Abstract
Children with chromosome 22q11.2 deletion syndrome (22q11.2DS) exhibit impaired ability to process and understand emotions in others. We measured structural connectivity in children and adolescents with 22q11.2DS (n = 28) and healthy controls (n = 29). Compared to controls, those with 22q11.2DS had poorer social skills and more difficulty recognizing facial emotions. Children with 22q11.2DS also had higher fractional anisotropic diffusion in right amygdala to fusiform gyrus white matter pathways. Right amygdala to fusiform gyrus fractional anisotropy values partially mediated the relationship between 22q11.2DS and social skills, as well as the relationship between 22q11.2DS and emotion recognition accuracy. These findings provide insight into the neural origins of social skills deficits seen in 22q11.2DS and may serve as a biomarker for risk of future psychiatric problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background
Chromosome 22q11.2 deletion syndrome (22q11.2DS), also known as velocardiofacial or DiGeorge syndrome, is a neurodevelopmental disorder that arises from a 1.5 to 3 megabase microdeletion on the long arm (q) of chromosome 22. Prevalence rates range from 1:2000 to 1:4000 live births (Howley et al., 2012; Weinberger et al., 2016). 22q11.2DS is associated with a high incidence of psychiatric and behavioral disorders, including depression, anxiety disorders, and attention deficit hyperactivity disorder (ADHD). Mortality rates for individuals with 22q11.2DS are higher than seen in typically developing (TD) populations. In a longitudinal sample of 1421 individuals from infancy to adulthood diagnosed with 22q11.2DS, Campbell et al. (2018) reported an overall mortality rate of 4%, with a median age of death of 5 months. Another study consisting entirely of adult participants found that individuals diagnosed 22q11.2DS with and without major congenital heart disease had a 72% and 95% probability of survival to age 45 years, respectively (Van et al., 2019).
Impaired social competence, such as difficulty forming and maintaining relationships, extremes of shyness or disinhibition, and mood instability are commonly reported in children and adolescents with 22q11.2DS (Fuerst et al., 1995; Woodin et al., 2001). To better understand the origin of social impairment, researchers have used theory of mind (ToM) tasks. However, such research has not been conclusive. While some authors point to a general ToM deficit as a core aspect of the social dysfunction in 22q11.2DS, others posit a delay in ToM development (Campbell et al., 2009, 2010; Niklasson et al., 2001). Nonetheless, difficulty interpreting and understanding the intentions of others is a common symptom of 22q11.2 deletion syndrome. This negatively impacts developing and maintaining social relationships as a child ages as the social environment becomes more complex (Angkustsiri et al., 2014; Loveland et al., 2001).
Problems with facets of social cognition, such as emotion processing, in children with 22q11.2DS are also reported in patients with schizophrenia. This commonality is of particular interest, as a 22q11.2 deletion confers a 30-fold increased risk for developing schizophrenia, compared to the general population (Bassett et al., 2014; Karayiorgou et al., 2010). Marked impairments in ToM and emotion processing are consistently noted in both first episode and chronic schizophrenia. Poorer or declining ability to process emotions of others is a likely behavioral marker of psychosis vulnerability (Jalbrzikowski et al., 2012; Penn et al., 2008). Moreover, poor social functioning in 22q11.2DS is associated with the presence of psychotic symptoms in these children (Baker & Skuse, 2005). Similar to patients with schizophrenia (Gur et al., 2002), children with 22q11.2DS do not recognize neutral or negative facial emotions, such as disgust, anger, and fear, as well as typically developing controls. There was no difference in recognition accuracy of positive emotions compared to the control group (Campbell et al., 2010).
Neural Correlates of Emotion Processing
Rapidly and accurately understanding emotions of others requires coordinated effort of brain regions involved in perceptual processing of facial features and interpreting the emotional significance of a stimulus. One such region, the fusiform face area (FFA) is located in the fusiform gyrus in the inferior temporal cortex. Kanwisher et al. (1997) noted that the FFA selectively responds more strongly to faces than to other objects. The FFA plays a crucial role in recognizing and discriminating between faces and displays stronger activation when faces convey emotion (Albohn & Adams, 2016; Tong et al., 2000; Vuilleumier & Pourtois, 2007). This increased activation to emotional faces has been attributed to the dense connections that the FFA has with the amygdala (Phillips et al., 2003).
The amygdala has long been implicated in affective learning, particularly in the context of Pavlovian fear conditioning (Critchley et al., 2002; LaBar et al., 1998). The amygdala receives and projects information from cortical and subcortical regions and is involved in the detection and evaluation of salient stimuli (Fossati, 2012). Evidence of this role includes amygdalar connections to the orbital lateral and medial PFC, regions that contribute to the processing of stimuli value and relevance (Miller & Cohen, 2001). The orbitofrontal cortex (OFC) is reciprocally connected to the amygdala and aids in the distinction between rewarding and punishing stimuli (Baxter et al., 2000). The ability to rapidly shift attention and potentiate responses to changes in the environment evolved as it is crucial for survival but also for navigating social interactions and the development of social skills. Stimulus–response associations are the basis of learned behavioral and emotional responses, making the OFC fundamental to social cognition (Buka et al., 2001). Liang et al. (2009) found that connectivity patterns between the OFC and amygdala differed as a function of varying emotional expression in adults looking at images of emotionally-charged faces. Particularly, metrics of functional connectivity from the right lateral OFC to amygdala varied as a function of facial emotion expressions that convey a message of approach (i.e., happy, neutral) versus threat (i.e., angry, fearful) to the receiver.
White matter microstructure anomalies of social and emotion processing regions have been reported in individuals with 22q11.2DS. Diffusion tensor imaging (DTI) metrics of white matter integrity include fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD). Studies of individuals with 22q11.2DS report overall decreased AD in the inferior fronto-occipital fasciculus (IFOF) and furthermore, note that this reduced diffusivity is linked to the presence of positive schizophrenia symptoms in this population (Jalbrzikowski et al., 2013; Kikinis et al., 2012; Radoeva et al., 2012). The IFO is the longest association tract in the brain, connecting multiple regions that are implicated in social cognition. Damage to the IFOF has been linked to poor emotion recognition in images of faces (Philippi et al., 2009). Additionally, significantly reduced AD in the superior longitudinal fasciculus, an association tract that plays a role in the understanding of emotion and overall emotional intelligence, has been reported in people with 22q11.2DS (Radoeva et al., 2012). A more recent study by Olszewski et al. (2017) examining the social brain of individuals with 22q11.2DS found evidence of white matter disruption in many tracts, such as increased FA within the left IFOF and right cingulum bundle and decreased RD within the left IFOF, right cingulum bundle, right thalamo-frontal tract, and right inferior longitudinal fasciculus compared to TD controls. Taken together, deficient white matter connectivity may play a role in reduced emotional awareness.
The Current Study
The aim of the current study was to examine structural connectivity patterns between brain regions involved in emotion processing, as well as to investigate how these patterns are associated with social and emotion processing skills in children with 22q11.2DS compared to typically developing controls. Andersson et al. (2008) found that when repeatedly presented with fearful faces, TD children exhibited reduced activation of the right amygdala over time. However, children with 22q11.2DS did not display this pattern of amygdalar suppression, suggesting that they do not adapt to fearful faces as well as their TD counterparts. Additionally, Campbell et al. (2010) reported that children with 22q11.2DS did not identify negative facial emotions (e.g., anger, fear) as well as TD controls. Thus, we first hypothesized that children with 22q11.2DS would demonstrate poorer performance on an emotion recognition task compared to TD controls when the distractor face portrayed fear and when the target face portrayed anger or fear. Second, we hypothesized that parents of children with 22q11.2DS would report lower social skills in their children compared to parents of TD children. Third, we predicted that there would be significant differences in DTI measures (i.e., FA, RD, AD, and MD) between the two groups in temporo-amygdala-orbitofrontal pathways associated with emotion processing. These pathways include the amygdala to fusiform (Amy-FG), amygdala to lateral orbitofrontal cortex (Amy-lOFC), and lateral orbitofrontal cortex to fusiform (lOFC-FG). Fourth, we hypothesized that FA in these pathways would be associated with emotion recognition accuracy and parent-reported social skills. Finally, we predicted that variations in FA would mediate the expected association of 22q11.2DS and measures of emotion recognition and social skills.
Materials and Methods
Participants
Participants were 57 children aged 7–14 years with 22q11.2DS (n = 28; 12 females) and typically developing controls (n = 29; 12 females) and their parents. Participants were excluded if they had a head injury, central nervous system infection, or other focal neurologic abnormality. Exclusion criteria limited to typically developing participants included parent reports of a known genetic disorder, a head injury, a learning disorder and behavioral or other known psychiatric disorders.
Procedure
As part of a larger ongoing study, families were recruited via national and state 22q11.2DS support groups, social media, fliers posted around the greater New Orleans area, and word-of-mouth. Upon arrival, families were briefed on all tasks and procedures to be conducted and gave informed consent, while children gave informed assent. The visit consisted of parents and children filling out questionnaires, completing computer-based tasks, and undergoing a series of MRI scans. All children completed the Wechsler Intelligence Scale for Children IV (WISC-IV). See Table 1 for more demographic information.
Measures
Behavioral Assessment System for Children Parent Rating Scale, Second Edition (BASC-2 PRS)
The BASC-2 PRS (Reynolds & Kamphaus, 2015) is a multidimensional parent-report questionnaire used to assess the adaptive and problem behaviors of children and young adults aged 2–25 years. The PRS is between 134 and 160 items and has a four-choice response format (i.e., Never, Sometimes, Often, and Almost Always). The test yields four domain score (externalizing problems, internalizing problems, behavioral symptoms index, and adaptive skills), as well as 14 subscales (e.g., anxiety, depression, atypicality, social skills, functional communication, etc.). T-scores of the social skills index were used as a measure of social cognition.
Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV)
The WISC-IV (Wechsler, 2003) is a cognitive ability assessment of verbal comprehension, perceptual reasoning, working memory, and processing speed. The WISC-IV Full Scale Intelligence Quotient (FSIQ) was used to assess general intellectual functioning in this study.
Face in the Cloud Task
High-resolution images of children ages 10–17 years were pulled from the NIMH Child Emotional Faces Picture Set (NIMH-ChEFS) and presented on a 27-in touchscreen monitor (Planar® Helium™) using E-prime Software (Psychology Software Tools, Inc., Pittsburgh, PA; (Schneider et al., 2002). Facial emotion stimuli included three emotional states (afraid, angry, happy, and neutral), and images were cropped to exclude hair and clothing. Facial stimuli were randomly selected from each emotion category; 48 happy faces, 39 angry faces, 38 afraid, and 32 neutral faces were available for selection.
Facial emotion stimuli (6.2 cm × 8.0 cm) were presented randomly, totaling in 32 images presented at once against a black background. Trials incorporated one stimulus displaying a target emotion against 31 stimuli exhibiting a distractor emotion. Emotion conditions included the following: happy amongst angry, happy amongst afraid, happy amongst neutral, angry amongst happy, angry amongst afraid, angry amongst neutral, afraid amongst happy, afraid amongst angry, and afraid amongst neutral. Participants were instructed to search for the face with the different emotion and tap on the screen where it resided. Twenty-four trials proceeded in the following event order (Fig. 1): target (2500 ms), fixation (1000 ms), and facial emotion stimuli (open ended).
Imaging Procedures
MRI Image Acquisition and Data Analysis
Structural data was collected using a 3.0 T Siemens Verio whole-body scanner (Siemens Medical Solutions, Erlangen, Germany) and an eight channel head coil at the Touro Imaging Center. Foam pads were added to reduce head movement, and participants wore headphones to limit scanner noise. Using the following parameters, a T1-weighted flash gradient-echo whole-brain anatomical image was acquired: 176 sagittal slices, 1.00 mm thick, field of view (FOV) = 100 mm, TR/TE = 1900/2.48 ms, 9° flip angle, within a 256 × 256 matrix.
Diffusion-weighted imaging acquisition was collected using the following SE-echo-planar imaging (EPI) sequence: 60 axial slices, 30 diffusion gradient directions, 3 mm thick, FOV = 100 mm, TR/TE = 5100/95 ms, 90° flip angle, 128 × 128 mm in-plane resolution, 2 acquisitions without diffusion weighting b0, b = 1200 s/mm2. Because we are concerned with obtaining detailed information for region-of-interest (ROI) analysis, we pursued high-resolution images 3D normalized to the brain atlas for anatomical reference (Talairach & Tournoux, 1988). Some participants were unable to complete their scanning session due to excessive movement and/or anxiety. Additionally, other were excluded due to motion-related artifacts that were too extreme for correction procedures. In total, 5 individuals with 22q11.2DS and 6 TD controls were removed from our final DTI analyses.
Preprocessing and Processing
BrainVoyager T1-weighted structural images were preprocessed using BrainVoyager 20.4 / BVQX 3.4.0 (Goebel et al., 2006) on a Lenovo computer with Ubuntu Linux (v14.04.1; 15.6 GB; 8-core Intel Xeon processor; 8 × 1.80 GHz; Gallium 0.4 on NVC3) Operating System. Raw images from the scanner were transformed to DICOM format and adjusted for contrast and brightness. Once images were iso-voxeled and corrected for inhomogeneity, we segregated the head from the brain tissue and manually cleaned all remaining meninges. Brains were aligned along a plane demarcated by the anterior and posterior commissures. Data was converted from DICOM to NIfTI (Neuroimaging Informatics Technology Initiative) format and inspected to ensure images did not change or flip orientation following conversion.
Freesurfer Manually skull-stripped images from BrainVoyager were converted to NIfTI format and run through FreeSurfer (v6.0). We utilized the ‘recon-all’ script in three segments (autorecon 1, 2, and 3) to reduce the processing time for brain reconstruction and segmentation. With the exception of motion correction, intensity normalization, and skull stripping, we utilized the following automated methods included within the process: cortical and subcortical structure segmentation, tessellation of the white and gray matter boundary, and topology correction. Segmentation and neuroanatomical labels were inspected for accuracy following processing, and errors were manually corrected prior to re-running segments of the ‘recon-all’ script. For a more detailed description, Fischl et al. (2002) provide an explanation of the automated segmentation algorithm. Whole brain tissue segmentation was completed by FreeSurfer, and the brain was reconstructed to the Desikan-Killiany and Destrieux anatomical atlases (Desikan et al., 2006).
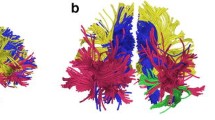
FSL Diffusion tensor imaging data was processed using FSL (v5.8.0) software (https://fsl.fmrib.ox.ac.uk/fsl/). Raw DICOM images were converted to NIfTI format using MRICron (v2.1.48–0) image viewer. Images were corrected for movement and eddy-current distortion using FSL’s Eddy Correct tool prior to registering the diffusion model to each voxel. Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques (BEDPOSTX) was conducted to allow each voxel to determine any number of crossing fibers. Anatomical T1 images were then aligned with diffusion data, followed by probabilistic tractography using tissue mask parcellations. Seed masks for left and right hemisphere amygdala, fusiform, and lateral orbitofrontal cortex were used to estimate tractography parameters between regions (see Fig. 2).
Statistical Analysis
Prior to analysis, the variables: BASC2 social skills subscale score, FSIQ, Face in the Cloud Task accuracy, face in the cloud reaction time, and structural connectivity values of associated emotion processing pathways were screened for missing data, normality of distribution, univariate outliers, and multivariate outliers. For variables that did not meet the assumption of normality, we applied a log transformation. Multicollinearity was evaluated by the variance inflation factors (VIF) for each model. The VIF of all variables in each model was < 2, suggesting that multicollinearity was not a point of concern. In the current study, bivariate correlations and means for variables of interest were examined using R version 4.0.3 (R Development Core Team, 2020), and figures were produced using the package ggplot2 (Wickham, 2009). Independent samples t-tests were used to test for group differences in FSIQ and parent-reported social skills. Emotion recognition accuracy between groups, controlling for age, sex, and reaction time, was examined using multiple regression with a Bonferroni correction for multiple comparisons (p < 0.007). Multivariate analyses of variance (MANOVAs) and when appropriate, follow-up analyses of variance (ANOVAs) were conducted to examine how DTI metrics varied by group. Age and sex were entered into each model as covariates. We decided not to use IQ as a covariate in the models due to the central role of cognitive impairments in the 22q11.2DS clinical profile. Due to this, the effect of lower FSIQ can be difficult to separate from other clinical and neural factors of 22q11.2DS (Dennis et al., 2009; Padula et al., 2015). Mediation analyses were conducted using the ‘psych’ package using the function ‘mediate’ (Revelle, 2019) in R version 4.0.3 (R Development Core Team, 2020). To test for the specificity of structural connectivity effects for social impairments and emotion recognition accuracy, we used the ‘ROCR’ (Sing et al., 2005) and ‘cutpointr’ (Thiele & Hirschfeld, 2020) packages in R.
Results
To characterize general intellectual functioning and test the hypothesis that as a group, children with 22q11.2DS would have lower parent-reported social skills, a series of independent samples t-tests were conducted. Parents of children with 22q11.2DS reported significantly lower levels of social skills in their children compared to what was reported by parents of TD children. Additionally, children with 22q11.2DS scored significantly lower in the WISC-IV Full Scale Intelligence Quotient (FSIQ) than their TD counterparts (see Table 1).
To test the hypothesis that children with 22q11.2DS would demonstrate poorer performance on an emotion recognition task compared to TD controls when the distractor face portrayed fear and when the target face portrayed fear or anger, we conducted a series of multiple regression analyses. Age, sex, and emotion recognition task reaction time were included as covariates in each model. To correct for multiple comparisons, a Bonferroni correction was applied for the seven tests (p < 0.007). During trials where the target emotion was angry or happy regardless of the distractor emotions, children with 22q11.2DS scored significantly lower than TD children. There was no difference in accuracy between groups when the target emotion was afraid. During trials where the distractor emotion was afraid, children with 22q11.2DS scored significantly lower than TD children. There were no differences in accuracy between groups when the distractor emotion was angry or happy. Children with 22q11.2DS scored significantly lower than TD children on overall emotion face target identification accuracy. There were no differences in reaction times between the groups (see Table 2).
A series of MANOVA tests were conducted to test the hypothesis that children with 22q11.2DS will have reduced white matter integrity, as measured by FA, AD, MD, RD, and pathway volume, in regions associated with emotion processing compared to typically TD children. Age and sex were entered into this analysis as covariates to ensure that any observed differences of group on DTI measures were independent of these variables. Three pathways were examined for left and right hemispheres separately and are as follows: Amy-FG, Amy-lOFC, and lOFC-FG. Diagnosis was entered as a fixed factor, while the three emotion processing pathways were entered simultaneously as dependent variables.
There was a significant effect of diagnosis on right hemisphere emotion processing pathway FA values, F (3, 27) = 3.02, p = 0.04, partial η2 = 0.25. Thus, a series of follow-up ANOVAs were conducted for each dependent variable. Box’s M was not significant (p = 0.47), indicating that there were no significant differences between the covariance matrices. Therefore, Wilk’s Lambda was used. Results indicated that there was a significant effect of diagnosis on right Amy-FG FA values, where children with 22q11.2DS had higher right Amy-FG FA values than TD controls. There were no other differences in right hemisphere FA values by diagnosis. There were no other significant differences by diagnosis for remaining FA, AD, MD, RD, or white matter pathway volumes for either hemisphere (See Table 3). To investigate if there was an association between DTI measures in control regions and emotion recognition accuracy and social skills, partial correlations controlling for age and sex were conducted. Control regions included the left and right superior frontal and superior parietal lobes, as they were not hypothesized to be associated with emotion processing or social skills. There were no significant relationships between left or right superior frontal-superior parietal FA, AD, MD, or RD values and emotion recognition accuracy scores or social skills (Table 4).
To investigate if variations in white matter integrity explain group differences in overall emotion recognition accuracy and social skills, two mediation analyses were conducted. Right Amy-FG FA values were included as the mediating variable, as this was the only measure of pathway integrity that differed between groups. The first model examined the mediating role of right Amy-FG pathway FA values in the relationship between diagnostic group and overall emotion recognition accuracy. Overall accuracy of the Face in the Cloud task was used as the observed variable Emotion Recognition. Age and sex were entered as covariates into the model.
The total effect of group on emotion recognition accuracy, controlling for age, sex, and task reaction time, was significant (β = − 0.48, p < 0.001). The direct effect of group on emotion recognition accuracy was not significant when controlling for right Amy-FG FA and age (β = − 0.26, p = 0.051), indicating that there was mediation. The indirect effect was tested using a percentile bootstrap estimation approach with 1000 samples. Results indicated that the indirect coefficient was significantly different from zero (β = − 0.22, boot = − 0.24, 95% CI − 0.70, − 0.02), suggesting partial mediation (see Fig. 3).
The second model examined the mediating role of right Amy-FG pathway FA values in the relationship between diagnostic group and parent-reported social skills. The BASC-2 PRS Social Skills T-score was used as the observed variable of Social Skills. Age was not entered as a covariate in the final model, as the BASC-2 PRS accounts for the child’s age when calculating the final T-scores. The total effect of group on social skills, controlling for sex, was significant (β = − 0.59, p < 0.001). The direct effect of group on social skills remained significant when controlling for right Amy-FG FA (β = − 0.37, p = 0.003), indicating that there was not complete mediation. The indirect effect was tested using a percentile bootstrap estimation approach with 1000 samples. Results indicated that the indirect coefficient was significantly different from zero (β = − 0.22, boot = − 0.24, 95% CI − 0.65, − 0.02), suggesting partial mediation (see Fig. 4).
To test the specificity of structural connectivity effects for social impairments and emotion recognition, we first determined the optimal cut-point for both of these outcome variables based on group using the Youden-Index method (see Table 5). As shown in Fig. 5A, the Receiver Operating Characteristic (ROC) curve suggests that right Amy-FG FA effects for emotion recognition accuracy had a true positive rate of > 80%, with an area under the curve (AUC) value of 0.90. Figure 5B displays the ROC curve for the right Amy-FG FA effects for social impairments. Again, the true positive rate is > 80%, while the AUC was 0.81. Taken together, results suggest good specificity and model predictive performance.
Discussion
Emotion Recognition, Social Skills, and 22q11.2DS
The aim of this study was to examine associations among emotional face processing and structural connectivity in children with 22q11.2DS because people with 22q11.2DS often have difficulty navigating social interactions. Consistent with our first hypothesis, parents of children with 22q11.2DS reported that their child had poorer social skills than the TD children in the control group. These findings replicate prior studies describing impaired social ability and lower FSIQ scores in children and adolescents with 22q11.2DS versus typically developing peers (Fuerst et al., 1995; Moss et al., 1999; Woodin et al., 2001). The neuropsychological profile of children and adolescents with 22q11.2DS is markedly varied. While gross intelligence scores range from moderately deficient to average, verbal IQ scores are significantly higher than performance IQ scores (Simon et al., 2002; Sobin et al., 2005; Swillen et al., 1997; Woodin et al., 2001). However, poor social competence remains a consistent hallmark of the syndrome (Campbell et al., 2009, 2010; Niklasson et al., 2001).
In accordance with hypothesis one, overall emotion recognition accuracy was significantly impaired in children with 22q11.2DS compared to TD children. Specific deficits were noted in recognizing angry and happy faces, as well as discrepancies in accuractly identifying other emotions when the distractor faces displayed fear. The origin of such perceptual impairments may be related to difficulties with featural and configural face processing (Mondloch et al., 2002). In typically developing individuals, neural systems such as fronto-temporal and occipital networks interpret facial cues in the context of social cognition. In 22q11.2DS, studies have noted that these regions are notably affected. Functional hyper- and hypoconnectivity has been observed in fronto-occipital, fronto-temporal, and limbic connections compared to control groups. These anomalies are thought to contribute to imapired social-emotional perception and are associated with positive symptoms of psychosis (Andersson et al., 2008; Campbell et al., 2011; Mattiaccio et al., 2018; Ottet et al., 2013).
The ability to recognize emotions emerges early in development, though adult-like proficiency is typically not achieved until age 10 years. While some emotions are identified by young children with accuracy comparable to adults, the capacity to discriminate between other more complex emotions does not emerge until late childhood. For example, happiness and sadness are easily recognized by age 6 years, while correctly labeling expressions of fear or digust proves more difficult until the child reaches 8 to 10 years of age (Boyatizis et al., 1993; Durand et al., 2007). Patterns for anger, however, are more varied. Thomas et al. (2007) found that young children performed significantly worse at correctly identifying angry facial expressions compared to older children and adolescents. They attributed this to the maturation of the PFC and amygdala, which continues through adolescence. Overall, a child’s ability to correctly identify specific emotions follows a general pattern, with increasing aptitude as the child ages.
The developmental pattern of emotion recognition in children with 22q11.2DS is less clear. Difficulties in making specific age-related conclusions are likely due to a number of social, perceptual, and communication problems commonly seen in this population. Campbell et al. (2010) found that these children displayed atypical visual scan path patterns, with more time spent focusing on the mouth and less time fixating on the eyes. Others have suggested that difficulties in recognizing facial expressions are related to emotional withdrawal, limiting their ability to effectively processes the emotional valence of faces (Schneider et al., 2012). Regarding specific emotions, findings suggest that children and adolescents with 22q11.2DS display poor recognition for faces displaying anger, disgust, and fear compared to TD controls (Campbell et al., 2010; McCabe et al., 2011, 2013). These perceptual differences have been attributed to poorer affective memory, attentional deficits, and differential brain structure and/or function compared to TD children (Andersson et al., 2008; Haxyby et al., 2002; Leleu et al., 2016; Norkett et al., 2017; Van Amelsvoort et al., 2006). In the present study, there were group performance differences only for faces expressing anger and happiness. Of note, children with 22q11.2DS had more difficulty recognizing and identifying target expressions in the task when the surrounding faces exhibited fear. As previously noted, children with 22q11.2DS have increased rates of anxiety disorders compared to their TD peers. Given the impact that anxiety has on attentional control, particularly in the context of bias to threatening stimuli, it is possible that children with 22q11.2DS displayed more vigilance toward faces displaying fear (Popa et al., 2019; Roy et al., 2008; Shapiro et al., 2014). This attentional bias would likely impede cognitive control, resulting in a compromised ability to accurately classify surounding emotions.
Altered Structural Connectivity and Social–Emotional Impairments
Another potential source of impaired emotion recognition is neurological abnormality. We found children with 22q11.2DS had significantly higher FA in right hemisphere Amy-FG white matter pathways. Additionally, our results indicate that the relationship between a 22q11.2DS diagnosis and emotion recognition, as well as the deletion’s relationship with impaired social skills, was partially mediated by this increase in FA. To briefly review, anisotropy is directly related to volume and orientation of neuronal fibers both parallel and perpendicular to the tract of interest (Stigler & McDougle, 2013; Thaler, 2018). Higher levels of anisotropy are typically associated with greater organization, yet methodological advancements suggest that this is a simplification. Rather, many white matter pathways run parallel to and overalap other tracts. Thus, increases in FA in one tract might be the result of higher anisotropy in overlapping pathways (Beaulieu, 2002; Rice et al., 1993). Aside from intravoxel fiber crossing, other parsimonious explanations for higher FA in abnormal brains are decreased axon diameter and reduced branching. Diffusion abnormalities characterized by increased FA have been reported in patients with schizophrenia. For example, Cheung et al. (2011) and Szeszko et al. (2008) found that drug naïve patients with recent onset schizophrenia showed a positive association between positive symptoms and FA in the ILF and fronto-occipital fasciculus, two association pathways involved in facial emotion processing. As Hoeft et al. (2007) stated, “more is not always better.”
Our findings of higher FA in right amygdala to fusiform gyrus pathways in children and adolecents with chromsome 22q11.2DS in relation to impaired social skills and emotion recognition point to atypical strucutral connectivity as a possible contributor to poorer socioemotional function. There are strong reciprocal projects between the amygdala and fusiform gyrus, and coupling between these two regions increases when viewing emotional faces (Fairhall & Ishai, 2007). While we are not able to determine the specific origins of increased FA in this pathway in children with 22q11.2DS, it may be a result of reduced branching or increased axonal packing. Others have similarly noted increased FA in relation to poor cognitive functioning in both healthy children and those with Williams syndrome (Hoeft et al., 2007; Tuch et al., 2005). In children with ADHD, Peterson and colleagues (2011) found increased FA in pathways associated with visual processing as a function of increasing ADHD symptom severity.
This study was not without limitations. First, this study includes a cross-sectional sample of an ongoing longitudinal study. This prevents us from analyzing within-subject differences in structural connectivity of regions associated with emotion processing over time. Second, we did not examine other brain regions that were not hypothesized to be affected by a 22q11.2DS diagnosis. This would be a particular concern if all pathways examined showed an effect on emotion processing accuracy scores and reported social skills. However, we saw evidence for specificity, as only right hemisphere Amy-FG FA was associated with 22q11.2DS and negatively associated with emotion processing task accuracy and social skills. Therefore, we argue that the inclusion of additional control regions was not vital to our conclusions, but future work should incorporate additional pathways to address this potential limitation. We do, however, contend that these results should be interpreted with the knowledge that other regions are associated with emotion processing and social skills, beyond what is implicated in 22q11.2DS. For example, the posterior superior temporal sulcus (face perception and recognition), the inferior frontal gyrus (emotion categorization), the anterior insula (recognition of motivationally relavent emotional faces), and the inferior parietal lobule (expression processing) are all implicated in effective emotion processing (Gobbini & Haxby, 2007; Jáni & Kašpárek, 2018; Keuken et al., 2011; Marrazzo et al., 2021; Pitcher, 2014). Given their global role in emotional face processing, future work would benefit from incorporating these regions as control variables when investigating diagnosis-specific brain alterations.
Third, we used the BASC-2 PRS Social Skill scale to evaluate parent-reported social skills of children with 22q11.2DS and TD controls. In a sample of children with high-functioning autism spectrum disorder (HFASD) and TD controls, Volker et al. (2010) found that those with HFASD scored significantly lower than TD children on the BASC-2 PRS Social Skills scale, suggesting that it captured social deficits of the group. However, they noted that the Social Skills scale may not be as sensitive to certain aspects of the skill deficits, such as a child’s asocial behaviors. Future work should, therefore, incorporate the Social Responsiveness Scale (SRS) (Constantino & Gruber, 2012) as a measure of social impairments in 22q11.2DS given its high sensitivity and specificity identifying social deficits. Fourth, we were unable to test for sensitivity based on psychotropic medications, as not all of our study participants documented current medication use. Future work should consider medication use to determine its effects on neural circuitry. Finally, to better characterize these white matter anomalies and understand their microstructural origins, more invasive ex vivo methods examining dendritic alterations and axonal tracing are currently necessary.
Conclusions
The present study contributes to the existing literature characterizing impaired social skills and emotion recognition in children with 22q11.2DS. We found that socioemotional impairment are partially influenced by atypical white matter connectivity between the right amygdala and fusiform gyrus. This partial mediation suggests that while there was a significant relationship between right Amy-FG connectivity and social-emotional impairment, it did not fully account for the observed relationship between diagnosis and measures of social skills and emotion recognition ability. Given the important role that these regions have in the interpretation of others’ facial emotions, this association was not surprising. This is, to the best of our knowledge, the first study characterizing the relationship between poorer emotion recognition and differences in white matter structural connectivity between these regions in children with 22q11.2DS. An important next step would be to follow these children longitudinally to measure changes in their developing brains in relation to both socioemotional maturation but also as a potential early biomarker of risk for developing a psychiatric disorder.
References
Albohn, D. N., & Adams, R. B. (2016). Social vision: At the intersection of vision and person perception. Neuroimaging personality, social cognition, and character (pp. 159–186). Elsevier.
Andersson, F., Glaser, B., Spiridon, M., Debbané, M., Vuilleumier, P., & Eliez, S. (2008). Impaired activation of face processing networks revealed by functional magnetic resonance imaging in 22q11.2 deletion syndrome. Biological Psychiatry, 63(1), 49–57.
Angkustsiri, K., Goodlin-Jones, B., Deprey, L., Brahmbhatt, K., Harris, S., & Simon, T. J. (2014). Social impairments in chromosome 22q11.2 deletion syndrome (22q11. 2DS): Autism spectrum disorder or a different endophenotype? Journal of Autism and Developmental Disorders, 44, 739–746.
Baker, K. D., & Skuse, D. H. (2005). Adolescents and young adults with 22q11 deletion syndrome: Psychopathology in an at-risk group. The British Journal of Psychiatry, 186(2), 115–120.
Bassett, A. S., Chow, E. W., AbdelMalik, P., Gheorghiu, M., Husted, J., & Weksberg, R. (2014). The schizophrenia phenotype in 22q11 deletion syndrome. American Journal of Psychiatry, 160(9), 1580–1586.
Baxter, M. G., Parker, A., Lindner, C. C., Izquierdo, A. D., & Murray, E. A. (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience, 20(11), 4311–4319.
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance in Vivo, 15(7–8), 435–455.
Boyatzis, C. J., Chazan, E., & Ting, C. Z. (1993). Preschool children’s decoding of facial emotions. The Journal of Genetic Psychology, 154(3), 375–382.
Buka, S. L., Tsuang, M. T., Torrey, E. F., Klebanoff, M. A., Wagner, R. L., & Yolken, R. H. (2001). Maternal cytokine levels during pregnancy and adult psychosis [Research Support, Non-U S Gov’t Research Support, U S Gov’t, Non-P H S]. Brain, Behavior, and Immunity, 15(4), 411–420.
Campbell, I. M., Sheppard, S. E., Crowley, T. B., McGinn, D. E., Bailey, A., McGinn, M. J., Unolt, M., Homans, J. F., Chen, E. Y., Salmons, H. I., Gaynor, J. W., & McDonald-McGinn, D. M. (2018). What is new with 22q? An update from the 22q and You Center at the Children’s Hospital of Philadelphia. American Journal of Medical Genetics. Part A, 176(10), 2058–2069.
Campbell, L. E., Azuma, R., Ambery, F., Stevens, A., Smith, A., Morris, R. G., & Murphy, K. C. (2010). Executive functions and memory abilities in children with 22q11.2 deletion syndrome [Research Support, Non-US Gov’t]. Australian and New Zealand Journal of Psychiatry, 44(4), 364–371. https://doi.org/10.3109/00048670903489882
Campbell, L. E., Stevens, A., Daly, E., Toal, F., Azuma, R., Karmiloff-Smith, A., & Murphy, K. C. (2009). A comparative study of cognition and brain anatomy between two neurodevelopmental disorders: 22q11.2 deletion syndrome and Williams syndrome [comparative study]. Neuropsychologia, 47(4), 1034–1044. https://doi.org/10.1016/j.neuropsychologia.2008.10.029
Campbell, L. E., Stevens, A. F., McCabe, K., Cruickshank, L., Morris, R. G., Murphy, D. G., & Murphy, K. C. (2011). Is theory of mind related to social dysfunction and emotional problems in 22q11.2 deletion syndrome (velo-cardio-facial syndrome)? Journal of Neurodevelopmental Disorders, 3(2), 152–161.
Cheung, V., Chiu, C. P. Y., Law, C. W., Cheung, C., Hui, C. L. M., Chan, K. K. S., Sham, P. C., Deng, M. Y., Tai, K. S., Khong, P. L., & McAlonan, G. M. (2011). Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychological Medicine, 41(8), 1709–1719.
Constantino, J. N., & Gruber, C. P. (2012). Social responsiveness scale: SRS-2. Western Psychological Services Torrance.
Critchley, H. D., Mathias, C. J., & Dolan, R. J. (2002). Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron, 33(4), 653–663.
Dennis, M., Francis, D. J., Cirino, P. T., Schachar, R., Barnes, M. A., & Fletcher, J. M. (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15(3), 331–343. https://doi.org/10.1017/S1355617709090481
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., Buckner, R. L., Dale, A. M., Maguire, R. P., Hyman, B. T., & Albert, M. S. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980.
Durand, K., Gallay, M., Seigneuric, A., Robichon, F., & Baudouin, J. Y. (2007). The development of facial emotion recognition: The role of configural information. Journal of Experimental Child Psychology, 97(1), 14–27.
Fairhall, S. L., & Ishai, A. (2007). Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex, 17(10), 2400–2406.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., Van Der Kouwe, A., Killiany, R., Kennedy, D., Klaveness, S., & Montillo, A. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Fossati, P. (2012). Neural correlates of emotion processing: From emotional to social brain. European Neuropsychopharmacology, 22, S487–S491.
Fuerst, K. B., Dool, C. B., & Rourke, B. P. (1995). Syndrome of nonverbal learning disabilities: Neurodevelopmental manifestations. Past and future. The Guilford Press.
Gobbini, M. I., & Haxby, J. V. (2007). Neural systems for recognition of familiar faces. Neuropsychologia, 45(1), 32–41.
Goebel, R., Esposito, F., & Formisano, E. (2006). Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping, 27(5), 392–401.
Gur, R. E., McGrath, C., Chan, R. M., Schroeder, L., Turner, T., Turetsky, B. I., Ragland, J. D., et al. (2002). An fMRI study of facial emotion processing in patients with schizophrenia. American Journal of Psychiatry, 159(12), 1992–1999.
Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51(1), 59–67.
Hoeft, F., Barnea-Goraly, N., Haas, B. W., Golarai, G., Ng, D., Mills, D., Korenberg, J., Bellugi, U., Galaburda, A., & Reiss, A. L. (2007). More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. Journal of Neuroscience, 27(44), 11960–11965.
Howley, S. A., Prasad, S. E., Pender, N. P., & Murphy, K. C. (2012). Relationship between reaction time, fine motor control, and visual–spatial perception on vigilance and visual-motor tasks in 22q11.2 deletion syndrome. Research in Developmental Disabilities, 33(5), 1495–1502.
Jalbrzikowski, M., Carter, C., Senturk, D., Chow, C., Hopkins, J. M., Green, M. F., Galván, A., Cannon, T. D., & Bearden, C. E. (2012). Social cognition in 22q11.2 microdeletion syndrome: relevance to psychosis? Schizophrenia Research, 142(1–3), 99–107.
Jalbrzikowski, M., Jonas, R., Senturk, D., Patel, A., Chow, C., Green, M. F., & Bearden, C. E. (2013). Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: Relationship with psychotic symptoms. Neuroimage Clinical, 3, 405–415.
Jáni, M., & Kašpárek, T. (2018). Emotion recognition and theory of mind in schizophrenia: A meta-analysis of neuroimaging studies. The World Journal of Biological Psychiatry, 19(sup3), S86–S96.
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17(11), 4302–4311.
Karayiorgou, M., Simon, T. J., & Gogos, J. A. (2010). 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nature Reviews Neuroscience, 11(6), 402.
Keuken, M. C., Hardie, A., Dorn, B. T., Dev, S., Paulus, M. P., Jonas, K. J., Van Den Wildenberg, W. P. M., & Pineda, J. A. (2011). The role of the left inferior frontal gyrus in social perception: An rTMS study. Brain Research, 1383, 196–205.
Kikinis, Z., Asami, T., Bouix, S., Finn, C. T., Ballinger, T., Tworog-Dube, E., & Kubicki, M. (2012). Reduced fractional anisotropy and axial diffusivity in white matter in 22q11.2 deletion syndrome: a pilot study [Multicenter Study Research Support, N I H, Extramural Research Support, Non-U S Gov’t Research Support, U S Gov’t, Non-P H S]. Schizophrenia Research, 141(1), 35–39.
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E., & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20(5), 937–945.
Leleu, A., Saucourt, G., Rigard, C., Chesnoy, G., Baudouin, J. Y., Rossi, M., Edery, P., Franck, N., & Demily, C. (2016). Facial emotion perception by intensity in children and adolescents with 22q11.2 deletion syndrome. European Child & Adolescent Psychiatry, 25(3), 297–310.
Liang, X., Zebrowitz, L. A., & Aharon, I. (2009). Effective connectivity between amygdala and orbitofrontal cortex differentiates the perception of facial expressions. Social Neuroscience, 4(2), 185–196.
Loveland, K. A., Pearson, D. A., Tunali-Kotoski, B., Ortegon, J., & Gibbs, M. C. (2001). Judgments of social appropriateness by children and adolescents with autism. Journal of Autism and Developmental Disorders, 31(4), 367–376.
Marrazzo, G., Vaessen, M. J., & de Gelder, B. (2021). Decoding the difference between explicit and implicit body expression representation in high level visual, prefrontal and inferior parietal cortex. NeuroImage, 243, 118545.
Mattiaccio, L. M., Coman, I. L., Thompson, C. A., Fremont, W. P., Antshel, K. M., & Kates, W. R. (2018). Frontal dysconnectivity in 22q11.2 deletion syndrome: an atlas-based functional connectivity analysis. Behavioral and Brain Functions, 14(1), 2. https://doi.org/10.1186/s12993-018-0134-y
Maurer, D., Le Grand, R., & Mondloch, C. J. (2002). The many faces of configural processing. Trends in Cognitive Sciences, 6(6), 255–260.
McCabe, K., Rich, D., Loughland, C. M., Schall, U., & Campbell, L. E. (2011). Visual scanpath abnormalities in 22q11.2 deletion syndrome: is this a face specific deficit? Psychiatry Research, 189(2), 292–298.
McCabe, K. L., Melville, J. L., Rich, D., Strutt, P. A., Cooper, G., Loughland, C. M., Schall, U., & Campbell, L. E. (2013). Divergent patterns of social cognition performance in autism and 22q11.2 deletion syndrome (22q11DS). Journal of Autism and Developmental Disorders, 43(8), 1926–1934.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function [research support, non-U S gov’t, research support, U S gov’t, P H S review]. Annual Review of Neuroscience, 24, 167–202.
Moss, E. M., Batshaw, M. L., Solot, C. B., Gerdes, M., McDonald-McGinn, D. M., Driscoll, D. A., Emanuel, B. S., Zackai, E. H., & Wang, P. P. (1999). Psychoeducational profile of the 22q11.2 microdeletion: a complex pattern. The Journal of Pediatrics, 134(2), 193–198.
Niklasson, L., Rasmussen, P., Óskarsdóttir, S., & Gillberg, C. (2001). Neuropsychiatric disorders in the 22q11 deletion syndrome. Genetics in Medicine, 3(1), 79–84.
Norkett, E. M., Lincoln, S. H., Gonzalez-Heydrich, J., & D’Angelo, E. J. (2017). Social cognitive impairment in 22q11 deletion syndrome: A review. Psychiatry Research, 253, 99–106.
Olszewski, A. K., Kikinis, Z., Gonzalez, C. S., Coman, I. L., Makris, N., Gong, X., & Kates, W. R. (2017). The social brain network in 22q11.2 deletion syndrome: a diffusion tensor imaging study. Behavioral and Brain Functions, 13(1), 4. https://doi.org/10.1186/s12993-017-0122-7
Ottet, M. C., Schaer, M., Debbané, M., Cammoun, L., Thiran, J. P., & Eliez, S. (2013). Graph theory reveals dysconnected hubs in 22q11DS and altered nodal efficiency in patients with hallucinations. Frontiers in Human Neuroscience, 7, 402.
Padula, M. C., Schaer, M., Scariati, E., Schneider, M., Van De Ville, D., Debbané, M., & Eliez, S. (2015). Structural and functional connectivity in the default mode network in 22q112 deletion syndrome. Journal of Neurodevelopmental Disorders, 7(1), 23. https://doi.org/10.1186/s11689-015-9120-y
Penn, D. L., Sanna, L. J., & Roberts, D. L. (2008). Social cognition in schizophrenia: An overview. Schizophrenia Bulletin, 34(3), 408–411.
Peterson, D. J., Ryan, M., Rimrodt, S. L., Cutting, L. E., Denckla, M. B., Kaufmann, W. E., & Mahone, E. M. (2011). Increased regional fractional anisotropy in highly screened attention-deficit hyperactivity disorder (ADHD). Journal of Child Neurology, 26(10), 1296–1302.
Philippi, C. L., Mehta, S., Grabowski, T., Adolphs, R., & Rudrauf, D. (2009). Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience, 29(48), 15089–15099. https://doi.org/10.1523/jneurosci.0796-09.2009
Phillips, M. L., Drevets, W. C., Rauch, S. L., & Lane, R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–514.
Pitcher, D. (2014). Facial expression recognition takes longer in the posterior superior temporal sulcus than in the occipital face area. Journal of Neuroscience, 34(27), 9173–9177.
Popa, A. M., Cruz, J. R., Wong, L. M., Harvey, D. J., Angkustsiri, K., Leckliter, I. N., Perez-Edgar, K., & Simon, T. J. (2019). Seeing eye to eye with threat: Atypical threat bias in children with 22q11.2 deletion syndrome. American Journal on Intellectual and Developmental Disabilities, 124(6), 549–567.
R Development Core Team. (2020). R: A language and environment for statistical programming. In (Version 3.6.2) R Foundation for Statistical Programming. R Development Core Team.
Radoeva, P. D., Coman, I. L., Antshel, K. M., Fremont, W., McCarthy, C. S., Kotkar, A., & Kates, W. R. (2012). Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings [Research Support, N I H, Extramural Research Support, non-U S Gov’t]. Behavioral and Brain Functions, 8(38), 1744–9081.
Revelle, W. (2019). psychTools: Tools to accompany the ‘psych’ package for psychological research. R Package Version, 1, 12.
Reynolds, & Kamphaus. (2015). Behavior Assessment System for Children, Second Edition (BASC-2). Wiley. https://doi.org/10.1002/9781118625392.wbecp447
Rice, M. E., Okada, Y. C., & Nicholson, C. H. A. R. L. E. S. (1993). Anisotropic and heterogeneous diffusion in the turtle cerebellum: Implications for volume transmission. Journal of Neurophysiology, 70(5), 2035–2044.
Roy, A. K., Vasa, R. A., Bruck, M., Mogg, K., Bradley, B. P., Sweeney, M., Bergman, R. L., McClure-Tone, E. B., & Pine, D. S. (2008). Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 47(10), 1189–1196.
Schneider, M., Debbané, M., Lagioia, A., Salomon, R., d’Argembeau, A., & Eliez, S. (2012). Comparing the neural bases of self-referential processing in typically developing and 22q11.2 adolescents. Developmental Cognitive Neuroscience, 2(2), 277–289.
Schneider, W., Eschman, A., & Zuccolotto, A. (2002). E-Prime: User's guide. Reference guide. Getting started guide. Psychology Software Tools, Incorporated.
Shapiro, H. M., Tassone, F., Choudhary, N. S., & Simon, T. J. (2014). The development of cognitive control in children with chromosome 22q11.2 deletion syndrome. Frontiers in Psychology, 5, 1–14. https://doi.org/10.3389/fpsyg.2014.00566
Simon, T. J., Bearden, C. E., Moss, E. M., McDonald-McGinn, D., Zackai, E., & Wang, P. P. (2002). Cognitive development in VCFS. Progress in Pediatric Cardiology, 15(2), 109–117.
Sing, T., Sander, O., Beerenwinkel, N., & Lengauer, T. (2005). ROCR: Visualizing classifier performance in R. Bioinformatics, 21(20), 3940–3941.
Sobin, C., Kiley-Brabeck, K., Daniels, S., Khuri, J., Taylor, L., Blundell, M., Anyane-Yeboa, K., & Karayiorgou, M. (2005). Neuropsychological characteristics of children with the 22q11 deletion syndrome: a descriptive analysis. Child Neuropsychology, 11(1), 39–53.
Stigler, K., & McDougle, C. (2013). Structural and functional MRI studies of autism spectrum disorders. Elsevier. https://doi.org/10.1016/B978-0-12-391924-3.00017-X
Swillen, A., Devriendt, K., Legius, E., Eyskens, B., Dumoulin, M., Gewillig, M., & Fryns, J.-P. (1997). Intelligence and psychosocial adjustment in velocardiofacial syndrome: A study of 37 children and adolescents with VCFS. Journal of Medical Genetics, 34(6), 453–458.
Szeszko, P. R., Robinson, D. G., Ashtari, M., Vogel, J., Betensky, J., Sevy, S., Ardekani, B. A., Lencz, T., Malhotra, A. K., McCormack, J., & Miller, R. (2008). Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology, 33(5), 976–984.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain (Vol. 270, pp. 132). Theime.
Thaler, A. (2018). Structural and functional MRI in familial Parkinson’s disease. International Review of Neurobiology, 142, 261–287.
Thiele, C., & Hirschfeld, G. (2020). cutpointr: Improved estimation and validation of optimal cutpoints in R. https://arxiv.org/abs/2002.09209
Thomas, L. A., De Bellis, M. D., Graham, R., & LaBar, K. S. (2007). Development of emotional facial recognition in late childhood and adolescence. Developmental Science, 10(5), 547–558.
Tong, F., Nakayama, K., Moscovitch, M., Weinrib, O., & Kanwisher, N. (2000). Response properties of the human fusiform face area. Cognitive Neuropsychology, 17(1–3), 257–280.
Tuch, D. S., Salat, D. H., Wisco, J. J., Zaleta, A. K., Hevelone, N. D., & Rosas, H. D. (2005). Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proceedings of the National Academy of Sciences, 102(34), 12212–12217.
Van, L., Heung, T., Graffi, J., Ng, E., Malecki, S., Van Mil, S., & Bassett, A. S. (2019). All-cause mortality and survival in adults with 22q11.2 deletion syndrome. Genetics in Medicine, 21(10), 2328–2335. https://doi.org/10.1038/s41436-019-0509-y
Van Amelsvoort, T., Schmitz, N., Daly, E., Deeley, Q., Critchley, H., Henry, J., Robertson, D., Owen, M., Murphy, K. C., & Murphy, D. G. (2006). Processing facial emotions in adults with velo-cardio-facial syndrome: Functional magnetic resonance imaging. The British Journal of Psychiatry, 189(6), 560–561.
Volker, M. A., Lopata, C., Smerbeck, A. M., Knoll, V. A., Thomeer, M. L., Toomey, J. A., & Rodgers, J. D. (2010). BASC-2 PRS profiles for students with high-functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(2), 188–199. https://doi.org/10.1007/s10803-009-0849-6
Vuilleumier, P., & Pourtois, G. (2007). Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia, 45(1), 174–194.
Wechsler, D. (2003). Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) administration and scoring manual. The Psychological Corporation.
Weinberger, R., Yi, J., Calkins, M., Guri, Y., McDonald-McGinn, D. M., Emanuel, B. S., & Michaelovsky, E. (2016). Neurocognitive profile in psychotic versus nonpsychotic individuals with 22q11.2 deletion syndrome. European Neuropsychopharmacology, 26(10), 1610–1618.
Wickham, H. (2009). Ggplot2: Elegant graphics for data analysis (2nd ed.). Springer. https://doi.org/10.1007/978-0-387-98141-3
Woodin, M., Wang, P. P., Aleman, D., McDonald-McGinn, D., Zackai, E., & Moss, E. (2001). Neuropsychological profile of children and adolescents with the 22q11. 2 microdeletion. Genetics in Medicine, 3(1), 34–39.
Funding
National Institute of Mental Health, 5R00MH086616-05, Elliott Alexander Beaton, National Institutes of Health, T32 MH100019, Ashley F.P. Sanders
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanders, A.F.P., Hobbs, D.A., Knaus, T.A. et al. Structural Connectivity and Emotion Recognition Impairment in Children and Adolescents with Chromosome 22q11.2 Deletion Syndrome. J Autism Dev Disord 53, 4021–4034 (2023). https://doi.org/10.1007/s10803-022-05675-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-022-05675-z