Abstract
Halomonas alkalicola CICC 11012s is an alkaliphilic and halotolerant bacterium isolated from a soap-making tank (pH > 10) from a household-product plant. This strain can propagate at pH 12.5, which is fatal to most bacteria. Genomic analysis revealed that the genome size was 3,511,738 bp and contained 3295 protein-coding genes, including a complete cell wall and plasma membrane lipid biosynthesis pathway. Furthermore, four putative Na+/H+ and K+/H+ antiporter genes, or gene clusters, designated as HaNhaD, HaNhaP, HaMrp and HaPha, were identified within the genome. Heterologous expression of these genes in antiporter-deficient Escherichia coli indicated that HaNhaD, an Na+/H+ antiporter, played a dominant role in Na+ tolerance and pH homeostasis in acidic, neutral and alkaline environments. In addition, HaMrp exhibited Na+ tolerance; however, it functioned mainly in alkaline conditions. Both HaNhaP and HaPha were identified as K+/H+ antiporters that played an important role in high alkalinity and salinity. In summary, genome analysis and heterologous expression experiments demonstrated that a complete set of adaptive strategies have been developed by the double extremophilic strain CICC 11012s in response to alkalinity and salinity. Specifically, four antiporters exhibiting different physiological roles for different situations worked together to support the strain in harsh surroundings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkaliphilic and halophilic bacteria from diverse lineages are distributed worldwide in natural and human environments, such as soda lakes (Ma et al. 2004), mill wastewater (Yang et al. 2010) and soap-making tanks (Tang et al. 2017). These surroundings allowed haloalkaliphilic bacteria to evolve adaptive mechanisms for survival under extreme alkaline and osmotic pressure (Padan et al. 2005).
To regulate pH homeostasis and cation concentrations in response to alkaline and saline stress, bacteria develop a variety of strategies, including adjustments to cell wall structure (Aono et al. 1999) and membrane lipid composition (Clejan et al. 1986); improvements in membrane transport activities and bioenergetics (Krulwich 1995); and accumulations of compatible solutes (Ono et al. 1998) and metabolic alterations for acid generation (Blankenhorn et al. 1999). Among these mechanisms, cation/proton antiporters play an indispensable role in cation tolerance and pH homeostasis by promoting the efflux of intracellular monovalent cations in exchange for external protons (Padan et al. 2005).
According to the sequence-based Transporter Classification Database (TCDB), cation/proton antiporters mainly belong to four superfamilies (Saier et al. 2006): the cation diffusion facilitator (CDF) superfamily, which includes members of the CaCA family (TC 2.A.19), such as ChaA and ChaB (Fujisawa et al. 2009; Sääf et al. 2001); the cation proton antiporter (CPA) superfamily (Chen et al. 2011), including the CPA1 (TC 2.A.36) and CPA2 (TC 2.A.37) families; the Na+ transporting Mrp superfamily, which includes members of the multi-subunit CPA3 antiporter (TC 2.A.63) family, such as the Mrp- and Pha-type antiporters (Kajiyama et al. 2007; Putnoky et al. 1998); and the ion transporter (IT) superfamily, including NhaA (TC 2.A.33), NhaB (TC 2.A.34), NhaC (TC 2.A.35), NhaD (TC 2.A.62) and NhaE (TC 2.A.111) families (Padan et al. 2015; Pinner et al. 1992; Ito et al. 1997; Liu et al. 2005; Sousa et al. 2013).
Although cation/proton antiporters are responsible for pH homeostasis, which allows bacteria to tolerate environmental stresses, different antiporters with diverse functions have evolved in neutrophilic and alkaliphilic bacteria. For example, NhaA and NhaB are major Na+/H+ antiporters in Escherichia coli and other enterobacteria. NhaA plays an essential role during alkaline stress by adjusting intracellular pH, whereas NhaB functions in neutral conditions (Pinner et al. 1992). The NhaD antiporter allows for survival in high salinity and alkalinity, and is mainly associated with haloalkaliphiles, such as Alkalimonas amylolytica (Liu et al. 2005), Halomonas elongata (Kurz et al. 2006), Halobacillus dabanensis (Zhang et al. 2014) and Halomonas alkaliphila (Wang et al. 2017). The Mrp antiporter, distinct from the single gene-encoded antiporter, is composed of six to seven subunits. This heterooligomeric antiporter usually exists in alkaliphilic Bacillus strains and has a crucial role at high alkalinity (Kajiyama et al. 2007). A similar antiporter complex, Pha, exists in Rhizobium meliloti and is mainly responsible for potassium proton exchange (Putnoky et al. 1998).
Halomonas alkalicola CICC 11012s, deposited at the China Center of Industrial Culture Collection (CICC), was isolated from a soap-making tank (pH > 10) in a household-product plant in China. The strain is able to grow in medium with 0–80 g/L NaCl and pH 7.0–12.5 (Tang et al. 2017). A striking feature of this strain is its extreme alkaliphilic tolerance. In this study, the genome of strain CICC 11012s was sequenced and analyzed to investigate the potential mechanisms for pH homeostasis. Through genome annotation and screening, four putative antiporters were characterized and their roles in response to alkaline stress were explored.
Materials and methods
Strains and plasmids
H. alkalicola CICC 11012s was previously isolated from a soap-making tank and cultured in tryptone soya agar (TSA). Two antiporter-deficient E. coli strains were selected to explore the functions of H. alkalicola antiporters: namely, E. coli KNabc, deficient in three Na+/H+ antiporter encoding genes (nhaA, nhaB and chaA), and E. coli TK2420, deficient in K+ uptake transporter encoding genes (kdp, kup and trk). E. coli KNabc (Nozaki et al. 1996) and E. coli TK2420 (Epstein et al. 1993) were cultured in LBK medium. E. coli Trans1-T1 and vector pUC19 were used for gene cloning. The strains and plasmids used in this study are listed in Table 1; the primers used in this study are listed in supplemental Table 1.
Growth experiments under alkaline and saline conditions
Triplicate growth studies were performed in 96-well plates for determining the alkaline and saline tolerance of H. alkalicola CICC 11012s. Tryptone soya broth (TSB; 200 μL) of various pH values (7.0–12.0) and NaCl concentrations (0–60 g/L) was aliquoted into the wells. Cells with exponential growth in TSB (OD600 = 0.5) were then inoculated into the broth. After 24 h incubation, absorbance data (OD600) were collected to illustrate growth under alkaline and salt conditions using SpectraMax® M2 multifunctional plate-reading machine (Molecular Device, Sunnyvale, USA).
Genome preparation, sequencing and annotation
Genomic DNA was extracted from cells during the exponential growth phase using the TIANamp Bacteria Genomic DNA Kit (Tiangen, Beijing, China) according to the manufacturer’s protocol. Qualification and quantification of the prepared DNA was measured using BioDrop μLite (BioDrop, Cambridge, UK). The integrity of the molecular weight fragments was verified on a 1% agarose gel. The genome of H. alkalicola CICC 11012s was sequenced on the Illumina HiSeq 2000 platform. Briefly, genomic DNA was randomly fragmented and then DNA fragments of appropriate lengths were retained by electrophoresis. Adapters were ligated to the fragments to construct the bacterial sequencing (BS) library. After quality testing, the qualified BS library was used for sequencing. Short reads were assembled into a genome sequence using SOAPdenovo (Li et al. 2010; Li et al. 2008b). The key parameter K, set at 79, was determined by optimal assembly results for the sample. The assembly results were then locally assembled and optimized according to paired-end and overlap relationships by mapping reads to contigs. The genes were predicted from the assembly results using Glimmer (Delcher et al. 1999; Salzberg et al. 1998; Delcher et al. 2007), which was developed for microorganisms, such as bacteria, archaea and viruses.
Tandem repeats were predicted using Tandem Repeat Finder (Benson 1999), and minisatellite and microsatellite DNAs were selected based on the number and length of repeated units. rRNAmmer (Lagesen et al. 2007), tRNAscan (Lowe and Eddy 1997) and Infernal software and the Rfam (Gardner et al. 2009) database were used to predict the rRNA, tRNA and sRNA, respectively.
Function annotation was accomplished by protein sequence analysis. The genes were aligned with databases to obtain the corresponding annotations. To ensure the biological meaning, the highest quality alignment was chosen as the gene annotation. Function annotation of genes was completed using Basic Local Alignment Search Tool (BLAST) against the non-redundant database (Version: 20121005), Cluster of Orthologous Groups of proteins (COGs) (Tatusov et al. 1997, 2003) and Kyoto Encyclopedia of Genes and Genomes (KEGGs) databases (Kanehisa 1997; Kanehisa et al. 2003, 2006).
The RAST and KEGG databases were used to analyze alkali-related genes, which are mainly responsible for the synthesis of cell wall structure, plasma membrane lipid composition, metabolisms for acid generation, bioenergetics and cation/proton antiporters that catalyze active proton transport, such as Na+ (Li+)/H+ antiporters and K+/H+ antiporters.
Characterization of monovalent cation/proton antiporters
Genes encoding HaNhaD, HaNhaP, HaPhaD and HaMrpD were amplified from genomic DNA by PCR. BamHI and HindIII restriction sites were inserted in the upstream and downstream regions, respectively, of the target genes. The PCR product was purified using Cycle-Pure kit (Omega, Norcross, USA), digested with BamHI and HindIII (Takara, Dalian, China), and ligated into the pUC19 plasmid at these two sites using T4 DNA ligase (Takara, Dalian, China). The resulting recombinant plasmids, designated as pUC19–HaNhaD, pUC19–HaNhaP, pUC19–HaPhaD and pUC19–HaMrpD, were transformed into competent E. coli Trans-T1 cells by chemical transformation. After PCR verification, the recombinant plasmids were isolated and transformed into E. coli KNabc and E. coli TK2420. Primers used in this work are listed in supplemental Table 1.
Characterizations of the four putative antiporters mentioned above were carried out in E. coli KNabc and E. coli TK2420. Recombinant cells harboring pUC19 in E. coli KNabc and E. coli TK2420 were considered as corresponding controls. LBK broths of different NaCl concentrations from 0 to 600 mM were used to investigate Na+ resistance in E. coli KNabc recombinants. Minimal medium with various KCl concentrations from 0 to 100 mM were chosen to explore K+ resistance using E. coli TK2420 recombinants. LBK medium containing 100 mM NaCl with different pH values were used to test pH resistance. The pH of the broth was adjusted incrementally with Tris–HCl buffer (50 mM) to 6.0, 7.0, 8.0 and 9.0 (Cheng et al. 2016).
The translated amino acid sequence of HaNhaD was analyzed using BLASTP software. Comparisons between HaNhaD and NhaD, a similar antiporter from close relatives, were carried out by ESPript 3.0 (Gouet et al. 1999).
Expression analysis in H. alkalicola CICC 11012s
Cells in mid-logarithmic phase (approximately, OD600 = 0.8–1.0) grown in 30 g/L NaCl at pH 7.5, 9.0 and 11.0 were collected. RNA was extracted from H. alkalicola CICC 11012s using the TRIzol-based method. Reverse transcription and gDNA removal were done with TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (Transgen Biotech, Beijing, China). The expression of HaNhaD, HaNhaP, HaPhaD and HaMrpD were quantified with real-time PCR on an ABI Prism 7500 System (ABI, Carlsbad, USA) using TransStart Green qPCR SuperMix (Transgen Biotech, Beijing, China). The 16S rRNA gene was used as a standard.
Results
Tolerance to alkalinity and salinity
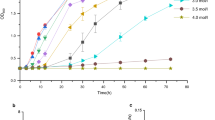
Triplicate growth tests were performed in 96-well plates with various pH values (7.0–12.0) and NaCl concentrations (0–60 g/L). As shown in Fig. 1, H. alkalicola CICC 11012s could tolerate a wide pH range, from pH 7.0 to 12.0, indicating the evolution of special adaptive mechanisms for high alkalinity. The species of genus Halomonas can generally withstand high salinity; however, strain CICC 11012s did not survive in a high salt environment. The growth of strain CICC 11012s in optimal conditions (pH 9.0 and 30 g/L NaCl) enabled genome and expression analysis.
Genome sequence and annotation
The genome sequence of H. alkalicola CICC 11012s has been deposited in NCBI database under accession number SRP 102919. Shotgun genome sequencing by Illumina HiSeq 2000 produced a total of 9,703,192 reads and 1288 Mb of data. Based on the assembly, the genome of H. alkalicola CICC 11012s is 3,511,738 bp and had a GC content of 67.67%. The number of scaffolds was 142 and the number of contigs was 243. No plasmids were found. Glimmer predicted 3295 coding sequences, with the total length of genes being 3,105,471 bp and representing 88.37% of the genome. The specific gene information, predictions of repeat regions and non-coding RNA are listed in Fig. 2 and Table 2.
Circular genome map of H. alkalicola CICC 11012s. From inner to outer: 1, GC skew (GC Skew is calculated using a sliding window, as (G − C)/(G + C), with the value plotted as the deviation from the average GC skew of the entire sequence); 2, GC content (plotted using a sliding window, as the deviation from the average GC content of the entire sequence); 3, tRNA/rRNA; 4 and 5, CDS (colored according to COG function categories, where 4 is the reverse strand and 5 is the forward strand)
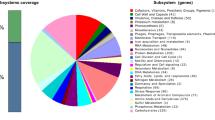
The COGs, KEGGs and non-redundant protein databases were used to annotate the predicted genes. Proteins were divided into twenty bins according to their functions in the COG database (Table 3). The adaptive strategies for bacteria in response to alkaline stress includes regulating cell wall structure and membrane lipid composition; improving membrane transport and bioenergetics activities; and promoting metabolic alterations to generate acids. Genome analysis indicated that the complete cell wall and plasma membrane lipid biosynthesis pathways, involving more than 200 genes, were found in strain CICC 11012s. Interestingly, genome analysis also identified a special pathway for teichuronic acid biosynthesis, which is usually present in Gram-positive bacteria, and unsaturated fatty acid biosynthesis pathways, which improves membrane fluidity and increases stress tolerance. Furthermore, 134 genes associated with ATPase activities and 57 genes involved in cytochrome action in the respiratory chain were annotated, thereby enabling energy production to deal with changing surroundings. An additional 17 genes were identified that encode deaminases, such as cytosine deaminase, l-serine deaminase, d-serine deaminase and adenosine deaminase, which produced acids that lowered intracellular pH in alkaline environments. Most importantly, four kinds of antiporters (HaNhaD, HaNhaP, HaPha and HaMrp) that played a crucial role in handling alkaline stress were identified by genome annotation.
Sequence analysis of four cation/proton antiporters
HaNhaD was homologous to NhaD antiporters from Halomonas shengliensis and Halomonas elongata with 89 and 87% sequence similarities, respectively. HaMrp was an antiporter complex that contains seven MrpA-G units, in which MrpA and MrpD were major components and might play an important role in sodium proton exchange. HaMrpD had 86% sequence identity with that from Halomonas daqingensis. HaNhaP was a putative K+/H+ antiporter and showed 86, 85 and 84% sequence similarities with NhaP from H. daqingensis, Halomonas aquamarina and Halomonas pantelleriensis, respectively. HaPha was similar to HaMrp and also composed of six units (PhaC-G and PhaA/B that were predicted as PhaA or PhaB), the difference being that HaPha was responsible for potassium efflux and proton influx. PhaD was the core unit and shared 81% identity with that from H. daqingensis. The specific sizes and arrangements of these antiporter genes or gene clusters within the genome were shown in supplemental Fig. 1. The GenBank accession numbers of HaMrpD, HaNhaD, HaNhaP and HaPhaD were MF488960 to MF488963 deposited in NCBI database.
The functions of four cation/proton antiporters
HaNhaD, HaNhaP, HaPhaD and HaMrpD were amplified and ligated into cloning vector pUC19. To confirm the antiporter function, the recombinant plasmids were expressed in antiporter-deficient E. coli KNabc and E. coli TK2420. E. coli KNabc and E. coli TK2420 recombinants carrying only pUC19 were used as controls.
To explore Na+ and Li+ transport capabilities, all E. coli KNabc strains mentioned above were grown in LBK medium with various NaCl and LiCl concentrations ranging from 0 to 600 mM. Results revealed that E. coli KNabc (pUC19–HaNhaD) and E. coli KNabc (pUC19–HaMrpD) exhibited enhanced Na+ tolerance in the presence of 100–500 mM NaCl over E. coli KNabc (pUC19), thereby indicating that these two antiporters are mainly responsible for Na+/H+ exchange (Fig. 3a). Furthermore, E. coli KNabc (pUC19–HaNhaD) grew better than E. coli KNabc (pUC19–HaMrpD) in 200–600 mM NaCl and it was the only strain that was able to tolerate Li+ even up to 500 mM (Fig. 3b). In contrast, the Na+ and Li+ resistance tests did not reveal any difference between E. coli KNabc (pUC19–HaNhaP), E. coli KNabc (pUC19–HaPhaD) and E. coli KNabc (pUC19) (Fig. 3a, b), which suggested that HaNhaP and HaPhaD antiporter activities were not associated with sodium.
Complementation assays of four antiporters in E. coli KNabc or E. coli TK2420 at different concentrations of NaCl, LiCl, KCl and different pH values. A, growth of E. coli KNabc recombinants with four antiporters in LBK medium at NaCl concentrations ranging from 0 to 600 mM; B, growth of E. coli KNabc recombinants with four antiporters in LBK medium at LiCl concentrations ranging from 0 to 600 mM; C, growth of E. coli TK2420 with four antiporters in minimum medium at various KCl concentrations ranging from 0 to 100 mM. D, growth of E. coli KNabc recombinants with four antiporters in LBK medium at different pH values
E. coli TK2420 strains expressing the four antiporters were selected to test K+ transport in minimal medium using various KCl concentrations from 0 to 100 mM. The expression of HaNhaP and HaPhaD resulted in weak growth of recombinant E. coli TK2420, compared with the control, at low potassium concentrations (0–40 mM; Fig. 3c), which implied that HaNhaP and HaPhaD were capable of exporting potassium. Moreover, E. coli TK2420 (pUC19–HaNhaD) showed remarkable growth in the presence of 20 mM potassium, indicating that HaNhaD contributes to potassium import; however, no difference was detected between E. coli TK2420 (pUC19–HaMrpD) and E. coli TK2420 (pUC19).
The influence of pH on the four antiporters was assessed using LBK medium, with 100 mM NaCl at different pH values. When compared to the control, E. coli KNabc (pUC19–HaNhaD) exhibited better complementation under acidic, neutral and alkaline conditions; however, HaMrpD expression supported growth of the antiporter-deficient E. coli strain only in neutral and alkaline conditions. Furthermore, no difference was detected between E. coli KNabc (pUC19–HaNhaP), E. coli KNabc (pUC19–HaPhaD) and E. coli KNabc (pUC19) at pH 6.0–9.0 (Fig. 3d).
Expression analysis in H. alkalicola CICC 11012s
The optimal growth conditions for strain CICC 11012s were pH 9.0 and 30 g/L NaCl. Cells grown to mid-logarithmic phase at pH 7.5, 9.0 and 11.0 in 30 g/L NaCl were collected and RNA was extracted for expression analysis. Real-time PCR analysis shows that the four antiporter genes were up-regulated with pH augmentation. HaNhaD exhibited high expression at pH 9.0, whereas HaMrpD displayed maximum expression levels under pH 11.0. Similar results were obtained for genes encoding K+/H+ antiporter activities; HaNhaP was up-regulated at pH 9.0 and HaPhaD showed maximum activity at pH 11.0 (Fig. 4).
Discussion
To accommodate alkaline pH and high external osmotic pressure, haloalkaliphiles have developed a series of mechanisms for cytoplasmic pH homeostasis and osmotic balance. They also have efficient energetic conversion systems to support survival and propagation in harsh environments. H. alkalicola CICC 11012s, isolated from a soap-making tank (pH > 10), is a typical haloalkaliphile whose growth requires 0.5 M of total Na+ as the lowest limit and pH > 8.5 (Banciu and Muntyan 2015). A comparison of pH and NaCl growth requirements between strain CICC 11012s and its closest relatives is shown in Table 4. Interestingly, H. alkalicola CICC 11012s is able to grow at pH 12.5, higher than any other Halomonas strains; however, it has the lowest observed Na+ tolerance and cannot survive above 80 g/L NaCl. The reasons for strain CICC 11012s’ survival in such high pH intrigued us to further explore the mechanisms of pH homeostasis.
In alkaline environments, membrane structure adjustments, intracellular pH maintenance and cation concentrations are important for bacteria. Whole genome sequences reveal that H. alkalicola CICC 11012s has developed potential adaptive strategies to cope with severe environments. For instance, strain CICC 11012s has developed complete pathways, involving 192 genes, for cell wall biosynthesis. Strikingly, a special pathway for teichuronic acid biosynthesis, which is usually present in the cell wall components of Gram-positive bacteria, was also found in strain CICC 11012s. Negative charges on teichuronic acid could give the cell surface the ability to absorb sodium and protons, as well as repulse hydroxide ions, thereby enabling the cell to grow in an alkaline environment (Aono 1987). Four genes encoding 3-hydroxyacyl-CoA dehydrogenases were annotated. These dehydrogenases are involved in fatty acid elongation to form unsaturated fatty acids with lower melting temperatures, thus improving membrane fluidity and increasing stresses tolerance (Banciu et al. 2005). A pathway for squalene biosynthesis also was detected. These products are located in the cell membrane and increase alkaline tolerance by compacting the bilayer to lower cation and proton permeability (Hauß et al. 2002).
Plasma membrane lipids and fatty acid components are also important for bacteria to cope with adverse conditions. Five cardiolipin synthases were identified in the genome. Cardiolipin plays a significant role in stabilizing respiratory chain components that assist in cytochrome c oxidase activity (Arias-Cartin et al. 2012), alkaliphilic adaptation (Krulwich 2006) and salt-stress response (De et al. 2009). Over 100 genes associated with ATPases and cytochromes, including cytochrome c oxidase, were identified, indicating that the strain has the potential to regulate energetic systems in extreme surroundings (Sorokin et al. 2013).
It is well known that monovalent cation/proton antiporters play an indispensable role in pH homeostasis. Antiporters of H. alkalicola, and its relatives, were searched in the genome profiles. Like other Halomonas members, H. alkalicola harbors proficient antiporters, such as NhaD, Mrp, NhaP and Pha (Table 3), to tolerate high alkalinity. To investigate the function of the four antiporters in response to alkalinity, HaNhaD, HaNhaP, HaPhaD and HaMrpD were expressed in antiporter-deficient E. coli KNabc and E. coli TK2420. Complementation assays indicate that HaNhaD and HaMrp are mainly responsible for Na+ and H+ exchange, while HaNhaP and HaPhaD exhibit K+/H+ antiporter activities.
The Na+ cycle is crucial for pH homeostasis; however, intracellular Na+ accumulation might be toxic to bacteria. Results demonstrate that HaNhaD is able to complement antiporter-deficient E. coli KNabc and confers Na+ and Li+ tolerance to strain CICC 11012s, thereby indicating that HaNhaD is mainly responsible for Na+ and H+ exchange. Meanwhile, HaNhaD exhibited antiporter activity in E. coli KNabc under acidic, neutral and alkaline conditions, with higher optical density maxima than other antiporters. HaNhaD was also up-regulated at high alkalinity, implying that it is the most significant H. alkalicola CICC 11012s antiporter.
The full-length HaNhaD nucleotide sequence is 1485 bp in length and is predicted to encode a protein of 494 amino acids with a molecular weight of 54 kDa. Amino acid sequence analysis shows that HaNhaD has the highest homology to NhaD from H. shengliensis and H. elongata with 89 and 87% similarities, respectively. Transmembrane topology predictions using online software HMMTOP indicate that HaNhaD has fourteen transmembrane helices that are characteristic of NhaD-type antiporters (Tusnády and Simon 2001). Furthermore, it possesses the conserved Asp (358) and Thr (359) residues (Fig. 5), which are crucial for the activity of antiporters in the NhaD family (Ostroumov et al. 2002). In addition, HaNhaD has a highly variable region at the N-terminus that is predicted to confer different pH and Na+ tolerances to bacteria. Further research focusing on HaNhaD-deficient mutants and HaNhaD structures is required.
Multiple alignment of amino acids sequences of HaNhaD with NhaD-type antiporters of other γ-proteobacteria. HaNhaD, H. alkalicola; HeNhaD, H. elongata; HcNhaD H. campaniensis; HsNhaD, H. shengliensis; HhNhaD, H. huangheensis; HkNhaD, H. korlensis; HscNhaD, H. saccharevitans; HspNhaD, H. sp. Y2; AaNhaD, A. amylolytica
The HaMrp complex, a Na+/H+ antiporter composed of HaMrp A-G subunits, is similar to Mrp from the alkaliphilic Bacillus strains, which have MrpA and MrpD as major subunits (Morino et al. 2008). Complementation and expression profiles reveal that HaMrpD functions in neutral and alkaline environments and achieves its highest expression level at pH 11.0, indicating that HaMrp probably participates in pH adjustment mainly under alkaline conditions. This might be an economic way for bacteria to handle harsh surroundings, such as high alkalinity and salinity. When strain CICC 11012s was subjected to alkaline condition, HaNhaD was likely triggered first because HaNhaD is encoded by a single gene and is easily up-regulated by bacteria. As pH increases, HaNhaD alone cannot handle the increase in protons; thus the HaMrp antiporter complex begins promoting the influx of protons in exchange with sodium. As a result, HaNhaD and HaMrp work together to regulate intracellular pH homeostasis and Na+ resistance in response to extreme alkalinity and salinity.
Potassium is another major monovalent cation involved in bacterial regulation of intracellular pH, enzyme activation and osmolality, as it is an important osmotic solute. Like sodium, excessive amounts of intracellular K+ are detrimental to bacteria (Epstein 2003). Complementation assays reveal that HaNhaP and HaPhaD are mainly responsible for K+/H+ antiporter activity. Although the expression levels of HaNhaP and HaPhaD were lower than that of HaNhaD and HaMrpD, a similar mechanism was detected in K+/H+ antiporter regulation. HaNhaP exhibits potassium and proton exchange abilities in neutral and alkaline environments, whereas HaPhaD plays a role in high alkalinity.
In summary, the genome of strain CICC 11012s, a double extremophile isolated from a soap-making tank, was sequenced and analyzed. Results show that this strain has developed a complete set of adaptive strategies in response to extreme alkalinity and salinity. Adjustments to the cell wall structure and plasma membrane lipid composition allow CICC 11012s to more easily adapt to harsh surroundings. Strikingly, four antiporters associated with Na+/H+ and K+/H+ tolerance were characterized. Results indicate that these four antiporters exhibit different physiological roles in different situations and work together to support the propagation of the strain in alkaline and saline environments.
References
Aono R (1987) Characterization of structural component of cell walls of alkalophilic strain of Bacillus sp. C-125. Preparation of poly (gamma-l-glutamate) from cell wall component. Biochem J 245:467–472. https://doi.org/10.1042/bj2450467
Aono R, Ito M, Machida T (1999) Contribution of the cell wall component teichuronopeptide to pH homeostasis and alkaliphily in the alkaliphile Bacillus lentus C-125. J Bacteriol 181:6600–6606
Arias-Cartin R, Grimaldi S, Arnoux P, Guigliarelli B, Magalon A (2012) Cardiolipin binding in bacterial respiratory complexes: structural and functional implications. Biochim Biophys Acta 1817:1937–1949. https://doi.org/10.1016/j.bbabio.2012.04.005
Banciu H, Muntyan M (2015) Adaptive strategies in the double-extremophilic prokaryotes inhabiting soda lakes. Curr Opin Microbiol 25:73–79. https://doi.org/10.1016/j.mib.2015.05.003
Banciu H, Sorokin D, Rijpstra WIC et al (2005) Fatty acid, compatible solute and pigment composition of obligately chemolithoautotrophic alkaliphilic sulfur-oxidizing bacteria from soda lakes. FEMS Microbiol Lett 243:181–187. https://doi.org/10.1016/j.femsle.2004.12.004
Benson G (1999) Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res 27:573–580. https://doi.org/10.1093/nar/27.2.573
Blankenhorn D, Phillips J, Slonczewski JL (1999) Acid- and base-induced proteins during aerobic and anaerobic growth of Escherichia coli revealed by two-dimensional gel electrophoresis. J Bacteriol 181:2209–2216
Chen JS, Reddy V, Chen JH et al (2011) Phylogenetic characterization of transport protein superfamilies: superiority of Superfamily Tree programs over those based on multiple alignments. J Mol Microbiol Biotechnol 21:83–96. https://doi.org/10.1159/000334611
Cheng B, Meng Y, Cui Y et al (2016) Alkaline response of a halotolerant alkaliphilic Halomonas strain and functional diversity of Its Na + (K +)/H + antiporters. J Biol Chem 291:26056–26065. https://doi.org/10.1074/jbc.m116.751016
Clejan S, Krulwich TA, Mondrus KR, Seto-Young D (1986) Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J Bacteriol 168:334–340. https://doi.org/10.1128/jb.168.1.334-340.1986
De LV, Catucci L, Ventrella A et al (2009) Cardiolipin increases in chromatophores isolated from Rhodobacter sphaeroides after osmotic stress: structural and functional roles. J Lipid Res 50:256–264. https://doi.org/10.1194/jlr.m800312-jlr200
Delcher AL, Harmon D, Kasif S et al (1999) Improved microbial gene identification with GLIMMER. Nucleic Acids Res 27:4636–4641. https://doi.org/10.1093/nar/27.23.4636
Delcher AL, Bratke KA, Powers EC et al (2007) Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23:673–679. https://doi.org/10.1093/bioinformatics/btm009
Epstein W (2003) The roles and regulation of potassium in bacteria. Prog Nucleic Acid Res 75:293–320. https://doi.org/10.1016/s0079-6603(03)75008-9
Epstein W, Buurman E, McLaggan D, Naprstek J (1993) Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem Soc Trans 21:1006–1010. https://doi.org/10.1042/bst0211006
Fujisawa M, Wada Y, Tsuchiya T, Ito M (2009) Characterization of Bacillus subtilis YfkE (ChaA): a calcium-specific Ca2+/H+ antiporter of the CaCA family. Arch Microbiol 191:649–657. https://doi.org/10.1007/s00203-009-0494-7
Gardner PP, Daub J, Tate JG et al (2009) Rfam: updates to the RNA families database. Nucleic Acids Res 37(Database issue):D136–D140. https://doi.org/10.1093/nar/gkn766
Gouet P, Courcelle E, Stuart D, Metoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15:305–308. https://doi.org/10.1093/bioinformatics/15.4.305
Hauß T, Dante S, Dencher NA, Haines TH (2002) Squalane is in the midplane of the lipid bilayer: implications for its function as a proton permeability barrier. Biochim Biophys Acta 1556:149–156. https://doi.org/10.1016/s0005-2728(02)00346-8
Ito M, Guffanti AA, Zemsky J, Ivey DM, Krulwich TA (1997) Role of the nhaC-encoded Na+/H+ antiporter of alkaliphilic Bacillus firmus OF4. J Bacteriol 179:3851–3857. https://doi.org/10.1128/jb.179.12.3851-3857.1997
Kajiyama Y, Otagiri M, Sekiguchi J, Kosono S, Kudo T (2007) Complex formation by the mrpABCDEFG gene products, which constitute a principal Na+/H+ antiporter in Bacillus subtilis. J Bacteriol 189:7511–7514. https://doi.org/10.1128/jb.00968-07
Kanehisa M (1997) A database for post-genome analysis. Trends Genet 13:375–376. https://doi.org/10.1016/s0168-9525(97)01223-7
Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M (2003) The KEGG resource for deciphering the genome. Nucleic Acids Res 32:277–280. https://doi.org/10.1093/nar/gkh063
Kanehisa M, Goto S, Hattori M et al (2006) From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res 34:D354–D357. https://doi.org/10.1093/nar/gkj102
Krulwich TA (1995) Alkaliphiles: ‘basic’ molecular problems of pH tolerance and bioenergetics. Mol Microbiol 15:403–410. https://doi.org/10.1111/j.1365-2958.1995.tb02253.x
Krulwich TA (2006) Alkaliphilic prokaryotes. Springer, New York
Kurz M, Brünig A, Galinski E (2006) NhaD type sodium/proton-antiporter of Halomonas elongata: a salt stress response mechanism in marine habitats? Saline Syst 2:10. https://doi.org/10.1186/1746-1448-2-10
Lagesen K, Hallin P, Rødland EA et al (2007) RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35:3100–3108. https://doi.org/10.1093/nar/gkm160
Li H, Zhang LP, Chen S (2008a) Halomonas korlensis sp. nov. a moderately halophilic, denitrifying bacterium isolated from saline and alkaline soil. Int J Syst Evol Microbiol 58:2582–2588. https://doi.org/10.1099/ijs.0.65711-0
Li R, Li Y, Kristiansen K, Wang J (2008b) SOAP: short oligonucleotide alignment program. Bioinformatics 24:713–714. https://doi.org/10.1093/bioinformatics/btn025
Li R, Zhu H, Ruan J et al (2010) De novo assembly of human genomes with massively parallel short read sequencing. Genome Res 20:265–272. https://doi.org/10.1101/gr.097261.109
Liu J, Xue Y, Wang Q et al (2005) The activity profile of the NhaD-type Na+ (Li+)/H+ antiporter from the soda lake haloalkaliphile Alkalimonas amylolytica is adaptive for the extreme environment. J Bacteriol 187:7589–7595. https://doi.org/10.1128/jb.187.22.7589-7595.2005
Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964. https://doi.org/10.1093/nar/25.5.955
Ma Y, Zhang W, Xue Y, Zhou P, Ventosa A, Grant WD (2004) Bacterial diversity of the Inner Mongolian Baer Soda Lake as revealed by 16S rRNA gene sequence analyses. Extremophiles 8:45–51. https://doi.org/10.1007/s00792-003-0358-z
Miao C, Jia F, Wan Y et al (2014) Halomonas huangheensis sp. nov. a moderately halophilic bacterium isolated from a saline–alkali soil. Int J Syst Evol Microbiol 64:915–920. https://doi.org/10.1099/ijs.0.056556-0
Morino M, Natsui S, Swartz TH, Krulwich TA, Ito M (2008) Single gene deletions of mrpA to mrpG and mrpE point mutations affect activity of the Mrp Na+/H+ antiporter of alkaliphilic Bacillus and formation of hetero-oligomeric Mrp complexes. J Bacteriol 190:4162–4172. https://doi.org/10.1128/jb.00294-08
Nozaki K, Inaba K, Kuroda T, Tsuda M, Tsuchiya T (1996) Cloning and sequencing of the gene for Na+/H+ antiporter of Vibrio parahaemolyticus. Biochem Biophys Res Commun 222:774–779. https://doi.org/10.1006/bbrc.1996.0820
Ono H, Okuda M, Tongpim S et al (1998) Accumulation of compatible solutes, ectoine and hydroxyectoine, in a moderate halophile, Halomonas elongata, KS3 isolated from dry salty land in Thailand. J Ferment Bioeng 85:362–368. https://doi.org/10.1016/s0922-338x(98)80078-0
Ostroumov E, Dzioba J, Loewen PC, Dibrov P (2002) Asp (344) and Thr (345) are critical for cation exchange mediated by NhaD, Na(+)/H(+) antiporter of Vibrio cholerae. Biochim Biophys Acta 1564:99–106. https://doi.org/10.1016/s0005-2736(02)00407-8
Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta 1717:67–88. https://doi.org/10.1016/j.bbamem.2005.09.010
Padan E, Danieli T, Keren Y et al (2015) NhaA antiporter functions using 10 helices, and an additional 2 contribute to assembly/stability. Proc Natl Acad Sci USA 112:E5575–E5582. https://doi.org/10.1073/pnas.1510964112
Pinner E, Padan E, Schuldiner S (1992) Cloning, sequencing and expression of the NhaB gene, encoding a Na+: H+ antiporter in Escherichia coli. J Biol Chem 267:11064–11068
Putnoky P, Kereszt A, Nakamura T et al (1998) The pha gene cluster of Rhizobium meliloti involved in pH adaptation and symbiosis encodes a novel type of K+ efflux system. Mol Microbiol 28:1091–1101. https://doi.org/10.1046/j.1365-2958.1998.00868.x
Romano I, Giordano A, Lama L, Nicolaus B, Gambacorta A (2005) Halomonas campaniensis sp. nov. a haloalkaliphilic bacterium isolated from a mineral pool of Campania Region, Italy. Syst Appl Microbiol 28:610–618. https://doi.org/10.1016/j.syapm.2005.03.010
Sääf A, Baars L, von Heijne G (2001) The internal repeats in the Na+/Ca2+ exchanger-related Escherichia coli protein YrbG have opposite membrane topologies. J Biol Chem 276:18905–18907. https://doi.org/10.1074/jbc.m101716200
Saier MH Jr, Tran CV, Barabote RD (2006) TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res 34:D181–D186. https://doi.org/10.1093/nar/gkj001
Salzberg SL, Delcher AL, Kasif S, White O (1998) Microbial gene identification using interpolated Markov models. Nucleic Acids Res 26:544–548. https://doi.org/10.1093/nar/26.2.544
Sorokin DY, Banciu H, Robertson LA et al (2013) Halophilic and Haloalkaliphilic Sulfur-Oxidizing Bacteria. Springer, Berlin, Heidelberg
Sousa PM, Videira MA, Vorburger T et al (2013) The novel NhaE-type Na+/H+ antiporter of the pathogenic bacterium Neisseria meningitidis. Arch Microbiol 195:211–217. https://doi.org/10.1007/s00203-012-0856-4
Tang X, Zhai L, Lin Y et al (2017) Halomonas alkalicola sp. nov., isolated from a household product plant. Int J Syst Evol Microbiol 67:1546–1550. https://doi.org/10.1099/ijsem.0.001757
Tatusov RL, Koonin EV, Lipman DJ (1997) A genomic perspective on protein families. Science 278:631–637. https://doi.org/10.1126/science.278.5338.631
Tatusov RL, Fedorova ND, Jackson JD et al (2003) The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4:41. https://doi.org/10.1186/1471-2105-4-41
Tusnády GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849. https://doi.org/10.1093/bioinformatics/17.9.849
Vreeland RH, Litchfield CD, Martin EL, Elliot E (1980) Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Evol Microbiol 30:485–495. https://doi.org/10.1099/00207713-30-2-485
Wang Y, Cai H, Chi C et al (2007) Halomonas shengliensis sp. nov. a moderately halophilic, denitrifying, crude-oil-utilizing bacterium. Int J Syst Evol Microbiol 57:1222–1226. https://doi.org/10.1099/ijs.0.64973-0
Wang Y, Song N, Yang L et al (2017) A novel NhaD-type Na+/H+ antiporter from the moderate halophile and alkaliphile Halomonas alkaliphila. Can J Microbiol 63:596–607. https://doi.org/10.1139/cjm-2017-0104
Xu X, Wu Y, Zhou Z et al (2007) Halomonas saccharevitans sp. nov. Halomonas arcis sp. nov. and Halomonas subterranea sp. nov. halophilic bacteria isolated from hypersaline environments of China. Int J Syst Evol Microbiol 57:1619–1624. https://doi.org/10.1099/ijs.0.65022-0
Yang C, Wang Z, Li Y et al (2010) Metabolic versatility of halotolerant and alkaliphilic strains of Halomonas isolated from alkaline black liquor. Bioresour Technol 101:6778–6784. https://doi.org/10.1016/j.biortech.2010.03.108
Zhang H, Wang Z, Wang L et al (2014) Cloning and identification of a novel NhaD-type Na+/H+ antiporter from metagenomic DNA of the halophilic bacteria in soil samples around Daban Salt Lake. Extremophiles 18:89–98. https://doi.org/10.1007/s00792-013-0600-2
Acknowledgements
This work was supported by the Fund of National Infrastructure of Microbial Resources (No. NIMR2017-4). The authors thank Professor Yanfen Xue (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) and Professor Jun Liu (Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin, China) for kindly providing E. coli KNabc strain and E. coli TK2420 strain.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Albers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhai, L., Xie, J., Lin, Y. et al. Genome sequencing and heterologous expression of antiporters reveal alkaline response mechanisms of Halomonas alkalicola . Extremophiles 22, 221–231 (2018). https://doi.org/10.1007/s00792-017-0991-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-017-0991-6