Abstract
As indicated by longitudinal observation, autism has difficulty controlling emotions to a certain extent in early childhood, and most children's emotional and behavioral problems are further aggravated with the growth of age. This study aimed at exploring the correlation between white matter and white matter fiber bundle connectivity characteristics and their emotional regulation ability in children with autism using machine learning methods, which can lay an empirical basis for early clinical intervention of autism. Fifty-five high risk of autism spectrum disorder (HR-ASD) children and 52 typical development (TD) children were selected to complete the skull 3D-T1 structure and diffusion tensor imaging (DTI). The emotional regulation ability of the two groups was compared using the still-face paradigm (SFP). The classification and regression models of white matter characteristics and white matter fiber bundle connections of emotion regulation ability in the HR-ASD group were built based on the machine learning method. The volume of the right amygdala (R2 = 0.245) and the volume of the right hippocampus (R2 = 0.197) affected constructive emotion regulation strategies. FA (R2 = 0.32) and MD (R2 = 0.34) had the predictive effect on self-stimulating behaviour. White matter fiber bundle connection predicted constructive regulation strategies (positive edging R2 = 0.333, negative edging R2 = 0.334) and mother-seeking behaviors (positive edging R2 = 0.667, negative edging R2 = 0.363). The emotional regulation ability of HR-ASD children is significantly correlated with the connections of multiple white matter fiber bundles, which is a potential neuro-biomarker of emotional regulation ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Autism spectrum disorder (ASD) refers to a group of severe neurodevelopmental disorders starting in early childhood, mainly manifested by social interaction, communication disorders and stereotypical repetitive interest behaviors [24, 48, 50]. Functional communication disorders associated with the core symptoms of ASD and associated mental deficits and nearly 71–80% of autism patients may be associated with emotional behavior problems are the major causes of severely impaired social functioning of autism [80]. The children of autism has been difficult to regulate emotions to a certain extent in early childhood, and most children's emotional behavior problems are further aggravated or even worsened with the growth of age [56, 79]. Mazefsky et al. believe that impaired emotion regulation may be inherent in autism themselves, and abnormal or impaired emotion regulation may be the more direct cause of behavioral disorders in autism (such as introverted behaviors like anxiety and extroverted behaviors like aggression and irritability) [59].
Notably, for emotion regulation (ER), studies have shown that children with autism have difficulties using goal-oriented and behavior-related strategies. They employ more emotion regulation strategies (e.g., avoidance and venting) and show a higher percentage of giving up and a longer duration of emotional arousal, which are also manifested as anger, aggression, self-injury, and other stimuli produced strong [72]. Emotional regulation is often defined in psychology as the process by which individuals influence, which emotions they have, when they have them, and how they experience and express those emotions [31]. The process of emotional response can be described as perception–valuation–action (PVA) [18, 81]. Emotional problems with autism can begin early in life [32]. An analysis of parents' memories and home videos of children with autism showed that early abnormal development usually appeared in the first year of life, including more negative emotions, irritability, poor eye contact, lack of interaction, and responses to parents' voices [37, 87]. The above-mentioned abnormalities may arise from deficits in emotion recognition, expression, and regulation in children with autism. Emotional regulation may serve as a potential mechanism to explain internalizing and externalizing behaviors in children with autism [55]. White et al. found that 11–84% of children with autism had varying levels of impaired anxiety, such as generalized anxiety disorder, dissociative anxiety disorder, and obsessive–compulsive disorder [84]. Carter et al. found that social anxiety symptoms increased with age in children with autism, while they decreased with age in children without autism [40]. Multiple studies of adolescents with autism have found that more than 25% have serious mood disorders [58, 74].
Children with autism may behave destructively during negative emotions or cope with emotional problems in ways that disturb others (being overexcited or depressed) [82]. However, Macari et al. did not support the negative emotion bias in children with autism. Their results suggested that children with autism had a weaker response to threat and a heavier response to target blocking, whereas the ability to express positive emotions was intact, suggesting the complexity of emotional expressions in autism [54]. The general recommendation is to use a combination of multi-source assessment methods, including mainly behavioral observations of children and parental questionnaires [1, 5]. Common observation methods comprise asking children to wait for surprises, free play in unstructured environments, as well as the disappointment paradigm [13]. In the task of emotional regulation paradigm, the emotional regulation strategies employed by children comprise constructive strategies and non-constructive strategies, and constructive strategies cover seeking help, reassessment, self-consolation, and so forth. Non-constructive regulation strategies involve distraction, self-talk, voice venting, body venting, and avoidance. Children with autism already have abnormalities in their ability to regulate emotions, either since they do not gain fully insights into the emotional expressions, or because they ignore the expression of emotions in others [67]. As depicted in the research tables, compared with TD children, besides experiencing more anger and anxiety, children with autism adopt more negative adaptive strategies such as repetitive behaviors and less constructive emotion regulation strategies (e.g., problem-solving) [39]. Konstantareas et al. coded children with autism using the low-stress paradigm and found that children with autism used less self-comforting strategies and more crying strategies [42]. Adolescents with autism are more likely to rely on obvious cues to describe emotional responses (e.g., “I’m upset because I'm crying”), providing them with non-specific emotional experiences [46]. Adults with autism describe using self-stimulating behaviors (e.g., rocking) or diverting attention to regulate their mood [47]. Overall, children with autism may face challenges in emotional regulation throughout the life cycle, causing distress to families, suggesting the need for early identification and intervention to provide direction for later emotional behavior problems and reduce the use of undesirable strategies. Accordingly, the exploration of emotional regulation in early childhood of autism becomes an important goal.
Few insights have been gained into the etiology of socio-emotional problems associated with autism, suggesting that underlying mechanisms include alterations in neurodevelopment and alterations in brain structure and functional connectivity [17, 44]. The emotional regulation mechanisms in people with autism are complex and may be partly similar to those in the normal population (e.g., physiological arousal, levels of negative and positive influence, changes in the amygdala and prefrontal cortex) and partly inconsistent with those in the normal population (e.g., information processing, differences in perception, and cognitive aspects) [55]. FMRI studies suggested that increasing indicators of positive and negative emotional responses in autism led to abnormal activation of the nucleus accumbens, amygdala, and dorsolateral prefrontal cortex [68], as well as abnormal frontal and amygdala responses during facial emotional processing [53, 85]. Clinical features of autism with special educational needs are associated with changes in fear facial emotional processing [57]. At present, most studies focus on differences in brain function, but there are few structural studies on emotional regulation in children, especially white matter and white matter fiber bundle connections. This is despite research suggesting that the structure of the amygdala in children with autism may be an important factor in later emotional and social problems [64]. For instance, amygdala enlargement is one of the first brain abnormalities identified in people with autism. Greater amygdala inhibition in emotional regulation may have a better ability to reduce pain [73], whereas more specific studies are lacking.
The neural hypothesis for specific imbalances in cognitive, emotional, and motor processes in children with autism refers to interactions between brain structures such as the prefrontal lobe, amygdala, and hippocampus, suggesting that such imbalances underlie the neural basis of many autism symptoms [25]. Studies have reported that the total brain volume and subcortical brain area volume increase in children with autism between 1 and 2 years of age [66, 75]. Circumstantial evidence from head circumference measurements at birth and MRI in infancy suggests that brain overgrowth does not occur at birth, but occurs later in the first year of life [33]. The above finding was later confirmed in a longitudinal MRI cohort of 55 infants aged 6 to 24 months [73]. At present, multiple studies have supported that autism has shown an increase in the volume of amygdala and hippocampus in early childhood, with the earliest finding at 6 months [61]. Other studies have found that the size of the amygdala increases from 2 to 4 years of age and is associated with the severity of symptoms in infants [81]. But from ages 8 to 14, this growth slowed down or even reversed, showing no significant difference from the control group [2, 3]. As revealed by some research, the amygdala is the core region of the social brain, and the amygdala and the prefrontal cortex play an important role in emotion regulation mechanisms. However, there are relatively few studies on the development of emotion-related amygdala and other subcortical brain regions in early childhood with autism [4]. The medial prefrontal cortex refers to a central region of inhibition control mechanisms while taking on critical significance in facial emotion recognition and emotion regulation [36]. A DTI study found an association between the projected microscopic properties of the prefrontal and temporal thalamus and social symptoms of children with autism [63]. The above-mentioned areas project into the anterior cingulate gyrus, orbitofrontal cortex, and amygdala, are involved in the regulation of memory, emotion, and drive in children with autism [14]. In general, the above-mentioned results help identify the underlying processes in the brains of children with autism that are associated with mood disorders. Thus, the neural mechanism of emotional regulation and related characteristics of children with autism should be investigated. The research based on neural mechanism is helpful to improve the emotional behavior problems of children with autism in terms of intervention and treatment, and improve the function of children with autism.
This study predicted children's emotional regulation ability based on the characteristics of subcortical nuclear mass volume, white matter and white matter network connectivity of children with autism. To screen out important white matter and white matter network characteristics related to emotional and behavioral problems with autism, which may further deepen our understanding of how brain structure affects the emotions of children with autism, to lay an empirical basis for early clinical intervention of autism.
Materials and methods
Subjects
The participants were members of the Children’s Mental Health Research Center of the Nanjing Brain Hospital affiliated with Nanjing Medical University from October 2017 to February 2020 who were invited to participate in a cohort study. A total of 107 children participated in this study. The sample included 55 infants at high risk for autism (45 boys; age range, 18–30 months) and 52 TD infants (35 boys; age range, 18–30 months). Magnetic resonance imaging (MRI) scans were performed on the enrolled children, and sequences due to incomplete sequences or head movements during MRI scanning were excluded.
Enrolment criteria
The ASD at-risk group were selected on a clinical assessment by two child psychiatrists based on of the following: (i) positive M-CHAT (modified checklist for autism in toddles) assessment results [43], (ii) scores above 30 on the Childhood Autism Rating Scale (CARS) [76], (iii) all cases satisfied either risk criteria for ASD (if under 24 months of age at intake, the diagnosis was repeated 24 months later, meeting the diagnostic criteria for ASD) or DSM-5 criteria for ASD diagnosis [49] if over 24 months of age at intake, and received both (iv) the Autism Diagnostic Observational Schedule (ADOS) [49] and (v) the Autism Diagnostic Interview-Revised (ADI-R) [51]. The exclusion criteria were as follows: (1) definite history of craniocerebral trauma; (2) history of visual or hearing impairment, severe neurological problems, or other somatic disorders; (3) genetic or metabolic diseases with clear etiology (e.g., Rett's syndrome and Fragile X syndrome).
The TD group included 52 infants (35 boys; age range, 18–30 months). The TD infants had no neuropsychiatric disorders and were matched with the HR-ASD group on mental age and the ratio of boys to girls. TD participants were screened for ASD using the M-CHAT and CARS. The groups were matched on the raw scores of the Gesell Developmental Assessment (DQ), a standardized test assessing IQ in children aged 6 months and older. The DQ scores in the TD group were higher than 80. The exclusion criteria were as follows: (1) presence of any kind of neurodevelopmental disorder or delay; (2) definite history of craniocerebral trauma; (3) history of visual or hearing impairment, severe neurological, or other physical disorders.
This study gained approval from the Medical Ethics Committee of Nanjing Medical University (Approval number: 2017-KY098-01), and all the participants’ legal guardians signed informed consent.
Clinical psychological assessment
Diagnosis and cognitive assessment
General demographic data, personal history, parenting history, past history and family history of the subjects were investigated using a self-compiled General Situation Questionnaire. The Gesell scale was used to evaluate the developmental level of all subjects [7]. The childhood autism rating scale (CARS) [76], autism diagnostic observation schedule-2nd edition, ADOS-2) and the autism diagnostic interview-revised (ADI-R) [51] were used to assess the severity of symptoms in the HR-ASD group.
Videos of still face (SFP) behavior paradigm in HR-ASD and TD groups
Still-face experimental paradigm diagram
A structured experiment was conducted in the behavioral observation room of the research base, and all subjects were required to complete a video recording the still-face paradigm (SFP) experiment when they were enrolled. Baseline period: Mother and child sat face to face, requiring mother and child to interact normally for 2 min; Still face phase: Then stop the interaction and keep the neutral face for 1 min (complete with fixed instructions) (Fig. 1).
Based on the interaction between children and caregivers, the code index rules [16, 29] are as follows: (1) Emotion response of children (interaction period): ① Negative emotion of children: Closing lips, whine, complain, frown, grimace, refusal to interact with the mother. ② Positive emotion of children: Showing positive emotion or vocalization, continuing to pay attention to the mother, and beginning or maintaining social interaction with the mother. clear and emotional tone of voice, or a happy laugh. ③ Neutral emotion of children: Neutral emotion, voice. (2) The emotion regulation ability of the children (Still-face period): Constructive adjustment strategies include: ① Help Seeking: Smiling or vocalizing toward the mother, with eyes and body movements toward the mother. Having body contact with the mother, enjoying the physical comfort of hugging, and moving into the lap of the mother. ② Self-soothing: Sucking the thumb, rubbing the arm, stroking the desk, appearing to be in a calm or low arousal state. ③ Deep expiratory relief: Making a deep breathing sound. Non-constructive adjustment strategies: ④ Self-stimulating: Repetitive or jerky body movements, hand flapping that seems to provide stimulation or sustain arousal, body swaying, tapping on the desk. ⑤ Distraction: Drawing attention to a task without using a toy, object, or person, or drawing attention away from the task (quickly). ⑥ Crying: The sound of pain, crying.
Behavioral coding was performed using Noldus Observer 12.0XT behavioral observation record analysis [61]. The video was independently coded by two trained research assistants with a confidence of 93%, and any differences in coding metrics were resolved through discussion.
Imaging data acquisition
The subjects in this study used GE 3.0 discovery MR750 and 32 channel coils for MRI image acquisition. Scanning parameters of 3D-T1 structural image: echo time 3.24 ms, scanning field 256 × 256, layer thickness 1 mm, layer number 156, scanning time 4 min. The parameters of diffusion tensor imaging (DTI) are: echo time 102 ms, repeat time 8000 ms, B value 1000 s/mm, gradient direction 25, scanning field 220 × 220, thickness 2 mm, number of layers 75 and scanning time 7 min. All subjects were given 10% chloral hydrate (0.5 ml/kg) orally and scanned during sleep.
Preconditioning and analysis of brain structure
Preprocessing of brain structural magnetic resonance data
First, the MRIcron tool kit (https://people.cas.sc.edu/rorden/mricron) was used for preprocessing and format conversion of original DICOM images, and Freesurfer6.0 (FreeSurferWiki-Free Surfer Wiki harvard.edu) software was adopted for subcortical segmentation and data extraction. Freesurfer cortex reconstruction and segmentation comprised skull removal, image segmentation, and cortical reconstruction in 30 steps. The main brain structural parameters analyzed included subcutaneous nuclear volume. For cortex segmentation, the left and right hemispheres of the brain fall into 34 brain regions using the Desikan–Killiany atlas. Cortical thickness refers to the average distance between the inner and outer surfaces of the cerebral cortex. For data processing quality control, the Freesurfer user manual and pipe network tutorial were used, involving the use of tkregister2, tkmedit, and tksurfer to conduct layer-by-layer quality checks and manual correction for the data of the respective subject (http://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction).
Analysis of brain structural MRI data
First, the support vector machine (support vector those, SVM) method was adopted based on Matlab to classify research including Libsvm software package (http://www.csie.ntu.edu.tw/~cjlin/libsvm). The classification process was performed using a linear kernel support vector machine combined with leave-one-out cross validation (LOOCV). The specific steps were as follows: (1) Read data: set as label information (sample is row, feature is column). (2) Use the leave-one method for cross-verification and leave the subject as the test set and the rest as the training set. (3) Data normalization as follows: all Eigenvalues/Euclidean norm (mapping eigenvalues from a large range to [0, 1] or [–1, 1]):
Use linear kernel function (no distortion occurs in space, hyperplanes obtained in the new space are oblique lines, will not change) calculation formula:
(5) Parameter adjustment: Parameters C and gamma of the SVM model, C is the penalty coefficient, namely the tolerance for error. The higher the C, the more errors cannot be tolerated, and it is easy to overfit. The smaller the C, the easier it is to underfit. If C is too large or too small, the generalization ability is poor. (6) Use the optimized hyperparameters to train the whole training set. (7) Classification accuracy test replacement times: 1000 times. (8) Prediction of test sets and accuracy of the current fold; Summary decision value and average the weights of all the folds; Receiver operating characteristic (ROC) curves were generated to calculate specificity, sensitivity, and accuracy (ACC).
Feature screening methods: (1) Using the dimensionality reduction method of principal component analysis (PCA), linear dimensionality reduction, the use of orthogonal transformation to convert the observation data represented by linearly correlated variables into several data represented by linearly independent variables, linearly independent variables into the principal component. PCA selects the variable with the most significant difference in the orthogonal transformation as the first PC, which is the axis with the largest sum of the squares of the coordinate values in the rotation transformation. k principal components y1, y2, … The cumulative variance contribution rate of yk is defined as the ratio of the sum of k variances to the sum of all variances. The formula is expressed as follows:
The feature selection method extracts the features that contribute significantly to classification while removing the redundant features to enhance the classification performance.
Preprocessing of brain diffusion magnetic resonance data
In Linus system, Matlab and PANDA software were adopted to preprocess DTI data (http://www.nitrc.org/projects/panda/). The fully automatic processing of MRI data is realized through interface operation. The main steps comprise data format conversion: ① The dcm2nii software tool in MRIcron software is used to convert the original data in DICOM format into NIFTI format. ② Resampling (2 × 2 × 3 mm3) was performed to remove the brain tissue to obtain the whole brain mask, eddy current, and head motion correction. ③ Calculation of dispersion index: Calculate the dispersion index on the Full Pipeline interface, use affine transformation to register the FA image and TIWI image in the native space, and then nonlinear transform the structural image of a single research object to customized anti-warping transform based on the average value of all research objects. The generated dispersion indicators comprise anisotropy fraction (fractional anisotropy, FA), mean diffusivity (mean diffusivity, MD).
Analysis of brain diffusion magnetic resonance data
In the environment of Linus system, DTI data preprocessing uses Matlab2017b and PANDA1.3.1 software (http://www.nitrc.org/projects/panda/), the fully automatic processing of MRI data is realized through the interface operation。The construction of the white matter network first needs to define the nodes and edges of the network. According to the standard brain map, the subjects' brains were divided into different brain regions and defined as nodes of the brain network. In this study, the infant brain structure map AAL90 brain template was used (http://bric.unc.edu/ideagroup/free-softwares/unc-infant-0-1-2-atlases/) Perform registration and network node definition. Network nodes are defined as nodes based on the AAL map, and the brain is divided into 90 brain regions according to the self-made group-level AAL partitioning template, representing 45 nodes on the left and right sides of the brain network. Network edge is defined as the edge of the network defined by (fiber number, FN) or FA threshold in most previous studies before the construction of brain network. Based on the weight calculation of the atlas, the average weight of each region was calculated. The white matter atlas divided the white matter region into 50 brain regions to obtain one weight for each brain region, and the corresponding position was sorted.
Based on Matlab, PRONTO (http://www.mlnl.cs.ucl.ac.uk/pronto/prtsoftware.html) and SPM (https://www.fil.ion.ucl.ac.uk/spm/software/download/) software were used for analysis. Multi-voxel pattern analysis (MVPA) was used to estimate the voxel array based on brain images. When SVM was applied through MVPA, the signal intensity representing the voxel in the array formed the corresponding feature vector, with the associated weight (w), describing the contribution of the respective voxel to the linear decision function defining the hyperplane. Detailed process: feature extraction; model training and testing; model evaluation; pattern positioning. The results showed how much of a brain region’s voxel contributed to the classification. The construction of the white matter network requires the definition of nodes and edges. In accordance with the standard brain atlas, the subjects’ brains were divided into different brain regions and defined as nodes of the brain network. In this study, the AAL90 brain template of the infant brain structure atlas was employed for registration and the definition of network nodes. The network node was defined as the node defined based on the AAL map, and the brain was divided into 90 brain regions according to the self-made group-level AAL partition template, representing 45 nodes on the left and right sides of the brain network. Network edge is defined as the edge of the network defined by FA threshold in most previous studies before the construction of the brain network. The average weight of each region was calculated based on the weight calculation of the atlas. The white matter atlas divided the white matter region into 50 brain regions to obtain one weight for the respective brain region, and the corresponding position was sorted.
Analysis of white matter fiber bundle junction based on DTI
Matlab and libsvm (Libsvm—A Library for Support Vector Machines ntu.edu.tw) were adopted. The white matter fiber bundle connections in all children were predicted using the SVM method. Procedure: ① Read the structural connection data based on DTI; The two groups of features are combined and sorted into a function whose samples are rows and features are columns. ② Using SVM classification, the outer layer leaves a cross-validation process. Subsequently, feature filtering; Start with 25 features and keep 25 more at a time. Next, the for loop is carried out to retain the feature and predict the result of the total precision. ③ The overall result is the curve of the total precision changing with the number of edges. Use linear tuning to record prediction accuracy and decision value; Support vector weight and AUC; Seeking interpretability: selecting consistency features and calculating the weight of survival features. The weight of each surviving edge; 1 is negative weight, 3 is positive weight; Perform 1000 substitution tests. The ability of white matter fiber bundle connections to predict emotional regulation was constructed by connectome-based predictive modeling (CPM).
The CPM process function is called by Matlab, and the whole white matter fiber bundle connection matrix is built based on the white matter fiber bundle connection, and whether there is fiber bundle connection. Detailed procedures: ① Prepare characteristics and scales for the respective subject. ② The respective link is correlated with the behavioral scale: CPM takes the connection matrix and data from the individual as input, and generates a predictive model of behavioral data from the connection matrix. Pick a significant correlation to stay. Input: subject connection matrix and predicted behavior score, set P value threshold. The data from the training data set were correlated by regression analysis and Pearson correlation was used to identify the positive and negative prediction network. According to the number of fibers, if there were no fibers, the connection would be 0. The more the number of connections, the more fiber pathways, and the tighter the structure. Output: positive/negative correlation edge prediction accuracy, significantly correlated edge; true prediction r (coefficient); get the relationship between the true value of r and the predicted value of R. ③ When the leave-one method is used for cross-verification, the output is formatted, the training set dimension becomes 90*90* (n−1), the training set scale is created, the output correlation coefficient and correlation coefficient P value, and an empty array is created to store significant positive and negative correlation edges mask. ④ Leave a significant edge, select features; sifting through 4050, select the most significant correlation remaining. Store the sum of positive and negative significant edges of each subject in the training set; extract the training set and correlate the mask with the remaining positive correlation. All subjects were significantly edge-to-edge extraction. ⑤ Simple linear regression model: \(y = mx + b\), Each feature and a behavior value is regression, that is, the CPM model is generated. The sum of connections extracted by positive and negative significant correlated edges predicts the scale. Replacement test: 2000 times, CPM function was called to establish a network connection, and significant correlation connection was displayed. Pearson correlation (ρ) and mean square error formula were used for the corresponding relationship between the predicted value and the actual value or model performance:
Cross-verify interpretation variance formula:
Statistical analysis
① SPSS 23.0 software was used to analyze the children in the HR-ASD group and the TD group, and \(\chi^{2}\) test was performed for gender comparison. Independent sample T test was used to compare the age, developmental quotient, and emotion regulation characteristics of the two groups, as well as the mean and standard deviation. The demographic characteristics and emotional characteristics of the two groups of children were compared. Independent sample T test was used for variables that met the normal distribution, and z score standardization was performed for data that did not meet the normal distribution, and the mean and standard deviation were expressed. ② Subcutaneous nuclear group product was classified by SVM, and structural features were extracted by PCA and Filter. Linear regression was used to analyze the relationship between emotion regulation ability and screening characteristics, relationship between ability to regulate emotions and ADOS severity. Freeview was used to visualize brain regions. ③ The SVC classification model and SVR regression model of white matter characteristics (FA and MD values) of emotion regulation ability in the HR-ASD group were built based on the MVPA method, and the weight brain regions with significant contributions were obtained, ADOS severity was incorporated into the model as a covariate. ④ The CPM method was employed to predict the emotional regulation ability based on the white matter fiber bundle connection, showing significant correlation connections. The corresponding correlation between the predicted value and the actual value or the model performance was examined using the Pearson correlation method. Using one method of cross-validation will disturb the rerun of the CPM of data analysis, such that BrainNetViewer (http://www.nitrc.org/projects/bnv/) and Circos (http://www.circos.ca/) software visualization results were employed.
Results
General demographic data and clinical information
The HR-ASD group comprised 45 males (45/55, 81.81%) and 10 females (10/55, 18.18%). The mean age reached 23.12 ± 3.44 months. The TD group covered 52 males (35/52, 67.31%) and 10 females (17/52, 32.69%). The mean age was 17.12 ± 5.231 months. Further comparative analysis indicated the developmental quotient (t = 1.297, P = 0.279) and sex (t = 2.983, P = 0.084) of the two groups (Table 1).
Characteristics of and differences in emotion regulation abilities between the HR-ASD and TD groups
Behavioral data analysis showed that, compared with the TD group, positive emotions in the HR-ASD group decreased (t = − 2.821, P = 0.009) and negative emotions increased (t = 2.809, P = 0.009), and the differences were significant. Among the behaviors related to emotion regulation, the self-comforting behavior (t = 2.290, P = 0.027) in the HR-ASD group was less than that in the TD group, the self-stimulating behavior (t = 2.629, P = 0.012), avoidance behavior (t = 2.398, P = 0.021) and crying behavior (t = 3.156, P = 0.003) were higher than those in the TD group, and the differences were significant (Table 2).
Impact of ADOS severity on emotional regulation ability of children in the HR-ASD group
The impact of ADOS severity on constructive emotion regulation strategies and help seeking represented 4.3% and 3.3%. The impact of ADOS severity on self-soothing and self-stimulating of children represented 0.1% and 3.6% (Table 3).
Effect of subcutaneous nuclear volume on emotional regulation in the HR-ASD group of children
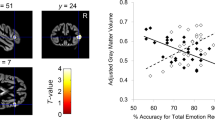
MRI scans were performed on children in both HR-ASD group and TD group. SVM method was used to establish the classification model of brain structure features (subcutaneous nuclei) in HR-ASD and TD groups. PCA and Filter methods are used as preliminary steps for classification models and linear regression that rely on MRI structural features. After feature screening, for the classification performance of a single parameter, an accuracy of 55.3%, specificity of 71.0% and an AUC of 0.622 were obtained based on subcutaneous nuclear population volume. Screening features include: left pallidum volume, left hippocampus.L volume, left amygdala volume, right hippocampus volume, right amygdala.R volume, right thalamus volume (Fig. 2, Table 4).
Linear regression analysis results showed that the volume of the right amygdala (R2 = 0.245) and the volume of the right hippocampus (R2 = 0.197) affected constructive emotion regulation strategies, and the volume of the right amygdala (R2 = 0.118) had an impact on mother-seeking, both of which were significant (Table 5).
To establish the classification and regression model of white matter characteristics in the HR-ASD group
Multi-voxel pattern analysis (MVPA) was used to estimate the voxel array based on brain images. We used SVM method to classify the white matter structure of children in high-risk ASD and TD groups. The total accuracy of FA value was 86.67%, and the prediction accuracy was 86.96%. The total precision of MD value was 88.89%, and the prediction accuracy was 91.30% (Table 6, Fig. 3). By evaluating the influence of features (FA and MD values) on classification, the ranking of contribution weight of FA values to classification was obtained. The Inferior.cerebellar.peduncle. R and Medial. lemniscus. L contributed more to classification, with weights of 7.587% and 6.907% (Supplement Table S1), respectively. According to the ranking of the MD value to the classification contribution weight, the Superior.cerebellar.peduncle.L and Cerebral.peduncle.R made a high contribution to the classification, with the weight of 9.585% and 7.136%, respectively (Supplement Table S2).
a, b To establish a SVM model of white matter characteristics (FA/MD) in HR-ASD group and TD group; c, d to establish a SVR model in of white matter characteristics (MD) and self-stimulating behavior in HR-ASD group. a Establishment of classification model in the HR-ASD group and TD group (a1/a2): probability distribution graph (FA/MD). (b1/b2): confusion matrix graph (FA/MD): confusion matrix of true and predicted values. (c1/c2): x and o represent HR-ASD group and TD group (FA/MD), respectively. (d1/d2): receiver characteristic curve (ROC). (b)The classification contribution weight (MD), A1/A2: Superior.cerebellar.peduncle.L; A3/A4: cerebral.peduncle.R; A5/A6: corticospinal.tract.L; A7/A8: superior.cerebellar.peduncle.R; A9/A10:medial.lemniscus.R; A11/A12: pontine.crossing.tract. c White matter characteristics (MD) and self-stimulating behavior: A1/A4: scatter diagram. A2/A5: prediction graph (red predicted value, black true value). A3/A6: prediction chart (red predicted value, blue target value). d Predictive feature weight: A1/A2: cerebral.peduncle.R; A3/A4: superior.cerebellar.peduncle.L; A5/A6: tapetum.R; A7/A8: inferior.cerebellar.peduncle.L; A9/A10: Superior.cerebellar.peduncle.R; A11/A12: Pontine.crossing.tract. L: left. R: right
ADOS severity was incorporated into the model as a covariate. The results are presented as follows: FA (R2 = 0.10, P < 0.01) and MD (R2 = 0.12, P < 0.01) had a certain ability to predict self-stimulating behavior (Table 7, Fig. 3). FA (R2 = 0.04, P < 0.01) and MD (R2 = 0.03, P < 0.01) showed a low predictive ability of constructive adjustment ability. FA (R2 < 0.01, P < 0.01) and MD (R2 < 0.01, P < 0.01) had higher predictive power for seeking a mother. By evaluating the influence of features (FA, MD value) on the predictive output, the ranking of MD value on the predictive feature weight of self-stimulating behavior was obtained. The brain regions with large contributions were as follows: right cerebral peduncle, left superior cerebellar peduncle, right tapetum, left inferior cerebellar peduncle, right superior cerebellar peduncle, and so forth (Supplement Table S3/S4, Fig. 3).
The classification and regression models of white matter fiber bundle connection in the HR-ASD group were built
The total accuracy of the HR-ASD group was 77.5%in the 106th link, with the highest level of 75.51%, with the upper edge of 77.5%, and the edge was 106–255 (from the 25 features, each more than 25 features, the training retained 2650 continuous), and the results were obtained by all the survival edges, the size and the important ranking of the weight, and the results of the results of the results of all the remaining sides, and the results of the results of the results of all the survival links, and eventually have 45 positive edges.
Using CPM method, the prediction model of the emotional adjustment ability of HR-ASD group based on white fiber beam connection was constructed, and the replacement test was the optimal result of 2000 iteration. Constructive strategy 20 is connected to 8 negative edges; The search for a mother is 10–24 (Table 8, Supplement Tables S5, S6, Fig. 4).
Visual diagram of fiber bundle connections associated with emotional regulation strategies in the HR-ASD group of young children. a, c, e, g Brain Graph: Built using BrainNetViewer, each node is represented as a sphere, where the sphere size represents the number of edges emanating from that node. Use CPM to build the eigenvalues and generate the edges. b, d, f, h Circular graph: Built using Circos. All brain regions are arranged in a circle, which roughly reflects the connection of two brain regions. The white matter brain network is constructed with brain regions as nodes and the number of fibers as edges. Positive and negative boundary values are shown in red and blue
Discussion
In this study, the mean square error model was adopted to encode the emotional regulation (help seeking, self-consolation and crying, etc.) of children in the HR-ASD group and the TD group by the behavioral paradigm of still face, and the emotional regulation levels of the two groups were explored. Then, we used machine learning methods to screen out important features of subcutaneous nuclear mass volume, white matter structure and white matter fiber bundle connections related to emotional regulation in HR-ASD infants.
Comparison of the characteristics of children’s emotional regulation ability between HR-ASD group and TD group
Our study found that compared with TD group, HR-ASD group children used less emotional regulation strategies, such as help-seeking and self-comforting behaviors; More self-stimulating behavior, such as repeatedly shaking the body, beating the table, and shaking the seat. Sequential analysis showed that children with autism used less social support strategies (toward the experimenter and verbally), and that the above-mentioned behavioral, vocal, and distraction strategies were all effective for TD children. In this paradigm, the children in the HR-ASD group showed less self-comforting behaviors and showed higher intensity of giving up or direct avoidance, while the children in the TD group could regulate their possible emotional responses through physical movements such as sucking the thumb, rubbing the arm, and touching the table. At the same time, children with better language development will carry out self-comforting speech behaviors through spontaneous vocal communication or deep breathing sound. Our study found that HR-ASD infants had less need to seek support from their mothers, and TD infants preferred to rely on caregivers for physical and verbal comfort. There is good evidence that children with ASD often exhibit negative emotions, including irritability and aggressive behavior, decreased positive emotions, less displays of joy, and more temper tantrums [61, 74]. Prospective and retrospective studies have shown that high-risk children who later develop autism exhibit more negative emotions than their peers, and parents observe that they are more likely to exhibit more distress and increase negative emotions [28, 30]. Our study found that children in the HR-ASD group used more distraction strategies, while children in the TD group were relatively focused. It has been found that the ability to maintain attention helps children deal with emotional distress, and the emergence of emotional recognition promotes concentration in children [20, 21].
The cognitive and behavioral capacity of young children to regulate their own arousal states are inherently limited, relying heavily on external regulation from caregivers. In instances where caregivers sensitively recognize increases in children's negative emotions, decreases in positive emotions, and the adoption of behaviors like crying and self-stimulation, those with higher sensitivity and responsiveness can significantly contribute to enhancing their young children's emotional and self-regulatory development [35]. For instance, when children employ more constructive adjustment strategies, such as seeking mothers, synchronous interactions can mitigate the impact of adverse environments on children. Early intervention and teaching children how to manage emotional impulses in their formative years are crucial. By instilling socially acceptable ways to navigate and transform emotions, children can develop the skills to balance their desires and needs with societal goals and demands [67].
Association between emotional regulation and subcutaneous nuclear mass volume in HR-ASD infants
Our research shows that SVM method was used to establish the classification model of brain structure features (subcutaneous nuclei) in the HR-ASD and TD groups. The left pallidum volume, left hippocampus volume, left amygdala volume and right hippocampus volume have great influence on the classification result. Regression model shows that the surface area of the left prefrontal cortex, the thickness of the right prefrontal cortex and the thickness of the right prefrontal basal medial cortex have effects on constructive emotion regulation in the HR-ASD group. The surface area of the prefrontal cortex and the thickness of the right medial temporal gyrus have influence on the regulatory strategies of seeking mothers.
The emotional regulation mechanisms in people with autism are complex and may be partly similar to those in the normal population (e.g., physiological arousal, levels of negative and positive influence, changes in the amygdala and prefrontal cortex) and partly inconsistent with those in the normal population (e.g., information processing, differences in perception, and cognitive aspects) [61]. Studies have reported abnormalities in the cortical limbic circuits of cognitive control (i.e., the prefrontal cortex) and emotional processing areas (i.e., limbic structures) in individuals with mood disorders [12, 22].
Neuroimaging studies have suggested changes in the function of the amygdala–prefrontal cortex circuits, but the results of studies on the above-mentioned changes are mixed [86]. Linear regression analysis showed that the volume of the left amygdala and the volume of the right hippocampus were negatively correlated with the influence of constructive emotion regulation strategies, and the volume of the amygdala was negatively correlated with the mother-seeking behavior of HR-ASD infants, which may indicate that the increase of amygdala volume decreases the ability of constructive regulation strategies of infants. For constructive regulation strategies, existing research has confirmed that constructive regulation strategies (e.g., "reappraisal") can regulate bilateral amygdala. When cognitive control is adopted to regulate emotional stimuli, amygdala activation will be weakened [8]. Although many studies have found an enlarged amygdala in children with autism, others have found no change or decrease in volume [38, 79]. The enlarged amygdala may be related not only to symptoms but also to emotional regulation in children with autism. Hazlett et al. supported the pathological overexpansion of cortical surface areas in autism dating back to the first year of life [34]. Previous research has suggested that children who develop autism have accelerated expansion of total cortical surface area, regional expansion in the occipital, temporal and frontal regions, and strong expansion in the visual cortex [34]. Studies of boys with ASD aged 2–5 years showed no difference in gray matter surface area, but increased thickness in some local cortical regions [71]. Previous studies reported that the prefrontal cortex is activated when processing vaguely expressed facial emotions [41], associated with the production of functional neurological symptoms [83]. There was a strong correlation between the volume of the amygdala and the volume of the neocortex, with input from the amygdala reaching 90% of the prefrontal area [19]. Accordingly, the surface area and thickness of the prefrontal cortex, the volume of the middle temporal gyrus, amygdala and hippocampus are clearly correlated with the emotional regulation ability of HR-ASD children.
Association of emotional regulation with white matter and white matter fiber bundle junction in HR-ASD infants
For white matter characteristics and white matter fiber bundle connections, the results of this study showed that FA and MD values were associated with self-stimulating behaviors, and the corpus callosum and cingulate gyrus had greater weight. One DTI study identified reduced FA values in specific fiber tracts, including the connections of the amygdala, fusiform area (FFA), and superior temporal sulcus, as well as the connections of the medial and lateral orbitofrontal cortex (OFC) to the uncinate tract of the anterior temporal lobe (e.g., the amygdala) [69]. One study showed that various regions of the anterior cingulate cortex are involved in cognition, emotion, and motivation [9]. The anterior cingulate cortex interacts with other cortical structures while taking on critical significance in the regulation of emotional experience [61]. The results of this study of white matter fiber tract junction showed that the connection between dorsolateral superior frontal gyrus and medial cingulate gyrus, middle frontal gyrus and medial paracingulate gyrus could predict constructive emotion regulation, the connection between dorsolateral superior frontal gyrus and supplementary motor area, and the connection between hippocampus and lingual gyrus could predict maternal seeking behavior, and also confirmed the close connection between white matter fiber tract junction and emotional regulation. Emotional experience involves associations between the ventral prefrontal cortex and the amygdala, and mood and anxiety disorders are associated with abnormalities in ventral prefrontal cortex/amygdala connectivity [27]. Neuromechanism-related studies suggest that social emotional dysfunction in autism patients is related to impaired white matter connectivity [6]. White matter FA in anterior cingulate gyrus and superior temporal gyrus was associated with repetitive restrictive behavior and adaptive function of autism, respectively [52]. One study suggesting that white matter pathology of autism may extend to near-range white matter junctions proposes that adolescents with autism exhibit lower FA values in the mid-frontal, temporal, and parietal lobes of long-range white matter throughout the brain compared to controls [77].
Studies have found differences in the temporal lobe, uncinate fiber tracts and white matter fiber tracts in the frontal area in children with autism [23], FA values in the amygdala were also significantly reduced, and the above-mentioned changes were largely associated with altered ventromedial prefrontal cortex connections [26, 62]. Conturo et al. found abnormalities in the white matter fiber tracts of the amygdala–fusiform cortex and the white matter tracts of the hippocampus—fusiform cortex in the autism group [15]. In DTI studies, the uncinate tract of autism was associated with impaired social function. The unhooked tract regulates the ventral limbic connections and is responsible for integrating complex social emotional structures, such as emotional recognition [45, 65]. Therefore, white matter fiber bundle connectivity is valuable for predicting emotional regulation and other aspects of HR-ASD children. Brothers proposed that a brain network consisting of three regions, the amygdala, orbitofrontal cortex (OFC) and superior temporal gyrus (STG), constitutes the neural basis of social intelligence, the so-called social brain [10]. Bickart et al. have shown that amygdala volume is positively correlated with the increase of network size and complexity, and that the amygdala is somewhat correlated with other social brain regions [11]. Therefore, neural mechanisms related to emotional regulation characteristics of patients with autism are necessary, especially in early childhood, to provide an empirical basis for early clinical intervention of autism.
Limitations of the study
This study had several limitations. First, due to the limitations of age, it was difficult to collect behavioral and imaging data for the subjects, and the sample size was relatively small. Follow-up of this study group is ongoing and will continue to school age to observe long-term outcomes and social development of the participants. Second, considering individual differences, we hope to increase the experimental paradigms in the future. Future studies should include data on daily parent–child interaction, emotion regulation strategies of children expressed under low-pressure conditions, and the emotion response of the parents. Focusing on multiple dimensions and utilizing multiple methods will improve our understanding of emotion regulation and improve the ecological validity of the experimental paradigm. Future studies should also focus on individual differences in children with autism to explore the characteristics of externalizing behaviors.
Conclusions
In brief, there is a significant correlation between the emotional regulation ability of HR-ASD children and multiple white matter fiber bundle connections (e.g., amygdala, hippocampal volume, and corpus callosum), which is conducive to identifying the potential processes related to abnormal emotional regulation in the brain of children with autism. By enhancing the emotional regulation ability, from the perspective of intervention and treatment, this study can lay a foundation for the follow-up study of the pathological mechanism of autism and prevent the possible accompanying emotional and behavioral problems.
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
References
Adrian M, Zeman J, Veits G (2011) Methodological implications of the affect revolution: a 35-year review of emotion regulation assessment in children. J Exp Child Psychol 110(2):171–197
Amaral DG, Schumann CM, Nordahl CW (2008) Neuroanatomy of autism. Trends Neurosci 31(3):137–145
Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, Reiss AL, Hardan AY (2014) A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuropsychopharmacol Biol Psychiatry 48(3):124–128
Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC (2000) The amygdala theory of autism. Neurosci Biobehav Rev 24(3):355–364
Berking M, Wupperman P (2012) Emotion regulation and mental health: recent findings, current challenges, and future directions. Curr Opin Psychiatry 25(2):128–134
Ameis S, Catani M (2015) Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex 62:158–181
Ball RS (1977) The gesell developmental schedules: Arnold Gesell (1880–1961). J Abnorm Child Psychol 5:233–239
Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2014) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 24(11):2981–2990
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4(6):215–222
Brothers L (1990) The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci 1:27–51
Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF (2011) Amygdala volume and social network size in humans. Nat Neurosci 14(2):163–164
Bebko G, Bertocci M, Chase H, Dwojak A (2015) Decreased amygdala-insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Res 231(1):77–86
Cole PM, Teti LO, Zahn-Waxler C (2003) Mutual emotion regulation and the stability of conduct problems between preschool and early school age. Dev Psychopathol 15(1):1–18
Catani M, Thiebaut de Schotten M (2012) Limbic pathways. In atlas of human brain connections. Oxford University Press Inc, New York
Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ (2008) Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc 14(6):933–946
Diener ML, Mangelsdorf SC (1999) Behavioral strategies for emotion regulation in toddlers: associations with maternal involvement and emotional expressions. Infant Behav Dev 22(4):1879
Dimitrova R, Pietsch M, Christiaens D, Ciarrusta J (2020) Heterogeneity in brain microstructural development following preterm birth. Cereb Cortex 30(9):4800–4810
Etkin A, Buchel C, Gross JJ (2015) The neural bases of emotion regulation. Nat Rev Neurosci 16(11):693–700
Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI (1997) Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J Comp Psychol 111(3):286–293
Feldman R (2006) From biological rhythms to social rhythms: physiological precursors of mother-infant synchrony. Dev Psychol 42(1):175–188
Feldman R (2009) The development of regulatory functions from birth to 5 years: insights from premature infants. Child Dev 80(2):544–561
Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D (2014) Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 45:202–211
Fingher N, Dinstein I, Ben-Shachar M, Haar S, Dale AM, Eyler L, Pierce K, Courchesne E (2017) Toddlers later diagnosed with autism exhibit multiple structural abnormalities in temporal corpus callosum fibers. Cortex 97:291–305
Giovagnoli G, Postorino V, Fatta LM, Sanges V, De Peppo L, Vassena L, Rose PD, Vicari S, Mazzone L (2015) Behavioral and emotional profile and parental stress in preschool children with autism spectrum disorder. Res Dev Disabil 45:411–421
Grossberg S, Kishnan D (2018) Neural dynamics of autistic repetitive behaviors and Fragile X Syndrome: basal ganglia movement gating and mglur-modulated adaptively timed learning. Front Psychol 13(9):269
Gibbard CR, Ren J, Skuse DH, Clayden JD, Clark CA (2018) Structural connectivity of the amygdala in young adults with autism spectrum disorder. Hum Brain Mapp 39(3):1270–1282
Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A (2012) Fractionation of social brain circuits in autism spectrum disorders. Brain 135:2711–2725
Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, Szatmari P (2009) Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. J Abnorm Child Psychol 37(1):59–78
Guo Y, Garfin DR, Ly A, Goldberg WA (2017) Emotion coregulation in mother-child dyads: a dynamic systems analysis of children with and without autism spectrum disorder. J Abnorm Child Psychol 45(7):1369–1383
Gomez CR, Baird S (2005) Identifying early indicators for autism in self-regulation difficulties. Focus Autism Other Dev Disabl 20(2):106–116
Gross JJ (2002) Emotion regulation: affective, cognitive, and social consequences. Psychophy siology 39(3):281–291
Gillberg C, Ehlers S, Schaumann H, Jakobsson G, Dahlgren SO, Lindblom R, Bagenholm A, Tjuus T, Blidner E (1990) Autism under age 3 years: a clinical study of 28 cases referred for autistic symptoms in infancy. J Child Psychol Psychiatry 31(6):921–934
Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J (2005) Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry 62(12):1366–1376
Hazlett HC, Gu H, Munsell BC (2017) Early brain development in infants at high risk for autism spectrum disorder. Nature 542(7641):348–351
Hastings PD, Kahle S, Fleming C, Lohr MJ, Katz LF, Oxford ML (2019) An intervention that increases parental sensitivity in families referred to child protective services also changes toddlers’ parasympathetic regulation. Dev Sci 22(1):12725
Hornberger M, Geng J, Hodges JR (2011) Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioral variant frontotemporal dementia. Brain 134(9):2502–2512
Hoshino Y, Kaneko M, Yashima Y, Kumashiro H, Volkmar FR, Cohen DJ (1987) Clinical features of autistic children with setback course in their infancy. Jpn J Psychiatry Neurol 41(2):237–245
Haar S, Berman S, Behrmann M, Dinstein I (2016) Anatomical abnormalities in autism? Cereb Cortex 26(4):1440–1452
Jahromi LB, Meek SE, Ober-Reynolds S (2012) Emotion regulation in the context of frustration in children with high functioning autism and their typical peers. J Child Psychol Psychiatry 53(12):1250–1258
Kuusikko-Gauffin S, Pollock-Wurman R, Mattila ML, Jussila K, Ebeling H, Pauls D, Moilanen I (2013) Social anxiety in parents of high-functioning children with autism and Asperger syndrome. J Autism Dev Disord 43(3):521–529
Kozlowska K, Griffiths KR, Foster SL, Linton J, Williams LM, Korgaonkar MS (2017) Grey matter abnormalities in children and adolescents with functional neurological symptom disorder. Neuroimage Clin 15(6):306–314
Konstantareas MM, Stewart K (2006) Affect regulation and temperament in children with Autism Spectrum Disorder. J Autism Dev Disord 36(2):143–154
Kleinman JM, Robins DL, Ventola PE, Pandey J, Boorstein HC, Esser EL et al (2008) The modified checklist for autism in toddlers: a follow-up study investigating the early detection of autism spectrum disorders. J Autism Dev Disord 38:827–839
Keunen K, Benders MJ, Leemans A, Stam PC, Scholtens LH, Viergever MA (2017) White matter maturation in the neonatal brain is predictive of school age cognitive capacities in children born very preterm. Dev Med Child Neurol 59(9):939–946
Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Janisse J, Chugani HT, Chugani DC (2010) Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb Cortex 20(9):2103–2113
Losh M, Capps L (2006) Understanding of emotional experience in autism: insights from the personal accounts of high-functioning children with autism. Dev Psychol 42(5):809–818
Lipsky D, Richards W, Lipsky D, Richards W (2010) Managing meltdowns: using the scared calming technique with children and adults with autism. J Autism Dev Disord 40(7):916–917
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392(10146):508–520
Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E (1989) Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 19(2):185–212
Lord C, Charman T, Havdahl A, Carbone P, Anagnostou E (2021) The lancet commission on the future of care and clinical research in autism. Lancet 6(21):1541–1545
Lord C, Rutter M, Le Couteur A (1994) Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Autism Dev Disord 24(5):659–685
Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, Lainhart JE (2010) Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res 3(6):350–358
Mulraney M, Sciberras E, Gulenc A, Efron D, Hazell P, Silk TJ (2021) Neural correlates of irritability in a community sample of children. J Affect Disord 292(1):223–226
Macari S, DiNicola L, Kane-Grade F, Prince E, Vernetti A, Powell K (2018) Emotional expressivity in toddlers with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 57(11):828–836
Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, White SW (2013) The role of emotion regulation in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 52(7):679–688
Matson JL, Cervantes PE (2014) Commonly studied comorbid psychopathologies among persons with autism spectrum disorder. Res Dev Disabil 35(5):952–962
McKechanie AG, Lawrie SM, Whalley HC, Stanfield AC (2022) A functional MRI facial emotion-processing study of autism in individuals with special educational needs. Psychiatry Res Neuroimaging 320(5):111426
Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S (2010) Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: a community- and clinic-based study. J Autism Dev Disord 40(9):1080–1093
Mazefsky CA, White SW (2014) Emotion regulation: concepts and practice in autism spectrum disorder. Child Adolesc Psychiatr Clin N Am 23(1):15–24
Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, Amaral DG (2012) Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry 69(1):53–61
Noldus L (1991) The observer: a software system for collection and analysis of observational data. Behav Res Methods Instrum Comput 23(3):415–429
Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y (2010) Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res 1362(29):141–149
Nair A, Treiber JM, Shukla DK, Shih P, Müller RA (2013) Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 136(6):1942–1955
Osterling J, Dawson G (1994) Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord 24(3):247–257
Poustka L, Jennen-Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B (2012) Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry 13(4):269–280
Pote I, Wang S, Sethna V, Blasi A, Daly E, BASIS Team (2019) Familial risk of autism alters subcortical and cerebellar brain anatomy in infants and predicts the emergence of repetitive behaviors in early childhood. Autism Res 12(4):614–627
Rieffe C, Meerum Terwogt M, Kotronopoulou K (2007) Awareness of single and multiple emotions in high-functioning children with autism. J Autism Dev Disord 37(3):455–465
Richey JA, Damiano CR, Sabatino A, Rittenberg A, Petty C, Bizzell J, Voyvodic J, Heller AS, Coffman MC, Smoski M, Davidson RJ, Dichter GS (2015) Neural mechanisms of emotion regulation in autism spectrum disorder. J Autism Dev Disord 45(11):3409–3423
Radua J, Via E, Catani M, Mataix-Cols D (2011) Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med 41(7):1539–1550
Raznahan A, Lenroot R, Thurm A, Gozzi M, Hanley A, Spence SJ, Swedo SE, Giedd JN (2012) Mapping cortical anatomy in preschool aged children with autism using surface-based morphometry. Neuroimage Clin 31(2):111–119
Sofronoff K, Attwood T, Hinton S, Levin I (2007) A randomized controlled trial of a cognitive behavioral intervention for anger management in children diagnosed with Asperger syndrome. J Autism Dev Disord 37(7):1203–1214
Shen MD, Nordahl CW, Young GS (2013) Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 136(9):2825–2835
Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG (2004) The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci 24(28):6392–6401
Simonoff E, Jones CR, Pickles A, Happé F, Baird G, Charman T (2012) Severe mood problems in adolescents with autism spectrum disorder. J Child Psychol Psychiatry 53(11):1157–1166
Swanson MR, Shen MD, Wolff JJ, Elison JT, Emerson RW, Styner MA, Hazlett HC, IBIS Network (2017) Subcortical brain and behavior phenotypes differentiate infants with autism versus language delay. Biol Psychiatry Cogn Neurosci Neuroimaging. 2(8):664–672
Schopler E, Reichler RJ, DeVellis RF, Daly K (1980) Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord 10(1):91–103
Shukla DK, Keehn B, Müller RA (2011) Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry 52(3):286–295
Schumann CM, Barnes CC, Lord C, Courchesne E (2009) Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry 66(10):942–949
Totsika V, Hastings RP, Emerson E, Lancaster GA, Berridge DM (2011) A population-based investigation of behavioural and emotional problems and maternal mental health: associations with autism spectrum disorder and intellectual disability. J Child Psychol Psychiatry 52(1):91–99
Tarver J, Palmer M, Webb S, Scott S, Slonims V, Simonoff E, Charman T (2019) Child and parent outcomes following parent interventions for child emotional and behavioral problems in autism spectrum disorders: a systematic review and meta-analysis. Autism 23(7):1630–1644
Thompson RA (2019) Emotion dysregulation: a theme in search of definition. Dev Psychopathol 31(3):805–815
Valentovich V, Goldberg WA, Garfin DR, Guo Y (2018) Emotion coregulation processes between mothers and their children with and without autism spectrum disorder: associations with children’s maladaptive behaviors. J Autism Dev Disord 48(4):1235–1248
Vuilleumier P (2014) Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin 44(4):323–337
White SW, Oswald D, Ollendick T, Scahill L (2009) Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev 29(3):216–229
Wiggins JL, Brotman MA, Adleman NE, Kim P, Oakes AH, Reynolds RC, Chen G, Pine DS, Leibenluft E (2016) Neural correlates of irritability in disruptive mood dysregulation and bipolar disorders. Am J Psychiatry 173(7):722–730
Young KS, Sandman CF, Craske MG (2019) Positive and negative emotion regulation in adolescence: links to anxiety and depression. Brain Sci 29(4):76
Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P (2005) Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci 23(2):143–152
Acknowledgements
The authors gratefully acknowledge the participants in this study. This study is based on the first author’s doctoral study project.
Funding
This study was supported by the National Key Research and Development Program of China (2016YFC1306200), the National Natural Science Foundation of China (81771478), the Project of Nanjing Rehabilitation Medical Center in JiangSu Province, China (2020), and the “Special Disease Cohort” Research Project of Nanjing Medical University (NMUC2018010A).
Author information
Authors and Affiliations
Contributions
XK and ND designed the study and performed the experiments; LF, LQ and BS also performed the experiments; ND analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
None.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, N., Fu, L., Qian, L. et al. The correlation between brain structure characteristics and emotion regulation ability in children at high risk of autism spectrum disorder. Eur Child Adolesc Psychiatry (2024). https://doi.org/10.1007/s00787-024-02369-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00787-024-02369-y