Abstract
Objective
To evaluate the efficacy of pregabalin and dexamethasone coadministration in preemptive analgesia and anxiety control in lower third molar surgery.
Materials and methods
A triple-blind, split-mouth clinical trial conducted with patients divided into two groups: control group, receiving placebo and dexamethasone, and test group, receiving pregabalin and dexamethasone preoperatively. The evaluated variables were pain, measured by the Visual Analog Scale (VAS), anxiety assessed through the State-Trait Anxiety Inventory (STAI) questionnaires, hemodynamic parameters [Blood Pressure (BP), Heart Rate (HR), Oxygen Saturation (SpO2)], and sedation assessed by the Ramsay scale.
Results
A total of 31 patients were included. The test group exhibited a significant reduction in pain at 2,4,6,8,12,16,24, and 48 h after surgery and in the consumption of rescue analgesics. Anxiety, evaluated by STAI and VAS, showed a significant decrease in the test group (p < 0.001). Additionally, there was a significant decrease in BP at most of the assessed time points (p < 0.05) and a significant reduction in HR at two different time intervals (p = 0.003 and p = 0.009), indicating a positive effect in the test group. There was no significant difference in SpO2 between the groups. Sedation assessment revealed a significant difference at all time points favoring the test group (p < 0.05). There were no significant postoperative adverse effects.
Conclusions
Pregabalin coadministered with dexamethasone demonstrated significant efficacy in controlling postoperative pain and anxiety, as well as a sedative effect.
Clinical relevance
The coadministration of pregabalin with dexamethasone may presents potential advantages in both pain modulation and psychological well-being of individuals undergoing third molar surgeries.
Trial registration
Brazilian Clinical Trials Registry (REBEC), No. RBR-378h6t6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective management of postoperative pain and minimization of anxiety associated with surgical procedures, such as third molar extractions, are crucial objectives in the context of dental practice [1]. In third molar surgeries, surgical trauma, often characterized as moderate to severe, has the potential to induce anxiety, as well as cause acute pain of nociceptive and inflammatory nature. The level of anxiety is correlated with individual predisposition or past experiences to surgical trauma, which can adversely impact patient’s quality of life and treatment success [2, 3]. Pain response is generated by the production and release of algogenic substances from neural tissues (free nerve endings) and supporting dental tissues (alveolar bone, ligaments, mucosa) [2]. Postoperative acute pain can lead to morbidity and affect patients’ oral function and quality of life [4]. If pain therapeutic approach is initiated after its stimulus, there is a possibility of developing peripheral hypersensitivity and central nervous system (CNS) hyperexcitability. This condition presents significant challenges in the context of postoperative pain management [5].
Preemptive analgesia is a strategic approach advocating the administration of analgesics before surgical procedures, aiming to decrease CNS sensitization by stimuli during surgery and/or postoperative inflammatory reactions. Additionally, effective management of acute pain and preoperative anxiety can also lead to reduced consumption of drugs for pain and inflammation control, promoting greater acceptance and comfort during the postoperative period. The use of drugs with analgesic and anti-inflammatory properties is a common practice in the context of third molar surgery, yielding positive clinical outcomes [5,6,7]. However, it is important to note that frequent or high concentrations of these drugs are associated with many adverse effects [4]. New antinociceptive strategies, such as multimodal preemptive analgesia, involving the simultaneous administration of medications with different mechanisms of action, have the potential to provide a synergistic and more effective pain control effect [8]. Recently, the combination of pregabalin with anti-inflammatory agents, such as dexamethasone, has shown promising prospects in the control of postoperative acute pain, both at peripheral and central levels, with a reduced incidence of associated adverse effects [9].
Pregabalin is a drug with analgesic, anticonvulsant, and anxiolytic properties, being a synthetic analog of the neurotransmitter Gamma-Aminobutyric Acid (GABA). Despite structural similarity, it does not have a direct functional relationship with GABA. Pregabalin is recognized as antiallodynic and anti-hyperalgesic, acting by inhibiting nerve membrane depolarization and afferent nerve conduction. This occurs through interaction with the α2-δ subunit of voltage-gated calcium channels, resulting in reduced release of excitatory neurotransmitters, such as glutamate, and sensitization of the central nervous system [4]. The main indications for pregabalin use include treatment of neuropathic pain associated with infections, cancer, or diabetes, control of epileptic episodes, and management of acute pain following orthognathic surgery [10] or third molar extractions [4]. Pregabalin also exhibits anxiolytic and sedative effects comparable to benzodiazepines, potentially exerting effective action in preoperative anxiety control [11, 12].
While pregabalin has demonstrated efficacy in the treatment of postoperative acute pain in various study models in the medical field [9, 13, 14], there is limited evidence from controlled studies on its efficacy when coadministered with dexamethasone in third molar surgeries. The search for pharmacological agents that not only provide analgesic relief but also contribute to reducing pre- and postoperative anxiety without significant adverse effects represents a significant advancement in the overall management of patients undergoing invasive dental surgical procedures. Therefore, this study aims to evaluate the efficacy of dexamethasone and pregabalin coadministration in preemptive analgesia and its influence on anxiety and sedation control in lower third molar extractions.
Materials and methods
Study design
A randomized, triple-blind clinical trial was conducted at the Clinical Research Center in Oral and Maxillofacial Surgery and Traumatology of the Oswaldo Cruz University Hospital (HUOC) at the University of Pernambuco (UPE), Brazil, from April 2022 to December 2023. The study adopted a split-mouth design, in which each patient underwent two lower third molar extractions at different surgical times, with each procedure performed on different hemiarches of the mandible, one side designated as the test group and the opposite side as the control group.
This study received approval from the Research Ethics Committee of the University of Pernambuco (UPE), as registered under approval number: 4,908,679. The study adhered to the principles of the Declaration of Helsinki. All voluntary participants provided their consent by signing an appropriate form informed consent form, in accordance with the precepts established in resolution no. 466/2012 of the national health council. The present research was duly registered in the Brazilian Clinical Trials Registry (REBEC) with identification RBR-378h6t6. The study conduct followed the guidelines advocated by the Consolidated Standards of Reporting Trials (CONSORT) [15], while bias control was performed by implementing predetermined methodological strategies, as stipulated in “The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials” [16].
Participants
Patients were selected for inclusion in this study based on predefined criteria aimed at undergoing surgery for impacted lower third molars. Inclusion criteria were adult individuals aged 18 to 35 years, of any gender and ethnicity, who were in good overall health (classified as ASA I and II by the American Society of Anesthesiologists) and demonstrated “high” or “very high” anxiety levels according to scores on the State-Trait Anxiety Inventory (STAI) questionnaire. Selected participants were required to have both lower third molars, right and left, partially or fully impacted, with similar positioning and root formation, showing healthiness and indication for extraction. Additionally, they were required not to have allergies to the pharmacological substances used in the study or their components and not to be taking other medications known to interact with pregabalin.
Exclusion criteria included the presence of pathological lesions associated with lower third molars, acute pericoronitis, recent history of chemotherapy and/or radiotherapy in the head and neck region, smoking, pregnancy, or lactation, failure to attend scheduled postoperative follow-up appointments, voluntary withdrawal during the research, presence of renal or hepatic insufficiency, surgery duration exceeding 40 min, difference in operative times exceeding 10 min, and development of postoperative infection.
Randomization
Randomization was performed by one of the researchers (D.S.B.) during the study. The generation of the random sequence was carried out using a computerized random number generator (Random, accessible at https://www.random.org/). Thus, participants undergoing lower third molar extractions were randomly assigned to receive therapy containing placebo + dexamethasone (control group) or pregabalin + dexamethasone (test group).
Allocation
This procedure was coordinated by a researcher not involved in patient evaluation or surgeries (D.S.B.). In order to ensure allocation concealment, opaque envelopes were used to store information regarding the medication combinations assigned to the groups (dexamethasone + placebo or dexamethasone + pregabalin), as well as the sequence of sides to be followed for the start of surgeries (right or left). After a period of 21 days, the second surgery was conducted on the contralateral side, with the second combination of medications administered.
Blinding
This clinical trial was conducted under triple-blinding, ensuring that during assessments, patients, the surgeon (J.A.D.), and the investigator (M.S.V.O.) remained unaware of the nature of the interventions. This approach aimed to ensure complete impartiality in assessing the effects of the drug employed, mitigating the risk of verification bias. In compliance with the need for masking (blinding) between groups to prevent differentiation of the drugs used in each surgery, placebo (100 mg of corn starch) was manipulated into capsules (Formula Certa®, Catolé do Rocha, PB, Brazil) with dimensions, shape, color, and aroma identical to pregabalin capsules (Lyrica® Pfizer Inc., New York City, NY, USA), packaged in containers of identical size and color. Each group also received two 4 mg dexamethasone tablets (Decadron®, Aché Laboratórios Farmacêuticos Ltda., São Paulo, SP, Brazil). The capsules and tablets were administered in equal amounts preoperatively, one hour before surgery, in both groups by D.S.B. Data collection and clinical examinations were conducted by the researcher M.S.V.O. who was not involved in the surgical procedure.
Interventions
All participants underwent preoperative clinical assessment, which included medical history, physical examination, and analysis of complementary exams. Surgical complexity was evaluated using the Pernambuco Index [2], which incorporates the Pell & Gregory classification, Winter’s dental impaction angle, root curvature and number, relationship with the second molar, age, and Body Mass Index (BMI). Surgical difficulty was categorized as low (scores of 8–12), moderate (scores of 13–17), or high (scores of 18–22), according to the obtained scores (Table 1).
In the preoperative period, one hour before surgery, all participants underwent rinsing with 0.12% chlorhexidine digluconate (15mL/1min; Perioxidin®, Lacer GlaxoSmithKline Brazil, Rio de Janeiro, RJ, Brazil). The experimental substances, dexamethasone + placebo or dexamethasone + pregabalin, were administered one hour before surgery, according to group assignment. In the postoperative phase, the prescription included amoxicillin (500 mg; Amoxil®, Lacer GlaxoSmithKline Brazil, Rio de Janeiro, RJ, Brazil) every 8 h for seven days and 0.12% chlorhexidine digluconate (15mL/1min/12 h) for the same period of time. Rescue medication employed was paracetamol (750 mg; Tylenol®, Johnson & Johnson©, New Brunswick, NJ, USA), indicated every 6 h when pain reached a score of 3 on the VAS.
All patients underwent identical operative procedures performed by the same team in both groups, following a standardized protocol. Surgeries were conducted during the early morning hours on the same weekday, in a quiet environment with only research team members present to maintain procedural consistency. The maximum predefined time of 40 min for the methodology was not exceeded in any of the surgeries, and the difference in operative times between the right and left hemiarches within the same patient did not exceed 10 min. Operative time was measured from the moment of incision until completion of the extraction.
The patients underwent local anesthesia with 2% lidocaine and 1:100,000 adrenaline (Alphacaine® 100, DFL, Rio de Janeiro, RJ, Brazil), respecting the maximum weight-dependent dose ratio of 7.0 mg/kg for each individual, for regional block of the inferior alveolar, buccal, and lingual nerves, using the direct technique. Surgical access was established through an incision in the region of the alveolar ridge posterior to the lower second molar, extending intrasulcularly to the mesial region of the lower first molar. After mucoperiosteal detachment, osteotomy was performed with a multilaminated 702 bur (SSWHITE©, Lakewood, NJ, USA) and/or odontosection with a Zekrya FG bur, 28 mm (Dentsply Sirona©, São José, SC, Brazil), using a high-speed surgical handpiece at 80,000 rpm under manual irrigation with 0.9% sodium chloride solution (Cristália©, Rio de Janeiro, RJ, Brazil), as needed for each case. Extraction was conducted with the aid of elevators and forceps. After cleaning and debridement of the socket with a curved curette, suturing was performed with Ethicon 4 − 0 silk suture (Ethicon®, Johnson & Johnson©, New Brunswick, NJ, USA), which was removed after a 7-day postoperative period. Variable values were collected preoperatively, intraoperatively, and postoperatively, and recorded in a database.
Outcomes and data collection
Primary outcomes
Pain assessment was conducted using the Visual Analog Scale (VAS), a 10-point tool that quantifies pain intensity, where 0 represents no pain and 10 denotes extreme pain. Interpretation of scores is categorized as follows: no pain (0); mild pain (1 to 2); moderate pain (3 to 7); and severe pain (8 to 10). Patients were instructed to record their VAS score at various postoperative time points, starting 30 min after surgery, and subsequently at the following intervals: 2,4,6,8,12,16,24,48, and 72 h. The number of rescue analgesics was documented up to the seventh postoperative day, including the time of administration for each dose. A comparison was made between the times of first rescue analgesic consumption in the two groups [6]. Additionally, any adverse effects encountered, such as dizziness, drowsiness, vomiting, and nausea, were recorded [17].
Anxiety assessment was performed using subjective tools. Additionally, changes in hemodynamic parameters were evaluated to objectively investigate the influence of anxiety on bodily functioning. Subjective assessment utilized the State-Trait Anxiety Inventory (STAI) and the Anxiety Visual Analog Scale (VAS). Objective assessment was conducted through hemodynamic parameters. The STAI is a tool that assesses both state anxiety (STAI-S) and trait anxiety (STAI-T). Trait anxiety is used to determine the baseline level of anxiety, while state anxiety corresponds to emotions typically associated with preoperative tension. Both questionnaires consist of 20 questions, each with 4 possible responses, resulting in a score ranging from 20 to 80. Higher scores indicate a higher level of anxiety at the time of assessment, while lower scores suggest a lower level of anxiety. Interpretation of these scores should take into account the patient’s gender and can be categorized as very low, low, normal, high, or very high [18]. The STAI-T questionnaire was used during the initial consultation for all patients. The STAI-S questionnaire was administered 1 h after medication administration.
The VAS was employed to enable the measurement of emotional states that are not easily captured by verbal or written language. This scale, with a length of 100 mm and 5 points, ranges from 0 (absence of anxiety) to 4 (maximum anxiety). Interpretation of scores is as follows: absence of anxiety (0), mild anxiety (1 to 2), moderate anxiety (3 to 7), and severe anxiety (8 to 10) [19]. The VAS was used at two distinct time points: during the initial consultation and 1 h after medication administration.
In the analysis of hemodynamic changes, the following were recorded: blood pressure (BP), expressed in millimeters of mercury (mmHg); heart rate (HR), measured in beats per minute (bpm); and oxygen saturation (SpO2), obtained as a percentage (%). An Ambulatory Blood Pressure Monitor (ABPM) CONTEC® ABPM50 (Contec medical systems - Hebei, China) was used to obtain BP and HR measurements. SpO2 was assessed using a pulse oximeter model OXP-10 Emai® (EMAI, São Paulo, Brazil).
During BP measurement, volunteers remained seated, with the left arm positioned parallel to the trunk and the cuff adapted to the arm, kept at the same level as the heart. The pulse oximeter had its cuff finger (digital sensor) adapted to the distal phalanx of the right index finger. After patient preparation and a 15-minute wait, HR, SpO2 values, and three BP readings were recorded to calculate the mean blood pressure, considered as the baseline value (A0). Hemodynamic parameters were recorded at the following time points, in addition to A0: immediately before medication administration (A1), one hour after medication administration (A2), immediately after local anesthesia application (A3), during the tooth extraction (A4), and at the end of suturing (A5).
From the data of Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP) was calculated. MAP represents the average pressure throughout the cardiac cycle, reflecting the overall pressure required to promote blood flow. For the calculation of MAP, we applied the formula: MAP = (2DBP + 1SBP) ÷ 3 [20]. Interpretation of the percentage of oxyhemoglobin (SpO2) in peripheral circulation was performed as follows: normal saturation (> 95%), mild hypoxemia (95 to 90%), moderate hypoxemia (89 to 86%), severe hypoxemia (< 86%) [21].
Secondary outcomes
The level of sedation was assessed using the Ramsay Sedation Scale, which quantifies the individual’s activity level by observing their responses to stimuli. The evaluated scores were categorized according to patients’ responses, using the following scale: 1 (anxious, agitated, or restless), 2 (cooperative, oriented, and calm), 3 (responds only to verbal command), 4 (displays active response to light touch on the glabella or to an auditory stimulus), 5 (displays weak response to light touch on the glabella or to an auditory stimulus), and 6 (does not respond to light touch on the glabella or to an auditory stimulus) [22]. Sedation level assessment was conducted at three different time points: 1 h after medication administration (S0), during tooth extraction (S1), and after suturing (S2).
The global assessment involved how patients categorized their satisfaction with the therapy used, using a 5-point Likert scale (0, poor; 1, fair; 2, good; 3, very good; and 4, excellent) [6]. Patients were instructed to provide their responses to the global assessment at the time of suture removal (7 days after the surgical procedure).
Calibration
The researchers responsible for clinical assessment and data collection (M.S.V.O.), as well as for surgical procedures (J.A.D. and S.M.C.M.F.), were previously calibrated to ensure consistency in data collection and surgical sequence. This calibration involved three data collection repetitions in 10 patients, ensuring the reproducibility of results. The validity of the results was confirmed by an agreement exceeding 80% for all variables, both categorical and numerical, as assessed by the intraclass correlation coefficient. Data collection and analysis were performed only when inter-rater agreement reached a level exceeding 80%.
Sample size
Based on the results of the study conducted by Degirmenci and Yalcin [23], which demonstrated superior efficacy of co-administration of ibuprofen + 150 mg of pregabalin compared to the exclusive use of ibuprofen in controlling postoperative pain after third molar removal (postoperative pain scores at 3 h [0-100] = 41.25 ± 23.75 vs. 22.50 ± 17.50), it was necessary to evaluate a sample composed of 28 patients. This sample was estimated to represent, with 90% power and 95% confidence, the alternative hypothesis outlined in this study. The statistical power of the tests was calculated using G*Power 3.1 software. This was done considering the α error probability [0.05], sample size, and effect size. Considering potential sample loss, a 10% increment was incorporated, totaling 31 patients, following a split-mouth study design. This approach aims to ensure the statistical robustness of the research, providing a faithful representation of the target population and reinforcing the reliability of the obtained results.
Statistical analysis
The data were presented in the form of absolute and percentage frequency and were analyzed using the McNemar test or mean and standard deviation, with subsequent Kolmogorov-Smirnov normality test. Group comparisons were performed using the Wilcoxon test, while within-group comparisons were conducted using the Friedman/Dunn test. Kaplan-Meier curves were generated to calculate the medication-free survival, and compared using the Mantel-Cox Log-Rank test. All analyses were performed with a confidence level of 95% using SPSS v20.0 software for Windows.
Results
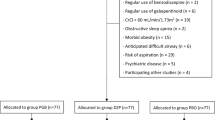
A total of 55 individuals were initially screened for possible inclusion in the study. Among these, 14 did not meet the pre-established inclusion and exclusion criteria, resulting in the participation of 41 individuals. During the follow-up period, ten patients were excluded due to loss to follow-up. The remaining participants were randomly allocated into two groups, as depicted in the CONSORT flowchart (Fig. 1). Detailed demographic information is available in Table 1.
A chronological account of recruitment, randomization/allocation concealment, interventions, outcome assessment, and loss to follow-up
Forty-one participants were considered for inclusion in the clinical trial, with impacted lower third molars randomly allocated to the test and control groups. At the end of the study, a total of 31 participants (with 62 impacted lower third molars) were actually included and completed the protocol; the loss to follow-up, totaling ten cases, mainly resulted from non-attendance at the first surgery (n = 5) and absence at the second surgery (n = 5). The chronological representation of the recruitment process is depicted in the CONSORT diagram (Fig. 1).
Demographic characteristics, number, and clinical features of each group
The mean age of the patients was 23.26 ± 4.06 years, with a male-to-female ratio of 0.41:1. Approximately half of the participants were pheoderma, with an average weight of 69.05 ± 16.74 kg and an average height of 1.66 ± 0.10 m. Most surgeries were classified as having moderate difficulty [48 teeth (77.42%)], according to the Pernambuco Index [2]. Both groups exhibited similar levels of operative difficulty, surgical time, and number of anesthetic cartridges (Table 2). A total of 54 surgical procedures (87.1%) required flap elevation, while 46 interventions (74.19%) involved osteotomy. Additionally, 38 teeth underwent odontosection during the surgeries.
The means of the pain variable were significantly lower in the test group at 2,4,6,8,12,16,24, and 48 h post-surgery [p < 0.001] (Fig. 2). The peak pain for patients occurred at 8 and 12 h for the control group (4.16 ± 1.86, p < 0.001) and test group (1.29 ± 0.82, p < 0.001), respectively. A greater number of patients in the control group required rescue analgesics [23(74.2%), p < 0.001]. Consequently, a higher amount of rescue medication was consumed by the control group (1.81 ± 1.58, p < 0.001) compared to the test group (0.39 ± 0.76, p < 0.001) (Table 3). Additionally, patients in the control group initiated, on average, rescue analgesic consumption earlier (24.19 ± 29.14) (Fig. 3).
Considering the assessment of anxiety through questionnaires, it was found that the mean scores of STAI-T (47.32 ± 7.29) indicated that the selected patients included in the study had high anxiety. The analysis of differences between the mean scores in the test (41.19 ± 6.45) and control (47.32 ± 6.47) groups regarding state anxiety (STAI-E) revealed a significant difference (p < 0.001). The means indicate that patients in the test group experienced anxiety considered normal, while the control group exhibited high anxiety (Table 4).
Analyzing anxiety through the EVA, a significant reduction in scores was observed between the initial consultation and 1 h after medication in both groups, as per intragroup analysis (p < 0.001). Furthermore, a significant reduction in anxiety was evidenced in the test group (1.58 ± 0.81) compared to the control group (2.61 ± 0.67) 1 h after medication (Table 4).
In the assessment of hemodynamic parameters, the combination of pregabalin and dexamethasone showed greater stability in BP and HR values, despite the significant increase observed in the intragroup analysis of both groups regarding SBP, MAP, and HR (p < 0.001) over the evaluated time periods. There was a significant reduction in SBP, DBP, and MAP in the test group compared to the control group at times A2 to A5 (p < 0.05) (Table 5). Additionally, a significant reduction in HR was observed in the test group patients at times A2 (p = 0.003) and A4 (p = 0.009). However, no differences in SpO2 were identified between the evaluated groups (p > 0.05) (Table 6).
The sedation assessment showed a significant difference between the groups at all analyzed times (S0, p = 0.002; S1, p < 0.001; S2, p = 0.003), indicating a positive effect of pregabalin on sedation. The most significant difference between the test group (2.42 ± 0.50) and the control group (1.32 ± 0.48) occurred at time S1 (p < 0.001) (Table 7). Intragroup analysis also revealed statistical differences in both groups across the evaluated times (S0 to S2), indicating that patients were mostly cooperative, oriented, and calm at the end of the procedures [p < 0.001].
The global assessment of patient satisfaction with the procedures, measured using the Likert scale, revealed a significant difference between the study groups (p < 0.001). The mean score of the test group (3.45 ± 0.68), classified as “very good,” was higher than that of the control group (2.58 ± 0.76), classified as “good.” These results indicate that the combination of pregabalin with dexamethasone provided greater comfort and satisfaction to patients at the end of the study.
Adverse effects/complications
Overall, three patients in the control group experienced adverse effects, including headache (n = 2) and nausea (n = 1). On the other hand, five patients in the intervention group reported adverse effects, such as drowsiness (n = 3), dizziness (n = 1), and nausea (n = 1). There were no reports of complications resulting from the surgical procedures in any of the patients in the study groups.
Discussion
In Oral and Maxillofacial Surgery, third molar extraction emerges as the most commonly performed procedure, characterized by triggering a series of postoperative clinical manifestations such as pain, swelling, and trismus, resulting from the inflammatory process. Additionally, due to its traumatic nature, this procedure often elicits anxiety at different levels in patients, which may anticipate the painful sensation or increase perceived pain levels postoperatively [6]. Approaching third molar extraction as a study model offers an advantage by minimizing interindividual variability in the analysis of pain and anxiety. This strategic choice allows for the comparison of distinct interventions using the same individual as a reference, eliminating potential idiosyncratic differences among participants that could interfere with the results. Therefore, the selection of this specific surgical procedure for the current study was based on the ease of participant recruitment, reproducibility of the model, and consistency provided for the analysis of the variables of interest [24].
Pregabalin in pain control and preemptive analgesia
Effective pain control is crucial for both patient well-being and surgical practice. Inadequate pain management can not only lead to unfavorable outcomes in the immediate postoperative period but also increase the risk of developing chronic and persistent pain conditions [25]. A deep understanding of the pathophysiological mechanisms of pain has sparked growing interest in investigating innovative approaches to achieve preemptive analgesia in acute tissue trauma contexts, such as third molar surgeries. Preemptive analgesia, defined as pharmacological treatment initiated preoperatively, active during intraoperative, and/or maintained postoperatively, aims to mitigate the physiological consequences of painful sensations. The primary goal of this therapy is to prevent nociceptor sensitization, both centrally and peripherally, aiming at reducing or preventing postoperative discomfort [26].
The strategy of multimodal preemptive analgesia involves the administration of therapy with two or more pharmacological agents, each with distinct mechanisms of action, which may exhibit additive or synergistic effects in pain prevention or reduction [9]. The combination of drugs aims to achieve three main objectives. First, it seeks to reduce acute pain during the surgical procedure and in the postoperative period. Secondly, it aims to prevent pathological pain modulation by the central nervous system (CNS), preventing the memorization of painful experiences. Thirdly, it aims to prevent the chronification of pain in the postoperative period. In cases where patients present with pain prior to the procedure, they may have developed nociceptor sensitization centrally, making preemptive analgesia less effective in these scenarios [27]. Thus, in order to assess the real efficacy of preemptive analgesia, none of the patients included in the sample of this study presented with pre-existing pain prior to the procedure in any of the third molars. However, a literature review conducted by Bhavaraju et al. [28] analyzed the effects of preemptive administration of gabapentinoids in oral and maxillofacial surgeries, such as third molar extractions, orthognathic surgery, and mandibular fracture surgery, the results showed that pregabalin was effective even in situations where painful stimuli were already present preoperatively.
In the systematic review and meta-analysis conducted by Zhang et al. [29], the authors investigated the effects of preemptive administration of pregabalin in laparoscopic cholecystectomy surgeries. The results suggested positive effects of the drug in reducing pain and a lower postoperative fentanyl consumption. The study by Degirmenci and Yalcin [23] addressed the preemptive use of pregabalin in third molar surgeries, observing positive effects on pain control, although no significant differences in the amount of rescue analgesics consumed were identified. Similarly, the results of the present study suggested a positive influence of the combination of pregabalin with dexamethasone on pain reduction in almost all analyzed periods when compared to placebo with dexamethasone (2,4,6,8,12,16,24, and 48 h) [p < 0.001]. Additionally, there was a decrease in the consumption of rescue analgesics and a longer period without the need for analgesics (61.03 ± 21.32) [p < 0.001].
In contrast to these findings, Cheung et al. [30] did not identify benefits of preemptive administration of pregabalin in third molar surgeries, associating the positive effects with postoperative administration. The clinical trial conducted by Olmedo-Gaya et al. [4] used pregabalin in surgeries for third molar extraction, both one hour before the procedure and one hour after, and found no positive effect on pain reduction, despite the evident impact on reducing the consumption of rescue analgesics. Similarly, the retrospective study by Sisa et al. [31] did not identify positive effects of preemptive administration of pregabalin on pain or on reducing opioid consumption in robot-assisted laparoscopic prostatectomy.
Following third molar surgeries, chemical pain mediators reach peak concentrations between the second and third postoperative days, triggering vasodilation and increased vascular permeability. This phenomenon results in fluid transudation and leukocyte diapedesis, processes directly associated with the onset of pain, edema, and limitation of oral function in surgical procedures involving impacted teeth [32,33,34]. Postoperative pain, characterized by its acute nature, typically peaks between 3 and 6 h after the procedure and may persist for a period of 48 to 72 h [26, 32, 35]. The results of the present study are not in line with the literature, as the peak of pain occurred at 8 and 12 h in the control and experimental groups, respectively. Additionally, after 72 h, a significant reduction in pain intensity was observed in both groups, indicating the possibility of a natural and progressive remission of pain during this period.
The mechanism of action of pregabalin as an anticonvulsant, analgesic, and anxiolytic is not yet fully elucidated, although the predominant hypothesis suggests blockade of calcium ion influx induced by depolarization in voltage-dependent calcium channels, specifically types P, Q, and N. This blockade results in the inhibition of the release of excitatory nociceptive neurotransmitters such as glutamate and noradrenaline [36]. Pregabalin exerts its effects by binding to the alpha-2/delta-1 (α2δ1) subunit of voltage-dependent calcium channels present in presynaptic neurons, widely distributed in the central and peripheral nervous systems [37].
Neurogenic pain is associated with postoperative pain, manifesting through hyperalgesia or allodynia. Physiological, clinical, and pharmacological similarities indicate a correlation between postoperative and neuropathic pain. Sensitization of primary afferent neurons (located at the site of stimulation) and secondary neurons (present in the dorsal horn of the spinal cord) plays a crucial role in the propagation and maintenance of both types of pain. Glutamate, an excitatory neurotransmitter, is directly involved in both pain processes. Furthermore, drugs such as opioids, local anesthetics, and gabapentinoids have shown efficacy in treating both neuropathic and postoperative pain, indicating an association in response to certain pharmacological treatments [38].
In the context of pharmacokinetics and pharmacodynamics, the literature highlights that plasma concentration of pregabalin is directly related to the administered dose, exhibiting rapid, predictable, and linear kinetics. Notably, pregabalin does not induce dependence, does not bind to plasma proteins, is excreted unchanged by the kidneys, undergoes no hepatic metabolism, and shows low potential for interaction with other drugs. The substance’s peak concentration is reached approximately 1 h after administration, justifying the choice of this timing in the present study to achieve maximum effects. The elimination half-life is on average 6.3 h, and its effects remain present for up to 24–48 h after ingestion when it is completely eliminated [30, 39]. The results of this clinical investigation demonstrate that the effects of pregabalin were able to influence pain reduction up to 48 h postoperatively, thus aligning with the existing literature.
The determination of the ideal concentration of pregabalin for preemptive analgesia and anxiety reduction is still not consensual in the literature. Higher concentrations are often associated with better effects but also with increased side effects. In many reports on the analgesic effects of pregabalin, the dose of 300 mg was considered high, and postoperative adverse effects such as dizziness and drowsiness were frequently observed [40]. The clinical trial conducted by Hill et al. [35] in third molar surgeries found a positive effect of 300 mg pregabalin on reducing postoperative pain, but a higher incidence of adverse effects such as dizziness, drowsiness, and vomiting was noted. Some studies suggest that the most clinically effective dose, with fewer adverse effects, is 150mg [17, 41]. The clinical study by Ahiskalioglu et al. [5] used 150 mg of pregabalin without association with anti-inflammatories in controlling pain in bimaxillary orthognathic surgery and obtained positive results. The study by Degirmenci and Yalcin [23] demonstrated significant effects on pain control with a concentration of 150 mg of pregabalin associated with intravenous ibuprofen, without significant adverse effects. Similarly, Cillo, Dattilo et al. [10] used 150 mg pregabalin in combination with 400 mg celecoxib in a clinical trial using orthognathic surgery for maxillomandibular advancement and obtained positive results in pain control and reduction of opioid analgesic consumption without considerable adverse effects.
In the present study, pregabalin 150 mg associated with 8 mg of dexamethasone, administered 1 h before surgery, demonstrated efficacy in pain control at nearly all postoperative time points compared to placebo associated with 8 mg of dexamethasone. However, adverse effects such as drowsiness, dizziness, and nausea were associated with pregabalin use. In contrast, Said et al. [42], in a retrospective cohort study involving patients undergoing orthognathic surgery, did not find a positive effect on pain in patients using pregabalin 150 mg compared to patients in the control group, with no significant associated adverse effects.
Pregabalin in anxiety control
Anxiety can trigger emotional changes and abrupt modifications in the activity of the autonomic nervous system, causing acute fluctuations in the circulatory system and vagal reflex. This phenomenon usually results in a prevalence of parasympathetic system action, triggering bradycardia, syncope, or even cardiac arrhythmias [43]. Dental treatment-related phobia can precipitate anxiety crises, leading to hemodynamic alterations and manifestations such as sweating, tremors, arrhythmias, and vasovagal reactions. Traditionally, benzodiazepines and antidepressants are the preferred substances for relieving anxiety symptoms, although they present long-lasting side effects such as cognitive impairment, physical dependence, and withdrawal crises. Additionally, selective serotonin reuptake inhibitors and serotonin-noradrenaline reuptake inhibitors demonstrate limited efficacy in reducing somatic anxiety [11].
The literature suggests that pregabalin may contribute to the clinical improvement of both psychic and somatic symptoms of anxiety, resembling the effect of benzodiazepines, however, over a shorter treatment period and with less prominent adverse effects [11]. Furthermore, in recent years, pregabalin has been explored in the treatment of anxiety disorders such as Generalized Anxiety Disorder (GAD) [44], as well as in the role of sleep modulator. Although the peak absorption of pregabalin is observed one hour after administration, it is suggested that the maximum anxiolytic effect occurs within 3 to 4 h after a single dose [45]. In contrast to the expectations derived from the literature, the present clinical study revealed an influence of pregabalin on anxiety reduction, even when only the period of 1 h after substance administration was considered.
The assessment of anxiety’s impact on dental treatment can be conducted through subjective tools. Additionally, monitoring hemodynamic parameters, such as BP, HR, and SpO2, can be performed at different pre and intraoperative time points [20, 46]. The clinical study by Jokela et al. [12], which addressed laparoscopic hysterectomy surgeries, demonstrated equivalent anxiolytic effects between doses of 75 or 150 mg of pregabalin and 5 mg of diazepam, administered orally. In this study, the group of patients receiving diazepam showed a higher incidence of adverse effects. A systematic review conducted by Torres-Gonzalez et al. [17] on the preoperative administration of pregabalin to control anxiety in patients undergoing surgical procedures concluded that a dose of 75 mg of pregabalin before surgery reduces anxiety and stabilizes intraoperative hemodynamic status. However, the administration of pregabalin 150 mg at least 1 h before surgery appears to provide more effective anxiety control without causing significant adverse effects. The results of the present study are consistent with the findings from the literature. Pregabalin 150 mg was associated with a significant reduction in mean BP (SBP, DBP, MAP) from A2 to A5 compared to the control group (p < 0.001). Additionally, a significant reduction was also observed in the experimental group in HR at times A2 (p < 0.003) and A4 (p < 0.009). However, it is noteworthy that, according to Gupta et al. [47], oral premedication with pregabalin 150 mg reduces mean BP but not HR after induction with propofol for laryngoscopy and intubation.
The State-Trait Anxiety Inventory (STAI), a subjective assessment instrument, evaluates state and trait anxiety in two distinct questionnaires [48]. The results of the present study suggest a positive influence on anxiety reduction in the test group (41.19 ± 6.45) compared to the control group (47.32 ± 6.47) regarding STAI-S (p < 0.001). This finding is inconsistent with the randomized clinical trial in elective outpatient surgeries by Nimmaanrat et al. [45], which compared the efficacy of 150 mg pregabalin with 10 mg diazepam and placebo through STAI-State and concluded that there was no significant reduction in preoperative anxiety after receiving pregabalin, diazepam, or placebo.
Another subjective approach to assess anxiety, employed in this study, was the Visual Analog Scale (VAS). The results indicate a significant reduction (p < 0.001) in anxiety levels in both groups between the initial consultation and 1 h after medication. Additionally, 1 h after medication, a significant reduction in anxiety was observed in the test group compared to the control group. In line with this, a study conducted by Spreng et al. [49], where pregabalin was administered to patients undergoing discectomy, showed a significant reduction in preoperative anxiety score assessed by VAS. In minor procedures performed under local anesthesia, Elrashidy et al. [50] found that patients scheduled for vitreoretinal surgery who received pregabalin preoperatively had lower anxiety compared to the placebo group. A systematic review conducted by Torres-González et al. [17] concluded that preoperative administration of a single dose of 150 mg pregabalin appears to be effective in significantly reducing anxiety. However, the systematic review by Mishriky et al. [41] did not find a positive association between preoperative pregabalin use and anxiety reduction measured by VAS in elective surgeries.
Pregabalin in sedation
Among gabapentinoids, pregabalin stands out as the agent with the most sedative effect when compared to gabapentin, without triggering significant intraoperative hemodynamic changes [51]. The sedative effect associated with pregabalin is often considered an adverse or unexpected phenomenon in clinical settings [12, 52]. However, the ability to induce sedation can be interpreted as an additional advantage in oral surgery procedures, especially for patients with high levels of anxiety and reduced cooperation during the procedure. The literature suggests the potential for increased perioperative sedation level provided by pregabalin, as well as its use as pre-anesthetic medication to attenuate pressor response during intubation [52], although some studies indicate that its preoperative administration does not result in reduced propofol requirement for general anesthesia induction [53]. However, the sedative effect of pregabalin has not been fully characterized, and its mechanism is not fully elucidated [40].
Oral administration of pregabalin induces a sufficient sedative effect during surgical procedures, as highlighted by Karube et al. [40]. In a randomized clinical trial conducted by Samarah et al. [54], where patients underwent surgeries in the lower lumbar spine region, researchers evaluated the sedative effects of pregabalin at a dose of 150 mg, concluding that the substance had positive effects without causing significant side effects. The systematic review and meta-analysis by Mishricky et al. [41] also supported the efficacy of preoperative pregabalin administration in postoperative analgesia and sedation. Similarly, the results of the present study suggest a positive effect of pregabalin at a dose of 150 mg compared to the control group at all evaluated time points. However, it is important to note that hemodynamic parameters showed significant changes between different evaluated time points. In contrast, in the study conducted by White et al. [55], pregabalin at a dose of 150 mg did not demonstrate efficacy in promoting sedation in outpatient surgeries, with only the concentration of 300 mg showing positive effects before anesthesia induction and in the early postoperative period.
Adverse effects
Adverse effects attributed to pregabalin are generally associated with its chronic use and at high doses, with common symptoms including drowsiness, dizziness, dry mouth, peripheral edema, blurred vision, weight gain, myoclonus, and gynecomastia [56]. In the context of Oral and Maxillofacial Surgery, a study conducted by Ahiskalioglu et al. [5] addressed the assessment of patients undergoing orthognathic surgery, highlighting that the placebo group showed a higher incidence of nausea and vomiting compared to the group treated with pregabalin at a dose of 150 mg. It is pertinent to note that despite this discrepancy, the observed disparity did not reach statistical significance.
In the specific scenario of third molar surgery, Hill et al. [35] identified adverse effects such as dizziness, drowsiness, and vomiting, being more prevalent in the group receiving pregabalin at a dose of 300 mg. Results from Olmedo-Gaya et al. [4] pointed out drowsiness and nausea as the most prominent adverse effects related to the use of pregabalin at a dose of 75 mg, showing a significant difference when compared to the control group. Conversely, Degirmenci and Yalcin [23] did not observe a significant difference between the studied groups, although adverse effects were more frequent in groups receiving pregabalin at doses of 75 mg and 150 mg. Additionally, Cheung et al. [30] reported that approximately 5.9% of individuals in the group receiving pregabalin at a dose of 75 mg experienced postoperative dizziness, although this rate was not sufficient to generate a significant difference compared to the control group.
Similarly, in the present study, five patients reported adverse effects such as drowsiness, dizziness, and nausea, while three patients in the control group also reported adverse effects, including headache and nausea. It is noteworthy that the adverse effects attributed to pregabalin were not considered significant. This finding reinforces the importance of a cautious approach in interpreting adverse effects associated with pregabalin, highlighting the need for broader analyses and consideration of different surgical contexts for a more precise understanding of the safety profile of this substance.
Limitations and future perspectives
Conducting clinical studies is not devoid of challenges, and the efficient management of variables emerges as a significant issue in much of this research. In the present study, participant heterogeneity emerges as a relevant aspect, encompassing differences in demographic characteristics and individual pain sensitivity, factors that may influence the response to pregabalin. These variations constitute a barrier to generalizing results to different patient groups. Another point to be carefully considered is the choice of assessment criteria for pain, anxiety, and sedation, as this selection can introduce bias into the results. The inherent subjectivity of measures such as the VAS may compromise data consistency, underscoring the need to consider objective approaches whenever possible.
The limited postoperative follow-up period emerges as a relevant gap, especially considering the long-term effects of pregabalin, particularly regarding anxiety. Studies with longer follow-up periods could provide additional insights into the continued efficacy and long-term safety of this intervention. Additionally, it is prudent to limit the generalization of the results of the present study to other surgical contexts or medical conditions, given the specificity of oral surgery. The efficacy and safety of pregabalin may manifest variability in different clinical scenarios, requiring a contextualized analysis. These considerations, based on scientific principles, are essential for a robust interpretation of the results of the clinical study in question and offer valuable insights for future research aimed at addressing these limitations more comprehensively and in depth.
The use of pregabalin as an integral part of multimodal preemptive analgesia approaches in third molar surgeries outlines various future perspectives that can be investigated to enhance the effectiveness and safety of this protocol. Among the avenues to be explored in subsequent studies, evaluating the efficacy of pregabalin in synergy with other analgesic and anxiolytic agents stands out. Multimodal protocols, which encompass different categories of medications, have the potential to potentiate analgesic effects, reducing the need for high doses and, consequently, mitigating potential adverse effects.
Expanding outcome assessments to cover not only pain intensity but also functional outcomes, oral health-related quality of life, and time to return to normal activities represents a more comprehensive approach to understanding the impact of pregabalin on post-surgical recovery. Deepening the understanding of pregabalin’s mechanisms of action in the context of preemptive analgesia, through neurophysiological studies, can provide crucial insights into the effects of this substance on central and peripheral sensitization, as well as on the modulation of emotional responses to pain. Furthermore, conducting comparative studies to assess the efficacy and safety of pregabalin compared to other pharmacological and non-pharmacological approaches for pain and anxiety control in third molar surgeries would contribute to establishing more informed and personalized therapeutic choices in this specific clinical context.
Conclusion
The results obtained in this study indicate that pregabalin co-administered with dexamethasone preoperatively demonstrated significant efficacy in controlling postoperative pain. The significant reduction in pain intensity, decreased rescue analgesic consumption, coupled with lower incidence of anxiety at the evaluated time points, suggests that pregabalin played a crucial role in mitigating the negative aspects of the postoperative period in third molar surgeries. Although a significant association between pregabalin use and sedative effects was observed, it is important to note that these effects were controlled and did not compromise patient safety or recovery. Pregabalin-induced sedation may be considered an advantage in surgical procedures, especially in cases where anxiety is a relevant factor. Therefore, the results of this clinical trial provide substantial support for the inclusion of pregabalin, especially in combination with dexamethasone, as an integral part of multimodal strategies for pain, anxiety, and sedation control in third molar surgeries. This approach offers potential benefits for both effective pain management and improvement of patients’ emotional comfort in the postoperative period.
Data availability
No datasets were generated or analysed during the current study.
References
Shah AV, Kumar KVA (2013) Comparative evaluation of pre-emptive analgesic efficacy of intramuscular Ketorolac Versus Tramadol following third molar surgery. J Maxillofac Oral Surg 12:197–202. https://doi.org/10.1007/s12663-012-0420-4
de Carvalho RWF, Vasconcelos BC (2018) Pernambuco index: predictability of the complexity of surgery for impacted lower third molars. Int J Oral Maxillofac Surg 47:234–240. https://doi.org/10.1016/j.ijom.2017.07.013
González-Martínez R, Jovani-Sancho MDM, Cortell-Ballester I (2017) Does Psychological Profile influence on third molar extraction and Postoperative Pain? J Oral Maxillofac Surg 75:484–490. https://doi.org/10.1016/j.joms.2016.09.023
Olmedo-Gaya MV, Manzano-Moreno FJ, Galvez-Mateos R, González-Rodriguez MP, Talero-Sevilla C, Vallecillo-Capilla M (2015) Oral pregabalin for postoperative pain relief after third molar extraction: a randomized controlled clinical trial. Clin Oral Invest 20:1819–1826. https://doi.org/10.1007/s00784-015-1657-3
Ahiskalioglu A, İnce İ, Aksoy M, Yalcin E, Ahiskalioglu EO, Kilinc A (2016) Effects of a single-dose of pre-emptive Pregabalin on Postoperative Pain and Opioid Consumption after double-Jaw surgery: a Randomized Controlled Trial. J Oral Maxillofac Surg 74:53e1–53e7. https://doi.org/10.1016/j.joms.2015.09.008
Barbalho JC, Vasconcellos RJH, de Morais HH, Santos LAM (2017) Effects of co-administered dexamethasone and nimesulide on pain, swelling, and trismus following third molar surgery: controlled clinical trial. Int J Oral Maxillofac Surg 46:236–242. https://doi.org/10.1016/j.ijom.2016.10.011
Almeida RDAC, Lemos CAA, de Moraes SLD, Pellizzer EP (2019) Efficacy of corticosteroids versus placebo in impacted third molar surgery: systematic review and meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg 48:118–131. https://doi.org/10.1016/j.ijom.2018.05.023
Au AHY, Choi SW, Cheung CW, Leung YY (2015) The efficacy and clinical safety of various analgesic combinations for Post-operative Pain after third molar surgery: a systematic review and Meta-analysis. PLoS ONE 10:e0127611. https://doi.org/10.1371/journal.pone.0127611
Demirhan A, Yasar U, Akcan T, Murat A (2013) Effect of Pregabalin and Dexamethasone Addition to Multimodal Analgesia on postoperative Analgesia following rhinoplasty surgery. Aesthetic Plast Surg 37:1100–1106. https://doi.org/10.1007/s00266-013-0207-0
Cillo Junior JE, Dattilo DJ (2014) Pre-emptive analgesia with Pregabalin and celecoxib decreases postsurgical pain following maxillomandibular advancement surgery: a randomized control clinical trial. J Oral Maxillofac Surg 72:1909–1914. https://doi.org/10.1016/j.joms.2014.05.014
Generoso MB, Trevizol AP, Kasper S, Cho HJ, Cordeiro Q, Shiozawa P (2017) Pregabalin for generalized anxiety disorder: an updated systematic review and meta-analysis. Int Clin Psychopharmacol 32:49–55. https://doi.org/10.1097/YIC.0000000000000147
Jokela R, Ahonen J, Tallgren M, Haanpa M, Korttila KA (2008) Randomized controlled trial of perioperative administration of Pregabalin for pain after laparoscopic hysterectomy. Pain 134:106–112. https://doi.org/10.1016/j.pain.2007.04.002
Choi YS, Jae-Kwang S, Jong WS, Jong CK, Young CY, Young LK (2013) Combination of Pregabalin and Dexamethasone for Postoperative Pain and functional outcome in patients undergoing lumbar spinal surgery. Clin J Pain 29:9–14. https://doi.org/10.1097/AJP.0b013e318246d1a9
Mathiesen O, Jørgensen DG, Hilsted KL, Trolle W, Stjernholm P, Christiansen H, Hjortsø NC, Dahl JB (2011) Pregabalin and dexamethasone improves post-operative pain treatment after tonsillectomy. Acta Anaesthesiol Scand 55:297–305. https://doi.org/10.1111/j.1399-6576.2010.02389.x
Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF (2008) CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med 5:e20. https://doi.org/10.1371/journal.pmed.0050020
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Torres-González MI, Manzano-Moreno FJ, Vallecillo-Capilla MF, Olmedo-Gaya MV, Manzano-Moreno FJ (2020) Preoperative oral pregabalin for anxiety control: a systematic review. Clin Oral Investig 24:2219–2228. https://doi.org/10.1007/s00784-020-03352-y
Torun AC, Yilmaz MZ, Ozkan N, Ustun B, Koksal E, Kaya C (2017) Sedative-analgesic activity of remifentanil and effects of preoperative anxiety on perceived pain in outpatient mandibular third molar surgery. Int J Oral Maxillofac Surg 46:379–384. https://doi.org/10.1016/j.ijom.2016.11.005
Aitken RCB (1969) A Growing Edge of Measurement of Feelings [Abridged]: Measurement of Feelings Using Visual Analogue Scales. Proceedings of the Royal Society of Medicine 62: 989–993. https://doi.org/10.1177/003591576906201005
de Morais HH, Vasconcellos RJH, Santos TS, Rocha NS, Araújo FAC, de Carvalho RW (2013) Clinical study of hemodynamic changes comparing 4% articaine hydrochloride with 1:100,000 and 1:200,000 epinephrine. Oral Surg Oral Med Oral Pathol Oral Radiol 116:e14–22. https://doi.org/10.1016/j.oooo.2011.10.043
Braz JRC (1996) Artigo Especial Monitorização Da Oxigenação E Da Ventilação. Rev Bras Anestesiol 46:223–240
Abdullah WA, Sheta SA, Nooh NS (2011) Inhaled methoxyflurane (Penthrox®) sedation for third molar extraction: a comparison to nitrous oxide sedation. Aust Dent J 56:296–301. https://doi.org/10.1111/j.1834-7819.2011.01350.x
Diniz JA, Barbirato DDS, do Nascimento EHL, Pontual ADA, Dourado ACAG, Laureano Filho JR (2022) Tomographic evaluation of the effect of simvastatin topical use on alveolar bone microarchitecture, pain and swelling after mandibular third molar extraction: a randomized controlled trial. Clin Oral Investig 26:3533–3545. https://doi.org/10.1007/s00784-021-04322-8
Lima CAA, Favarini VT, Torres AM, da Silva RA, Sato FRL (2017) Oral dexamethasone decreases postoperative pain, swelling, and trismus more than diclofenac following third molar removal: a randomized controlled clinical trial. Oral Maxillofac Surg 21:321–326. https://doi.org/10.1007/s10006-017-0635-0
Costa FW, Esses DF, de Barros Silva PG, Carvalho FS, Sá CD, Albuquerque AF, Bezerra TP, Ribeiro TR, Sá Roriz Fonteles C, Soares EC (2015) Does the preemptive use of oral nonsteroidal anti-inflammatory drugs reduce Postoperative Pain in Surgical removal of third molars? A Meta-analysis of Randomized clinical trials. Anesth Prog 62:57–63. https://doi.org/10.2344/0003-3006-62.2.57
Shah R, Mahajan A, Shah N, Dadhania AP (2012) Preemptive analgesia in third molar impaction surgery. Natl J Maxillofac Surg 3:144–147. https://doi.org/10.4103/0975-5950.111368
Bhavaraju SA, Vorrasi JS, Talluri S, Kalladka M, Khan J (2022) Pre-emptive administration of gabapentinoids to reduce postoperative pain and opioid usage following oral and maxillofacial surgical procedures. Oral Surg 15:106–115. https://doi.org/10.1111/ors.12617
Zhang Y, Wang Y, Zhang X (2017) Effect of pre-emptive pregabalin on pain management in patients undergoing laparoscopic cholecystectomy: a systematic review and meta-analysis. Int J Surg 44:122–127. https://doi.org/10.1016/j.ijsu.2017.06.047
Degirmenci A, Yalcin E (2019) The effect of pregabalin and ibuprofen combination for pain after third molar surgery. Niger J Clin Pract 22:503–510. https://doi.org/10.4103/njcp.njcp_492_18
Cheung CW, Choi WS, Leung YY, Lui F, Ng JK, Hei-Ho AM, Irwin MG (2012) A double-blind randomized crossover study to evaluate the timing of Pregabalin for third molar surgery under local anesthesia. J Oral Maxillofac Surg 70:25–30. https://doi.org/10.1016/j.joms.2011.03.056
Sisa K, Huoponen S, Ettala O, Antila H, Saari TI, Uusalo P (2021) Effects of pre-emptive pregabalin and multimodal anesthesia on postoperative opioid requirements in patients undergoing robot-assisted laparoscopic prostatectomy. BMC Urol 21:14. https://doi.org/10.1186/s12894-021-00785-9
Ong CK, Seymour RA (2003) Pathogenesis of postoperative oral surgical pain. Anesth Prog 50:5–17
Laureano Filho JR, Maurette PE, Allais M, Cotinho M, Fernandes C (2008) Clinical comparative study of the effectiveness of two dosages of Dexamethasone to control postoperative swelling, trismus and pain after the surgical extraction of mandibular impacted third molars. Med Oral Patol Oral Cir Bucal 13:E129–E132
da Costa Araújo FA, de Santana Santos T, de Morais HH, Laureano Filho JR, de Oliveira E, Silva ED, Vasconcellos RJ (2012) Comparative analysis of preemptive analgesic effect of tramadol chlorhydrate and nimesulide following third molar surgery. J Craniomaxillofac Surg 40:e346–e349. https://doi.org/10.1016/j.jcms.2012.01.018
Hill CM, Balkenohl M, Thomas DW, Walker R, Mathé H, Murray G (2001) Pregabalin in patients with postoperative dental pain. Eur J Pain 5:119–124. https://doi.org/10.1053/eujp.2001.0235
Chincholkar M (2018) Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth 120:1315–1334. https://doi.org/10.1016/j.bja.2018.02.066
Karaca O, Pınar HU, Turk E, Dogan R, Ahiskalioglu A, Solak SK (2019) Effects of single-dose preemptive Pregabalin and intravenous ibuprofen on postoperative opioid consumption and Acute Pain after laparoscopic cholecystectomy. J Invest Surg 32:189–195. https://doi.org/10.1080/08941939.2017.1386738
Gilron I (2006) Review article: the role of anticonvulsant drugs in postoperative pain management: a bench-to-bedside perspective. Can J Anaesth 53:562–571. https://doi.org/10.1007/BF03021846
Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P (2010) A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 49:661–669. https://doi.org/10.2165/11536200-000000000-00000
Karube N, Ito S, Sako S, Hirokawa J, Yokoyama T (2017) Sedative effects of oral pregabalin premedication on intravenous sedation using propofol target-controlled infusion. J Anesth 31:586–592. https://doi.org/10.1007/s00540-017-2366-7
Mishriky BM, Waldron NH, Habib AS (2015) Impact of Pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 114:10–31. https://doi.org/10.1093/bja/aeu293
Said AM, Zubovic E, Ha AY, Skolnick GB, Naidoo SD, AuBuchon J, Patel KB (2021) Effects of single-dose preoperative Pregabalin on Postoperative Pain and Opioid Consumption in Cleft Orthognathic surgery. J Craniofac Surg 32:517–520. https://doi.org/10.1097/SCS.0000000000007109
Gadve VR, Shenoi R, Vats V, Shrivastava A (2018) Evaluation of anxiety, Pain, and hemodynamic changes during Surgical removal of Lower Third Molar under local anesthesia. Ann Maxillofac Surg 8:247–253. https://doi.org/10.4103/ams.ams_216_18
Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D (2015) Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol 30:183–192. https://doi.org/10.1097/YIC.0000000000000078
Nimmaanrat S, Charuenporn B, Jensen MP, Geater AF, Tanasansuttiporn J, Chanchayanon T (2023) The anxiolytic effects of preoperative administration of pregabalin in comparison to diazepam and placebo. Sci Rep 13:9680. https://doi.org/10.1038/s41598-023-36616-0
Kupeli I, Gülnahar Y (2020) Comparing different music genres in decreasing Dental anxiety in young adults who underwent third molar surgery in Turkey: Randomized Controlled Trial. J Oral Maxillofac Surg 78. https://doi.org/10.1016/j.joms.2019.11.029. 546.e1-546.e7
Gupta K, Bansal P, Gupta PK, Singh YP (2011) Pregabalin premedication - a new treatment option for hemodynamic stability during general anesthesia: a prospective study. Anesth Essays Res 5:57–62. https://doi.org/10.4103/0259-1162.84192
Yamashita K, Kibe T, Aoyama K, Ohno S, Kohjitani A, Sugimura M (2020) The state anxiety inventory is useful for Predicting the Autonomic Nervous System State of patients before the extraction of an impacted Mandibular Third Molar. J Oral Maxillofac Surg 78:538–544. https://doi.org/10.1016/j.joms.2019.11.030
Spreng UJ, Dahl V, Raeder J (2011) Effect of a single dose of pregabalin on post-operative pain and pre-operative anxiety in patients undergoing discectomy. Acta Anaesthesiol Scand 55:571–576. https://doi.org/10.1111/j.1399-6576.2011.02410.x
Elrashidy A, Khattab AM, Elseify ZA, Oriby ME (2021) Perioperative Anxiolytic and Analgesic effects of Pregabalin in Vitreo-Retinal surgery: a Randomized, double-blind study. Anesth Pain Med 11:e117414. https://doi.org/10.5812/aapm.117414
Thakur N, Aggarwal V, Patidar S (2019) Comparison of post-operative analgesic effect of oral gabapentin and oral pregabalin in patients undergoing total abdominal hysterectomy under spinal anaesthesia. Int J Sci Res 8:2277–8179. https://doi.org/10.36106/ijsr
Ibrahim E, Sultan W, Helal S, Abo-Elwafa H, Abdelaziz A (2019) Pregabalin and dexmedetomidine conscious sedation for flexible bronchoscopy: a randomized double-blind controlled study. Minerva Anestesiol 85:487–493. https://doi.org/10.23736/S0375-9393.18.12685-X
Moreau-Bussière F, Gaulin J, Gagnon V, Sansoucy Y, de Médicis E (2013) Preoperative pregabalin does not reduce propofol ED(50): a randomized controlled trial. Can J Anaesth 60:364–369. https://doi.org/10.1007/s12630-013-9885-y
Samarah BM, Shehada FA, Qaddumi J, Almasry NA, Alkhawaldeh A, ALBashtawy M, Alyahya M, ALBashtawy S, Al-Awamreh K, Saifan A, ALBashtawy B, Abdalrahim A, ALBashtawy Z (2023) A comparison of the preemptive effects of oral pregabalin and gabapentin on acute postoperative sedation and complications in patients undergoing lumbar spine surgery. J Perioper Pract 33:358–364. https://doi.org/10.1177/17504589221141799
White PF, Tufanogullari B, Taylor J, Klein K (2009) The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg 108:1140–1145. https://doi.org/10.1213/ane.0b013e31818d40ce
Bafna U, Rajarajeshwaran K, Khandelwal M, Verma AP (2014) A comparison of effect of preemptive use of oral gabapentin and Pregabalin for acute post-operative pain after surgery under spinal anesthesia. J Anaesthesiol Clin Pharmacol 30:373–377. https://doi.org/10.4103/0970-9185.137270
Acknowledgements
The authors express their gratitude to the University of Pernambuco (UPE) and the Oswaldo Cruz University Hospital (HUOC) for their support, as well as to the research team and study participants.
Funding
This study was funded by the authors themselves.
Author information
Authors and Affiliations
Contributions
All authors contributed and share responsibility for the conception, design, and execution of the research, as well as for the analysis, interpretation of the results, and writing of the article. J.A.D. conceived the study. J.A.D. and D.S.B. conducted literature searches and compiled the data. J.A.D., D.S.B., A.C.A.G.D., and J.R.L.F. devised the research methodology. J.A.D. and K.G.S. performed formal analysis. J.A.D. and S.M.C.M.F. conducted third molar surgeries. M.S.V.O. and V.L.B.O.L. managed and stored study data. J.A.D. and D.S.B. drafted the original manuscript, and prepared tables and figures. J.A.D., A.C.A.G.D., J.R.L.F., and D.S.B. critically reviewed and edited the manuscript. A.C.A.G.D. and J.R.L.F. supervised the research process.
Corresponding author
Ethics declarations
Consent to publish
Patients provided informed consent for the publication of their data and photographs.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diniz, J.A., Dourado, A.C.A.G., Barbirato, D. et al. Evaluation of the effects of pregabalin and dexamethasone coadministration on preemptive multimodal analgesia and anxiety in third molar surgeries: a triple-blind randomized clinical trial. Clin Oral Invest 28, 304 (2024). https://doi.org/10.1007/s00784-024-05700-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05700-8