passive lateral advection and vertical mixing in the water column (Hamilton et al. 2008; Zhang et al. 2012, 2019; Sigler et al. 2017). Each water mass can be considered as a habitat with its own protists; even rare organisms have a biogeography that is best explained by their water mass of origin (Galand et al. 2009). Water mass was also the primary factor determining community composition and diversity of picoeukaryotes in the southern Chukchi Sea. Variations found in some picoeukaryote communities reflected variations in water masses. Some species of Trebouxiophyceae and Bathycoccus (Mamiellophyceae) were probably carried by the ACW, especially from the Alaskan Coastal Current (Grebmeier et al. 2006; Pisareva et al. 2015). Prasinoderma (Prasinophyceae), Bolidomonas (Bolidophyceae), Diaphanoeca (Choanoflagellatea) and some species of Chrysophyceae, Dictyochophyceae, and Spirotrichea were brought by the BSW because they showed relatively high abundance in waters deeper than 10 m at station R2 with upwelling.
Seasonal changes, varying depths, and varying stations (latitudes) at the same water mass also had a significant influence on the picoeukaryotic community. Some phylotypes are ubiquitous in surface waters (Kirchman et al. 2010) and others are predominantly found in deeper waters (Galand et al. 2010). We found clear changes such as replacement of species at different regions as well in different seasons, which presents changes in community functions and indicates a great effect on the whole system (Lovejoy et al. 2011). In different seasons, the assemblages in the same water mass may be less similar to those in different water masses which are located close by. This observation is different from the results of Hamilton et al. (2008).
As temperatures of the Arctic Ocean are increasing with climate change, its physiochemical environment is changing. These changes will continue to have an impact on the microbial community in the Chukchi Sea. The Chukchi Sea may become a more flagellate-based system, especially a picophytoplankton-based system, favored by warm temperatures and strong vertical stratification of the upper water column (Li et al. 2009; Lovejoy et al. 2011), as the phytoflagellates were primarily supported by regenerated nutrients (Carmack 2007; Tremblay et al. 2009). The success of macrozooplankton is tied to higher trophic levels (Hatun et al. 2009); these organisms are dependent on phytoplankton and are sensitive to species composition (Vargas et al. 2006). Consequently, the changes in microbial communities have a great impact on trophic levels, which might change along with the changing climate.
Conclusion
The community distribution and composition of picoeukaryotes had distinct seasonal features. Contribution of picophytoplankton, especially chlorophytes increased in early autumn compared with their contribution in midsummer (July). Water mass was the primary factor determining the community composition and diversity of picoeukaryotes. Seasonal changes, varying depths, and varying stations (latitudes) at the same water mass also had a significant influence on the picoeukaryotic community. The Chukchi Sea will become a smaller phytoflagellate-based system along with Arctic warming. This will change the whole pelagic ecosystem in this region. Long-term monitoring of biodiversity and community composition of picoeukaryotes is necessary to evaluate the effects of warming of waters. Such studies will also provide essential primary data to study changes in the whole pelagic ecosystem of the Arctic Ocean.
Abbreviations
- ACW:
-
Alaskan Coastal Water
- ANOVA:
-
Analysis of variance
- BSW:
-
Summer Bering Sea Water
- Chl a :
-
Chlorophyll a
- DCA:
-
Detrended correspondence analysis
- DO:
-
Dissolved oxygen
- OTUs:
-
Operational taxonomic units
- PCR:
-
Polymerase chain reaction
- RDA:
-
Redundancy analysis
References
Aerts LAM, McFarland A, Watts B, Lomac-MacNair K, Seiser P, Wisdom S, Kirk AV, Schudel CA (2013) Marine mammal distribution and abundance in the offshore sub-region of the northeastern Chukchi Sea during the open-water season. Cont Shelf Res 67:116–126. https://doi.org/10.1016/j.csr2.2013.04.020
Ardyna M, Gosselin M, Michel C, Poulin M, Tremblay JÉ (2011) Environmental forcing of phytoplankton community structure and function in the Canadian High Arctic: contrasting oligotrophic and eutrophic regions. Mar Ecol-Prog Ser 442:37–57
Arrigo KR, Perovich DK, Pickart RS, Brown ZW, vanDijken GL, Lowry KE et al (2012) Massive phytoplankton blooms under Arctic Sea Ice. Science 336:1408
Belevich TA, Ilyash LV, Milyutina IA, Logacheva MD, Goryunov DV, Troitsky AV (2017) Photosynthetic picoeukaryotes in the land-fast ice of the white Sea, Russia. Microbial Ecology
Blanchard AL, Feder HM (2014) Interactions of habitat complexity and environmental characteristics with macrobenthic community structure at multiple spatial scales in the northeastern Chukchi Sea. Deep Sea Res II 102:32–143. https://doi.org/10.1016/j.dsr2.2013.09.022
Bråte J, Logares R, Berney C, Ree DK, Klaveness D, Jakobsen KS, Shalchian-Tabrizi K (2010) Freshwater Perkinsea and marine-freshwater colonizations revealed by pyrosequencing and phylogeny of environmental rDNA. ISME J 4:1144–1153. https://doi.org/10.1038/ismej.2010.39
Brown ZW, Casciotti KL, Pickart RS, Swift JH, Arrigo KR (2015) Aspects of the marine nitrogen cycle of the Chukchi Sea shelf and Canada Basin. Deep Sea Res II 118(PA):73–87. https://doi.org/10.1016/j.dsr2.2015.02.009
Carmack EC (2007) The alpha/beta ocean distinction: a perspective on freshwater fluxes, convection, nutrients and productivity in high latitude seas. Deep Sea Res II 54:2578–2598. https://doi.org/10.1016/j.dsr2.2007.08.018
Charvet S, Vincent WF, Comeau A, Lovejoy C (2012) Pyrosequencing analysis of the protist communities in a High Arctic meromictic lake: DNA preservation and change. Front Microbiol 3:1–14. https://doi.org/10.3389/fmicb.2012.00422
Clarke JK, Stafford SE, Moore B, Rone LA, Crance J (2013) Subarctic cetaceans in the southern Chukchi Sea: evidence of recovery or response to a changing ecosystem. Oceanography 26(4):136–149. https://doi.org/10.5670/oceanog.2013.81
Coachman LK, Aagaard K, Tripp RB (1975) Bering Strait: The Regional Physical Oceanography. University of Washington Press, Seattle
Danielson SL, Eisner L, Ladd C, Mordy C, Sousa L, Weingartner TJ (2017) A comparison between late summer 2012 and 2013 water masses, macronutrients, and phytoplankton standing crops in the northern Bering and Chukchi Seas. Deep Sea Res II 135:7–26. https://doi.org/10.1016/j.dsr2.2016.05.024
Eddie B, Juhl A, Krembs C, Baysinger C, Neuer S (2010) Effect of environmental variables on eukaryotic microbial community structure of land-fast Arctic sea ice. Environ Microbiol 12(3):797–809
Feder HM, Jewett SC, Blanchard A (2005) Southeastern ChukchiSea (Alaska) epibenthos. Polar Biol 28:402–421
Galand PE, Casamayor EO, Kirchman DL, Lovejoy C (2009) Ecology of the rare microbial biosphere of the Arctic Ocean. Proc Natl Acad Sci USA 106:22427–22432
Galand PE, Potvin M, Casamayor EO, Lovejoy C (2010) Hydrography shapes bacterial biogeography of the deep Arctic Ocean. ISME J 4:564–576
Gall AE, Day RH, Weingartner TJ (2013) Structure and variability of the marine-bird community in the northeastern Chukchi Sea. Cont Shelf Res 67:96–115. https://doi.org/10.1016/j.csr.2012.11.004
Grasshoff K, Erhardt M, Kremling K (1983) Methods of seawater analysis, 2nd revised and extended edition. Verlag Chemie GmbH, Weiheim
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and Sub-Arctic seas. Annu Rev Mar Sci 4:63–78
Grebmeier JM, Cooper LW, Feder HM, Sirenko BI (2006) Ecosystem dynamics of the Pacific-influenced northern Bering and Chukchi Seas in the Amerasian Arctic. Prog Oceanogr 71(2):331–361
Grebmeier JM, Moore SE, Overland JE, Frey KE, Gradinger R (2010) Biological response to recent Pacific Arctic sea ice retreats. Eos 91:161–162
Häder DP, Villafañe VE, Helbling EW (2014) Productivity of aquatic primary producers under global climate change. Photochem Photobiol Sci 13(10):1370–1392
Hamilton AK, Lovejoy C, Galand PE, Ingram RG (2008) Water masses and biogeography of picoeukaryote assemblages in a cold hydrographically complex system. Limnol Oceanogr 53:922–935
Hatun H, Payne MR, Beaugrand G, Reid PC, Sandø AB, Drange H (2009) Large biogeographical shifts in the north-eastern Atlantic Ocean: from the subpolar gyre, via plankton, to blue whiting and pilot whales. Prog Oceanogr 80:149–162
Kilias ES, Nöthig EM, Wolf C, Metfies K (2014) Picoeukaryote plankton composition off West Spitsbergen at the entrance to the Arctic Ocean. J Eukaryot Microbiol 61:569–579
Kirchman DL, Cottrell MT, Lovejoy C (2010) The structure of bacterial communities in the western Arctic Ocean as revealed by pyrosequencing of 16S rRNA genes. Environ Microbiol 12:1132–1143
Kuletz KJ, Ferguson MC, Hurley B, Gall AE, Labunski EA, Morgan TC (2015) Seasonal spatial patterns in seabird and marine mammal distribution in the eastern Chukchi and western Beaufort seas: identifying biologically important pelagic areas. Prog Oceanogr 136:175–200. https://doi.org/10.1016/j.pocean.2015.05.012
Laney SR, Sosik HM (2014) Phytoplankton assemblage structure in and around a massive under-ice bloom in the Chukchi Sea. Deep-Sea Res II 105:30–41. https://doi.org/10.1016/j.dsr2.2014.03.012
Le F, Hao Q, Jin H, Li T, Zhuang Y, Zhai H, Liu C, Chen J (2014) Size structure of standing stock and primary production of phytoplankton in the Chukchi Sea and the adjacent sea area during the summer of 2012. Acta Oceanol Sin 36(10):103–115 (in Chinese)
Lee SH, Whitledge TE, Kang S-H (2007) Recent carbon and nitrogen uptake rates of phytoplankton in Bering Strait and the Chukchi Sea. Cont Shelf Res 27:2231–2249
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Li WKW, McLaughlin FA, Lovejoy C (2009) Smallest algae thrive as the Arctic Ocean freshens. Science 326:539
Linders J, Pickart RS, Björk G, Moore GWK (2017) On the nature and origin of water masses in Herald Canyon, Chukchi Sea: Synoptic surveys in summer 2004, 2008, and 2009. Prog Oceanogr 159:99–114. https://doi.org/10.1016/j.pocean.2017.09.005
Lovejoy S (2013) What Is Climate? Eos 94:1–2. https://doi.org/10.1002/2013EO010001
Lovejoy C, Potvin M (2011) Microbial eukaryotic distribution in a dynamic Beaufort Sea and the Arctic Ocean. J Plankt Res 33(3):431–444
Lovejoy C, Legendre L, Martineau MJ, Bacle J, von Quillfeldt CH (2002) Distribution of phytoplankton and other protists in the North Water. Deep-Sea Res II 49:5027–5047
Lovejoy C, Massana R, Pedròs-Aliò C (2006) Diversity and distribution of marine microbial eukaryotes in the Arctic Ocean and adjacent seas. Appl Environ Microbiol 2:3085–3095
Lovejoy C, Vincent WF, Bonilla S, Roy S, Martineau M-J, Terrado R, Potvin M, Massana R, Pedrós-Alió C (2007) Distribution, phylogeny, and growth of cold-adapted picoprasinophytes in Arctic Seas. J Phycol 43:78–89
Lovejoy C, Galand PE, Kirchman DL (2011) Picoplankton diversity in the Arctic Ocean and surrounding seas. Mar Biodivers 41(1):5–12
Lowry KE, van Dijken GL, Arrigo KR (2014) Evidence of under-ice phytoplankton blooms in the Chukchi Sea from 1998 to 2012. Deep Sea Res II 105:105–117
Lowry KE, Pickart RS, Mills MM, Brown ZW, van Dijken GL, Bates NR, Arrigo KR (2015) The influence of winter water on phytoplankton blooms in the Chukchi Sea. Deep Sea Res II 118:53–72. https://doi.org/10.1016/j.dsr2.2015.06.006
Majaneva M, Blomster J, Müller S, Autio R, Majaneva S, Hyytiäinen K, Nagai S, Rintala J-M (2017) Sea-ice eukaryotes of the Gulf of Finland, Baltic Sea, and evidence for herbivory on weakly shade-adapted ice algae. Eur J Protisto 57:1–15. https://doi.org/10.1016/j.ejop.2016.10.005
Martini KI, Stabeno PJ, Ladd C, Winsor P, Weingartner TJ, Mordy CW, Eisner LB (2016) Dependence of subsurface chlorophyll on seasonal water masses in the Chukchi Sea. J Geophys Res Oceans 121:1755–1770. https://doi.org/10.1002/2015JC011359
Moore SE, Laidre K (2006) Trends in sea ice cover within habitats used by bowhead whales in the western Arctic. Ecol Appl 16:932–944
Mueter FJ, Reist JD, Majewski AR, Sawatzky C, Christiansen D, Hedges JS, et al (2013) Marine fifishes of the arctic in arctic report card: Update for 2013 – tracking recent environmental changes. https://www.arctic.noaa.gov/reportcard/marine_fifish.html
Nielsen KM, Johnsen PJ, Bensasson D, Daffonchio D (2007) Release and persistence of extracellular DNA in the environment. Environ Biosaf Res 6(1–2):37–53. https://doi.org/10.1051/ebr:2007031
Pan X, Shao C, Ma H, Fan X, Al-Rasheid KAS, Al-Farraj SA, Hu X (2011) Redescriptions of two Marine Scuticociliates from China, with notes on Stomatogenesis in Parauronema longum (Ciliophora, Scuticociliatida). Acta Protozool 50(4):301–310. https://doi.org/10.4467/16890027AP.11.027.0064
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Pedrós-Alió C, Potvin M, Lovejoy C (2015) Diversity of planktonic microorganisms in the Arctic Ocean. Prog Oceanogr 139:233–243
Perrette M, Yool A, ,Quartly GD, Popova EE (2011) Near-ubiquityofice-edge blooms in the Arctic. Biogeosciences 8:515–524
Piepenburg D, Archambault P, Ambrose W, Blanchard A et al (2011) Towards a pan-Arctic inventory of the species diversity of the macro- and megabenthic fauna of the Arctic shelf seas. Mar Biodivers 41:51–70
Pisareva MN, Pickart RS, Spall MA, Nobre C, Torres DJ, Moore GWK, Whitledge TE (2015) Flow of Pacific water in the western Chukchi Sea: results from the 2009 RUSALCA expedition. Deep Sea Res I 105:53–73. https://doi.org/10.1016/j.dsr2.2015.08.011
Polyakov IV, Timokhov LA, Alexeev VA, Bacon S, Dmitrenko IA, Fortier L (2010) Arctic Ocean warming contributes to reduced polar ice cap. J Phys Oceanogr 40:2743–2756
Poulin M, Daugbjerg N, Gradinger R, Ilyash L, Ratkova T, von Quillfeldt C (2011) The pan-Arctic biodiversity of marine pelagic and sea-ice unicellular eukaryotes: a first-attempt assessment. Mar Biodivers 41(1):13–28
Questel JM, Clarke C, Hopcroft RR (2013) Seasonal and interannual variation in the planktonic communities of the northeastern Chukchi Sea during the summer and early fall. Cont Shelf Res 67:23–41
Roach AT, Aagaard K, Pease CH, Salo SA, Weingartner T, Pavlov V, Kulakov M (1995) Direct measurements of transport and water properties through the Bering Strait. J Geophys Res 100:18443–18457
Rozanska M, Poulin M, Gosselin M (2008) Protist entrapment in newly formed sea ice in the coastal Arctic Ocean. J Mar Syst 74:887–901
Sakshaug E (2004) Primary and secondary production in the Arctic Seas. In: Stein R, MacDonald RW (eds) The organic carbon cycle in the Arctic Ocean. Springer, Berlin
Sigler MF, Mueter FJ, Bluhm BA, Busby MS, Cokelet ED, Danielson SL et al (2017) Late summer zoogeography of the northern Bering and Chukchi seas. Deep Sea Res II 139:168–189. https://doi.org/10.1016/j.dsr2.2016.03.005
Soldo AT, Dogoy DA, Laria F (1978) Purine-excretory nasture of refractile bodies in the marine ciliate Parauronema acutum. J Protozool 25:416–418. https://doi.org/10.1111/j.1550-7408.1978.tb03917.x
Springer AM, McRoy CP, Turco KR (1989) The paradox of pelagic food webs in the northern Bering Sea-II. Zooplankt Communities Cont Shelf Res 9:359–386
Steele M, Ermold W, Zhang J (2008) Arctic Ocean surface warming trends over the past 100 years. Geophys Res Lett 35:L02614
Stevenson DE, Lauth RR (2012) Latitudinal trends and temporal shifts in the catch composition of bottom trawls conducted on the eastern Bering Sea shelf. Deep-Sea Res II 65–70:251–259. https://doi.org/10.1016/j.dsr2.2012.02.021
Terrado R (2011) Diversité et succession des protistes dans l’océan Arctique. Ph.D. thesis, Faculté des sciences et de génie, Doctorat interuniversitaire en océanographie Université Laval, Numéro unique: 27849
Terrado R, Vincent WF, Lovejoy C (2009) Mesopelagic protists: diversity and succession in a coastal Arctic ecosystem. Aquat Microb Ecol 56:25–39
Thaler M (2014) Diversity and distribution of heterotrophic flagellates in the Arctic Ocean. [dissertation/PHD’s thesis]. Université Laval, Chicago (IL)
Tragin M, Vaulot D (2019) Novel diversity within marine Mamiellophyceae (Chlorophyta) unveiled by metabarcoding. Sci Rep 9:5190. https://doi.org/10.1038/s41598-019-41680-6
Tremblay G, Belzile C, Gosselin M, Poulin M, Roy S, Tremblay JÉ (2009) Late summer phytoplankton distribution along a 3500 km transect in Canadian Arctic waters: strong numerical dominance by picoeukaryotes. Aquat Microb Ecol 54:55–70
Vargas CA, Escribano R, Poulet S (2006) Phytoplankton food quality determines time windows for successful zooplankton reproductive pulses. Ecology 87:2992–2999
Vaulot D, Eikrem W, Viprey M & Moreau H (2008) The diversity of small eukaryotic phytoplankton (≤3 μm) in marine ecosystems. FEMS Microbiol Rev 32:795–820
Vaulot D, Romari K, Not F (2002) Are autotrophs less diverse than heterotrophs in marine picoplankton? Trends Microbiol 10(6):266–267. https://doi.org/10.1016/s0966-842x(02)02366-1
Whitman WB, Coleman DC, Wiebe WJ (1998). Prokaryotes: The unseen majority. Proc Natl Acad Sci USA 95:6578–6583
Winter C, Moeseneder MM, Herndl GJ, Weinbauer MG (2008) Relationship of geographic distance, depth, temperature, and viruses with prokaryotic communities in the eastern tropical Atlantic Ocean. Microb Ecol 56:383–389
Woodgate RA, Aagaard K (2005) Revising the Bering Strait freshwater flux into the Arctic Ocean. Geophy Res Lett 32:L02602
Woodgate RA, Aagaard K, Weingartner T (2005) A year in the physical oceanography of the Chukchi Sea: Moored measurements from autumn 1990–1991. Deep-Sea Res II 52:3116–3149
Yun MS, Whitledge TE, Kong M, Lee SH (2014) Low primary production in the Chukchi Sea shelf, 2009. Cont Shelf Res 76:1–11
Zhang F, Ma Y, Lin L, Zhang J, He J (2012) Hydrophysical correlation and water mass indication of optical physiological parameters of picophytoplankton in Prydz Bay during autumn 2008. J Microbiol Methods 91:559–565. https://doi.org/10.1016/j.mimet.2012.09.030
Zhang F, He J, Lin L, Jin H (2015) Dominance of picophytoplankton in the newly open surface water of the central Arctic Ocean. Polar Biol 38:1081–1089. https://doi.org/10.1007/s00300-015-1662-7
Zhang F, Lin L, Gao Y, Cao S, He J (2016) Ecophysiology of picophytoplankton in different water masses of the northern Bering Sea. Polar Biol 39:1381–1397. https://doi.org/10.1007/s00300-015-1860-3
Zhang F, Cao S, Gao Y, He J (2019) Distribution and environmental correlations of picoeukaryotes in an Arctic fjord (Kongsfjorden, Svalbard) during the summer. Polar Res 8:3390–3400. https://doi.org/10.33265/polar.v38.3390
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41206189 and 41476173), Shanghai Natural Science Foundation (16ZR1439800), the Foreign Cooperation supported by CAAA (IC201307) and the Chinese Projects for Investigations and Assessments of the Arctic and Antarctic (CHINARE2012-16 for 04-03, 04-04 and 03-05). The data were issued by the Data-sharing Platform of Polar Science (http://www.chinare.org.cn) maintained by Polar Research Institute of China (PRIC) and Chinese National Arctic and Antarctic Data Center (CN-NADC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., He, J., Jin, H. et al. Comparison of picoeukaryote community structures and their environmental relationships between summer and autumn in the southern Chukchi Sea. Extremophiles 25, 235–248 (2021). https://doi.org/10.1007/s00792-021-01222-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-021-01222-3