Abstract
Objectives
This study aimed to investigate the regulatory roles of lncRNA MALAT1, miR-124-3p, and IGF2BP1 in osteogenic differentiation of periodontal ligament stem cells (PDLSCs).
Materials and methods
We characterized PDLSCs by employing quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analyses to evaluate the expression of key osteogenic markers including ALPL, SPP1, and RUNX2. Manipulation of lncRNA MALAT1 and miR-124-3p expression levels was achieved through transfection techniques. In addition, early osteogenic differentiation was assessed via Alkaline phosphatase (ALP) staining, and mineral deposition was quantified using Alizarin Red S (ARS) staining. Cellular localization of lncRNA MALAT1 was determined through Fluorescence In Situ Hybridization (FISH). To elucidate the intricate regulatory network, we conducted dual-luciferase reporter assays to decipher the binding interactions between lncRNA MALAT1 and miR-124-3P as well as between miR-124-3P and IGF2BP1.
Results
Overexpression of lncRNA MALAT1 robustly promoted osteogenesis in PDLSCs, while its knockdown significantly inhibited the process. We confirmed the direct interaction between miR-124-3p and lncRNA MALAT1, underscoring its role in impeding osteogenic differentiation. Notably, IGF2BP1 was identified as a direct binding partner of lncRNA MALAT1, highlighting its pivotal role within this intricate network. Moreover, we determined the optimal IGF2BP1 concentration (50 ng/ml) as a potent enhancer of osteogenesis, effectively countering the inhibition induced by si-MALAT1. Furthermore, in vivo experiments utilizing rat calvarial defects provided compelling evidence, solidifying lncRNA MALAT1’s crucial role in bone formation.
Conclusions
Our study reveals the regulatory network involving lncRNA MALAT1, miR-124-3p, and IGF2BP1 in PDLSCs’ osteogenic differentiation.
Clinical relevance
These findings enhance our understanding of lncRNA-mediated osteogenesis, offering potential therapeutic implications for periodontal tissue regeneration and the treatment of bone defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental plaque-induced periodontitis, a chronic infectious disease, enhances the slow deterioration of both hard and soft periodontal tissues. Currently, it affects almost 90% of the world’s population and is the main cause of tooth loss [1,2,3,4]. The restoration of periodontal tissue, with a focus on alveolar bone regeneration, presents the biggest obstacle in the treatment of periodontitis. Periodontal ligament stem cells (PDLSCs), which stand out for their strong capacity for self-renewal and adaptable multilineage differentiation potential, are essential stem cell repositories in periodontal tissues. Due to their features and accessibility, PDLSCs play a role as the best option for promoting periodontal tissue regeneration [5]. However, inflammation, senescence, and hypoxia are only a few of the multiple factors that might affect PDLSCs’ ability to produce bone [6,7,8]. Hence, a meticulous exploration into the determinants impinging on the osteogenic differentiation of PDLSCs promises to enhance the prospect of periodontal tissue regeneration.
Only 2% of the human genome is translated into proteins, despite the startling 80% transcription into RNA, indicating that the majority of the transcriptome is non-coding RNA [9]. The cell nucleus or cytoplasm is the home of long non-coding RNAs (lncRNAs), which are characterized by transcript lengths of more than 200 bp and orchestrate their functions by subcellular localization [10]. A growing amount of evidence currently highlights the diverse participation of lncRNAs across a range of cellular activities, despite their initial dismissal as transcriptional noise devoid of functional importance [11], including cell cycle, apoptosis, developmental differentiation, and genomic imprinting [12,13,14,15]. Additionally, lncRNAs actively participate in controlling the proliferation, differentiation, apoptosis, and activity of osteoblasts and osteoclasts while acting as crucial stewards of gene expression [16].
MiRNA, short for microRNA, is a class of endogenous, single-stranded, non-coding small RNAs encoded by genes. Typically ranging in length from approximately 20 to 24 nucleotides, miRNAs have been found to regulate about one-third of the human genome. They play crucial regulatory roles in various cellular processes within organisms, including proliferation, migration, and stem cell differentiation. The binding of miRNA to complementary sequences in the target mRNA results in post-transcriptional regulation, leading to the inhibition of the target mRNA’s expression. This interaction contributes to the formation of intricate regulatory networks [17, 18]. These lncRNAs commonly interact with miRNAs to take on crucial regulatory roles during the osteogenic process [19, 20]. The lncRNA MALAT1 has become a focus of investigation due to the wide range of biological functions it plays. It is able to regulate gene transcription, consequently playing crucial roles in a variety of fields including tissue inflammatory disease, oncogene development, cardiovascular remodeling, hepatic fibrosis, diabetes, and more [21,22,23,24]. The expression of lncRNA MALAT1 is significantly lower in patients with osteoporosis compared to healthy individuals [25]. In a whole-genome study on the Qatari population, low bone density was found to be closely associated with intronic variations in the lncRNA MALAT1 gene locus [26]. Research by Song et al. identified lncRNA MALAT1 as a biomarker for osteoporosis [27]. Additionally, lncRNA MALAT1 has been shown to positively regulate osteogenesis through mediating the miR-485-5p/WNT7B axis, miR-214-3p/BMP2 axis, miR-204/Smad4 axis, and miR-124-3p/IGF2BP1 axis [25, 28,29,30]. Bao et al. discovered that lncRNA MALAT1, by competitively inhibiting miR-140-5p, promotes odontogenic differentiation of dental pulp stem cells, suggesting its potential as a target for future tooth regeneration [31]. Furthermore, lncRNA MALAT1, by mediating the miR-155-5p/ETS1 signaling pathway, promotes osteogenic differentiation of periodontal ligament stem cells, providing a novel strategy for treating periodontitis [32]. Other studies indicate that multiple lncRNAs regulate the osteogenic differentiation of periodontal ligament stem cells [33].
Existing literature reports that lncRNA MALAT1 exerts its biological effects by targeting and inhibiting the expression of miR-124-3P, which is known for its significant inhibitory role in osteogenic differentiation [34,35,36,37]. Analysis using the starBase database revealed miR-124-3P as a potential target of lncRNA MALAT1, and insulin-like growth factor 2-RNA-binding protein 1 (IGF2BP1) can bind to miR-124-3P, forming a competitive endogenous RNA (ceRNA) network [25]. IGF2 is highly expressed in various tumors, participating in tumor cell survival, proliferation, and apoptosis [38]. miR-124-3p can inhibit the growth and migration of cervical cancer cells by targeting IGF2BP1 [39], and IGF2BP1 is involved in the progression of osteosarcoma [40, 41]. Previous studies have associated IGF2 with aging and osteogenesis [42, 43], as it can regulate bone cortical and trabecular characteristics by affecting osteoblasts and osteoclasts, thereby influencing radial bone growth [44]. RhIGF2 at 5 ng/mL promotes the proliferation and osteogenic/odontogenic differentiation potential of apical papilla stem cells [45]. However, limited research has been conducted on whether IGF2BP1 affects the osteogenic differentiation of PDLSCs. Based on this literature review, the focus of this study is to investigate whether lncRNA MALAT1 regulates the osteogenic differentiation of PDLSCs through the miR-124-3p/IGF2BP1 axis.
Materials and methods
Cell culture
PDLSCs were obtained from healthy premolars extracted for orthodontic reasons. The premolars were collected with informed consent from five physically healthy donors, whose ages ranged between 14 and 22 years (average age 18 years). The experimental protocol was approved by the Ethics Committee of the School of Stomatology, Jilin University (Approval number: SJDKQ2024017). The isolation and culture of PDLSCs were conducted following the previously described procedure [46].
The process involved gently scraping the periodontal ligament from the proximal 1/3 of the premolar, followed by a 5-min digestion with pancreatic enzyme. PDLSCs were then cultured in a growth medium (GM), comprising alpha minimum essential medium supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin and streptomycin, in a 5% CO2 environment at 37 °C. For osteogenic induction, hPDLSCs were cultured in an osteogenic medium (OM), which consisted of GM supplemented with b-glycerophosphate (10 mM), dexamethasone (100 nM), and vitamin C (200 mM). The culture medium was refreshed every two days.
Flow cytometry for cell phenotype identification
PDLSCs from the P3-P5 generation in the logarithmic growth phase were enzymatically digested and centrifuged at 1200 rpm for 6 min. The cell pellet was resuspended to prepare a cell suspension with a density of 1 × 106 cells/ml. The cell suspension was divided into 6 tubes, and antibodies CD31 (303105, Biolegend), CD34 (343503, Biolegend), CD45 (304005, Biolegend), CD46 (361011, Biolegend), STRO-1 (MA5-28636, Thermo), and Vimentin (699309, Biolegend) were added separately. After mixing, the tubes were kept in the dark at room temperature and incubated for 20 min. After washing twice with PBS, the cells were resuspended in 500 μL of PBS and analyzed using a flow cytometer.
Construction and transfection of cells with plasmids
The full-length cDNA of lncRNA MALAT1 was amplified and cloned into the pCDNA3.1 vector to generate the pCDNA3.1-MALAT1 construct (over-MALAT1) (Hanheng Biotech, Shanghai). siRNA-lncRNA MALAT1(si-MALAT1) and si-NC was supplied by Hanheng Biotech, Shanghai. miR-124-3p mimics, mimics NC, miR-124-3p inhibitor, and inhibitor NC were obtained from Ribobio Corporation, Guangzhou. when the cell density of PDLSCs reached 30–50%, the culture medium was replaced with Opti-MEM for transfection. Lipofectamine® 2000 was used as the transfection reagent. After 24–72 h of cell culture, cells were collected for subsequent experiments.
CCK8
The Cell Counting Kit-8 (CCK-8) (Solarbio, Beijing) assay was employed to assess cell proliferation. Transfected PDLSCs with overexpressed MALAT1, siRNA-MALAT1, and their respective control groups were digested using trypsin, washed with PBS, and seeded in 6-well plates at equal cell numbers. The cells were cultured in a 37 °C, 5% CO2 incubator for 24 h. Subsequently, 800 μL of culture medium was added to each well, gently shaken for even distribution, and then incubated at 37 °C for 2 h. Then, cells were returned to a 37 °C, CO2 incubator for 3 days. After that, on days 1, 2, and 3, the optical density (OD) value at 450 nm was measured using an enzyme-linked immunosorbent assay (ELISA) reader.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Cellular RNA was extracted using the TRIzol method (Invitrogen, USA). The RNA was then reverse transcribed into cDNA using the M-MLV reverse transcription kit (Promega, USA). Amplification was performed using the ABI StepOne Plus real-time fluorescence quantitative PCR system. GAPDH served as the internal reference for mRNA and lncRNA MALAT1, while U6 was used as the internal reference for miRNA. Data analysis was conducted using the 2–ΔΔCT method [47]. The primer sequences are provided in Supplementary Table S1.
Western blot analysis
Cells were lysed with cell lysis buffer, and the supernatant was collected after centrifugation. The protein concentration was determined using the BCA assay kit (Promega, USA). Samples were loaded into a 10% SDS–polyacrylamide gel for electrophoresis. Proteins were separated by electrophoresis and transferred to a PVDF membrane using a wet transfer method. The membrane was then blocked with BSA for 60 min. Primary antibodies including ALPL (Ab194297, 1:2000, Abcam), SPP1 (Af0227, 1:1000, Affinity), RUNX2 (Af5186, 1:1000, Affinity), and β-actin (Af5186, 1:2000, Abcam) were incubated overnight, followed by secondary antibodies (1:5000, Abcam) incubation. Protein bands were detected using an ECL imaging system and analyzed using Image J.
Alkaline phosphatase (ALP) staining and activity
ALP staining was performed using the ALP Staining Kit (Solarbio, Beijing). PDLSCs (5 × 104) were seeded in a 12-well plate and induced for osteogenesis for 7 days. Subsequently, the cells were fixed in 4% paraformaldehyde for 10 min, stained with the ALP Staining Kit, and then incubated at 37 °C, 5% CO2 for 30 min. The stained cells were observed after avoiding light. For the corresponding group, the culture medium was aspirated, and the cells were washed with PBS. Then, 1 mL of cell lysis buffer was added, and the cells were left at room temperature for 3 h. The cell lysate (1.5 ml) was collected in an EP tube, and 100 μL of the lysate was mixed with an equal volume of ALP reaction substrate. After incubating at room temperature for 30 min, the absorbance was measured using a spectrophotometer at 405 nm. Additionally, 20 μL of PDLSC cell lysate was used for total protein quantification, and the absorbance was measured at 630 nm. The ALP activity was calculated by dividing the ALP absorbance value by the total protein value.
Alizarin Red S (ARS) staining and quantification
Mineralized nodule formation was determined by ARS staining. The PDLSCs were inoculated into 12-well plates. After 14 days of osteogenic induction, PDLSCs were fixed in 4% polyoxymethylene for 10 min, washed with distilled water, and then stained with 0.1% ARS for 20 min. To quantitatively assess mineralized nodules, they were dissolved in 1 ml of 10% cetylpyridinium chloride for 1 h and then quantified spectrophotometrically at 570 nm. ARS intensity was normalized to total protein concentration.
Fluorescence in situ hybridization (FISH) experiment
To initiate the experiment, cells are seeded in a 24-well plate at a density of 1 × 104 cells/well to achieve 60%-70% confluence. Following this, the cells are fixed with 4% paraformaldehyde and permeabilized using a pre-cooled permeabilization buffer at 4 °C. For probe detection, the process begins with a 30-min prehybridization step at 37 °C. A hybridization solution is prepared at the same temperature, incorporating a 20 μM lncRNA FISH probe (Ribobio, Guangzhou). The cells are then incubated overnight at 37 °C in the dark. Subsequent steps involve washing the cells with hybridization wash solutions at 42 °C to reduce background interference. Following this, a 1X DAPI staining is performed in the dark for 10 min. The cells are finally mounted on slides under dark conditions for fluorescence detection.
Dual-luciferase assay
Binding sites between lncRNA MALAT1 and miR-124-3p, as well as between miR-124-3p and IGF2BP1, were predicted using starBase database. Wild-type (lncRNA MALAT1-WT) and mutant-type (lncRNA MALAT1-MUT) constructs of the lncRNA MALAT1, along with wild-type (IGF2BP1-WT) and mutant-type (IGF2BP1-MUT) constructs of IGF2BP1, were generated (GeneCopoeia, USA) and cloned into luciferase reporter gene vectors. PDLSCs were seeded into a 6-well plate and cultured until reaching 60–80% confluency. After detachment using trypsin–EDTA, the cells were seeded into a 24-well plate and transfected with miR-124-3p mimics, miR-NC, lncRNA MALAT1-WT, lncRNA MALAT1-MUT, IGF2BP1-WT, and IGF2BP1-MUT. After 24 h, the Dual-Luciferase Reporter Assay System (Saiweier, Wuhan) was employed to measure the relative luciferase activity in each group.
Animal experiment
The experiment was conducted under the supervision of the Institutional Animal Care and Use Committee of Jilin University. The number of permits was SY202104016. Twelve SD male rats weighing about 200 g were used for the experiment, which were obtained from Liaoning Changsheng Laboratory Animal Co. After intraperitoneal anesthesia with 10% chloral hydrate, the heads of the rats were dehairing and disinfected and the skin was incised in the middle of the head for about 5–8 mm. A 5-mm-diameter drill was used to create a 5-mm-diameter cranial defect symmetrically on both sides of the midline of the skull. PDLSCs in the control group and si-MALAT1 PDLSCs in the experimental group were mixed 1:1 with Matrigel matrix and placed on both sides of the defects respectively and then the skin was sutured layer by layer. The samples were taken by necropsy after 8 weeks for further analysis.
Micro-CT imaging, Masson staining, and HE staining
After an 8-week survival period, the rats were euthanized, and calvarial tissues were collected and fixed in 4% paraformaldehyde. Micro-CT imaging was employed to assess bone regeneration, and software analysis quantified bone volume fraction (BV/TV). Following micro-CT analysis, the tissues were decalcified for 8 weeks in a decalcification solution, followed by automated dehydration, paraffin embedding, and sectioning into 5 μm-thick paraffin sections. Masson staining and HE staining were subsequently performed. The same blue color in all Masson staining photos was used as the unified standard for the determination of collagen fibers by Image J software, and the collagen volume fraction (CVF) (%) was calculated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. Data are presented as mean ± standard deviation (SD) of at least three independent experiments. For comparisons involving more than two groups, one-way ANOVA followed by Tukey’s multiple comparisons test was employed. Unpaired Student’s t-test was used for other data analyses, as appropriate. A significance level of p < 0.05 was considered statistically significant, and significance was denoted in the figures.
Results
Primary cell isolation and characterization of periodontal ligament stem cells (PDLSCs)
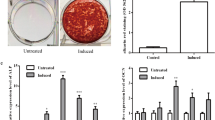
Following a two-week culture of periodontal ligament tissue blocks, primary cells were observed either dispersed at the culture bottle’s base or gradually migrating along the tissue block’s periphery (Fig. 1A). Upon achieving 80%-90% confluency of cells encircling the tissue block, subculturing was operated to facilitate subsequent investigations. To delineate the surface marker expression profile of PDLSCs, flow cytometry was performed and analysis unveiled robust expression of the stem cell markers CD146 (98.2%), STRO (99%), and Vimentin (98.6%), contrasted by negative expression of CD31, CD34, and CD45 (Fig. 1B). These findings confirm the mesenchymal stem cell-like properties of the cultured PDLSCs. Following osteogenic induction for 5, 7, and 14 days, noticeable elevations in the expression levels of ALPL, SPP1, and RUNX2 mRNA were observed in PDLSCs, substantiating their successful osteogenic differentiation process (Fig. 1C).
Periodontal ligament stem cells (PDLSCs) identification. (A) Phenotype of the PDLSCs after 5, 7, and 14 days of cell culture. (B) Positive expression of the PDLSCs surface markers including CD146 (98.2%), STRO (99%), and Vimentin (98.6%) are revealed by flow cytometry; whereas CD31, CD34, and CD45 were found to be negative, indicating the mesenchymal stem cell like properties of the cultured PDLSCs. (C) Significantly increased expression of ALPL, SPP1, and RUNX2 mRNA after 14 days of osteogenic induction detected by qRT-PCR demonstrates successful differentiation of PDLSCs into osteogenic lineages. The cell experiments were repeated three times; Scale bar, 500 um; Data were indicated as mean ± SD; P < 0.05 was marked as *
Osteogenic differentiation of PDLSCs induced by lncRNA MALAT1
The results of the CCK8 experiment demonstrated that the overexpression or inhibition of lncRNA MALAT1 did not exhibit significant proliferation effects on PDLSCs, as depicted in Fig. 2A. After 48 h of osteogenic induction, PDLSCs conducted transfection with si-MALAT1 and over-MALAT1 plasmids. Subsequent qRT-PCR analyzed the mRNA levels of osteogenesis-related genes, including ALPL, SPP1, and RUNX2, which revealed significant elevation within the over-MALAT1 group (Fig. 2B). Western blot analysis further confirmed amplified protein levels of ALPL, SPP1, and RUNX2 in the overexpression group, whereas their suppression was evident in the knockdown group (Fig. 2C).
lncRNA MALAT1 promoted osteogenic differentiation of PDLSCs. (A) CCK8 was performed to identify the cell viability of various groups in 1, 2 and 3 days. (B) The mRNA expression of ALPL, SPP1, and RUNX2 were performed by qRT-PCR after transfection of PDLSCs with over-expressed MALAT1 or silenced MALAT1. (C) The protein expression of ALPL, SPP1, and RUNX2 was performed by Western blot after transfection of PDLSCs with over-expressed MALAT1 or silenced MALAT1. (D) ALP staining images and quantitative analysis of PDLSCs after 7 Days of osteogenic induction. (E) ARS staining images and quantitative analysis of PDLSCs after 14 days of osteogenic induction. Con: control; over-NC: negative control for over-MALAT1; si-NC: negative control for si-MALAT1. The cell experiments were repeated three times; Scale bar, 200 um; Data were indicated as mean ± SD. P < 0.05 was marked as *, P < 0.01 was marked as **
Upon seven days of osteogenic induction, ALP staining, and activity exhibited attenuated levels in the si-MALAT1 group, with converse elevation associated with lncRNA MALAT1 overexpression (Fig. 2D). After a 14-day osteogenic induction period, the intensity of ARS staining demonstrated analogous trends, suggesting that the matrix mineralization of PDLSCs was under the regulatory influence of lncRNA MALAT1 (Fig. 2E).
miR-124-3p plays a role as a target of lncRNA MALAT1
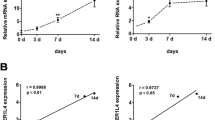
StarBase database substantiated the binding interaction between lncRNA MALAT1 and miR-124-3p, signifying a potential regulatory axis (Fig. 3A). To validate these computational predictions, we generated distinct plasmids that were named the wild-type (lncRNA MALAT1-WT) and mutant (lncRNA MALAT1-MUT) forms of lncRNA MALAT1. These plasmids were co-transfected with miR-124-3p mimic (referred to as “mimics”) and control mimics (miR-NC) to facilitate a dual luciferase reporter assay. Intriguingly, the results demonstrated that miR-124-3p profoundly attenuated the luciferase activity driven by lncRNA MALAT1 wild-type (P < 0.05). Conversely, this inhibitory effect was conspicuously absent in the context of the mutant reporter gene (Fig. 3B). This compelling evidence proves the direct targeting of miR-124-3p by lncRNA MALAT1, wherein the latter exerts a regulatory influence over the former, culminating in the suppression of miR-124-3p’s gene expression. The subcellular distribution of lncRNA MALAT1 emerges as a pivotal factor governing its mechanistic involvement. Employing FISH, we scrutinized the cellular localization of lncRNA MALAT1 within PDLSCs (Fig. 3C). Remarkably, our FISH experiments unveiled a predominant cytoplasmic residence of lncRNA MALAT1 within the PDLSCs.
The miR-124-3p was a target miRNA of lncRNA MALAT1. (A) The binding sites between lncRNA MALAT and miR-124-3p predicted by bioinformatics tool. (B) Fluorescent luciferase reporter gene assay detecting binding between LncRNA MALAT1 and miR-124-3p. (C) Localization of lncRNA MALAT1 in PDLSCs detected through RNA-FISH analysis. The cell experiments were repeated three times; Scale bar, 100 um; Data were indicated as as mean ± SD. P < 0.0001 was marked as ****
miR-124-3p mediates suppression of osteoblastic differentiation
To explore the potential role of miR-124-3p in osteogenesis, we conducted the transfection of PDLSCs with miR-124-3p mimics and inhibitor. After 48 h of osteogenic induction, qRT-PCR and Western blot analyses revealed a significant decrease in the expression levels of key osteogenic markers-ALPL, SPP1, and RUNX2, in the miR-124-3p mimics group. In contrast, the inhibitor group exhibited a marked enhancement in the expression of these markers (Fig. 4A, B). Evidently, these findings showed the pivotal role of miR-124-3p in negating the process of osteogenic differentiation within PDLSCs. This observation is further supported by the demonstrable decrease in ALP staining and activity upon miR-124-3p overexpression, and the conversely increased staining and activity when miR-124-3p is downregulated (Fig. 4C). A parallel trend is also evident in matrix mineralization, corroborated by ARS staining (Fig. 4D).
miR-124-3p inhibited osteoblast differentiation of PDLSCs. (A) The qRT-PCR detected mRNA expression of ALPL, SPP1, and RUNX2 after PDLSCs transfected with miR-124-3p mimics and miR-124-3p inhibitor. (B) The protein expression of ALPL, SPP1, and RUNX2 in PDLSCs after transfection with miR-124-3p mimics and miR-124-3p inhibitor detected by Western blot. (C) ALP staining images and quantitative analysis of PDLSCs undergoing osteogenic induction for 7 days. (D) ARS staining images and quantitative analysis of PDLSCs after 14 days of osteogenic induction. Con: control; miR-NC: negative control for mimics; inhibitor-NC: negative control for inhibitor; The cell experiments were repeated three times; Scale bar, 200 um; Data were indicated as mean ± SD. P < 0.05 was marked as *, P < 0.01 was marked as **
Impact of miR-124-3p and lncRNA MALAT1 on osteogenic differentiation
To further confirm whether lncRNA MALAT1 mediated the osteogenic differentiation of PDLSCs by regulating miR-124-3p, we transfected miR-124-3p mimics or inhibitor into PDLSCs stably overexpressing lncRNA MALAT1, and osteogenic induction was carried out 24 h later. Remarkably, our results showed a hierarchical progression in the expression levels of osteogenesis-related markers, including ALPL, SPP1, and RUNX2. Specifically, the over-MALAT1 + inhibitor group exhibited the most substantial increase in these markers, followed by the over-MALAT1 group, as demonstrated in Fig. 5A, B. Furthermore, ALP and ARS staining showed that miR-124-3p mimics partially reversed the ability of lncRNA MALAT1 to promote PDLSCs osteoblast differentiation, which was further facilitated by miR-124-3p inhibitor (Fig. 5C, D).
miR-124-3p partially blocked the pro-osteogenic function of lncRNA lncRNA MALAT1. (A) ALPL, SPP1, and RUNX2 mRNA expression in over-expressing-MALAT1 PDLSCs transfected with miR-124-3p mimics and inhibitor by qRT-PCR. (B) The ALPL, SPP1, and RUNX2 protein expression in over-expressing-MALAT1 PDLSCs transfected with miR-124-3p mimic and inhibitor by Western blot. (C) ALP staining images and quantitative analysis of PDLSCs undergoing osteogenic induction for 7 days. (D) ARS staining images and quantitative analysis of PDLSCs after 14 days of osteogenic induction. Con: control; The cell experiments were repeated three times; Scale bar, 200 um; Data were indicated as mean ± SD. P < 0.05 was marked as *, P < 0.01 was marked as **
IGF2BP1 regulation by lncRNA MALAT1/miR-124-3p
The binding site between IGF2BP1 and miR-124-3p was predicted using a bioinformatics platform (Fig. 6A). To further explore this finding, two distinct types of IGF2BP1 plasmids were engineered: IGF2BP1 wild type (IGF2BP1-WT) and mutant type (IGF2BP1-MUT), both integrated into dual luciferase vectors. These constructs were co-transfected with miR-124-3p mimics (mimics) and control mimics (miR-NC) for dual luciferase reporter assays. The results showed that miR-124-3p effectively attenuated the luciferase activity of the IGF2BP1 wild-type construct (P < 0.05), while presenting a sight influence on the mutant-type reporter, thereby confirming miR-124-3p’s targeting of IGF2BP1 and consequent gene expression inhibition (Fig. 6B). Furthermore, in exploring IGF2BP1’s role in enhancing osteogenic differentiation of PDLSCs, two concentrations (50 ng/ml and 100 ng/ml) were assessed. The qRT-PCR and Western blot analyses demonstrated that under osteogenic induction conditions, 50 ng/ml of IGF2BP1 significantly enhanced PDLSCs’ osteogenic differentiation compared to 100 ng/ml. Notably, the presence of si-MALAT1 in the osteogenic medium significantly impeded PDLSCs’ osteogenic capacity, consistent with findings from earlier sections. In addition, the introduction of 50 ng/ml of IGF2BP1 rescued PDLSCs’ osteogenic potential compromised by si-MALAT1, suggesting partial restoration of osteogenesis by IGF2BP1(Fig. 6C, D). Furthermore, following with 7 days of osteogenic differentiation, ALP staining revealed maximal ALP expression in the OM + 50 ng/ml IGF2BP1 group, followed by OM + 100 ng/ml IGF2BP1. Furthermore, the OM + si-MALAT1 + 50 ng/ml IGF2BP1 group displayed slightly elevated ALP levels compared to OM + si-MALAT1, implying a partial reversal of si-MALAT1 induced osteogenesis inhibition by 50 ng/ml IGF2BP1 (Fig. 6E). Similarly, the experimental groups were evaluated after 14 days of osteogenic differentiation, leading to ARS staining. Both OM + 50 ng/ml IGF2BP1 and OM + 100 ng/ml IGF2BP1 groups exhibited abundant mineralized nodules, with the former displaying the highest number. Meanwhile, 50 ng/ml IGF2BP1 counteracted the osteogenesis inhibition evoked by si-MALAT1 (Fig. 6F).
IGF2BP1 was regulated by lncRNA MALAT1/miR-124-3p. (A) Prediction of binding sites between IGF2BP1 and miR-124-3p using bioinformatics tool. (B) Fluorescent luciferase reporter gene assay detecting binding between IGF2BP1 and miR-124-3p. (C) The mRNA expression of ALPL, SPP1, and RUNX2 in PDLSCs under normal growth medium (GM), osteogenic medium (OM), and after the addition of varying concentrations of IGF2BP1 detected by qRT-PCR. (D) The protein expression of ALPL, SPP1, and RUNX2 in PDLSCs under normal growth medium (GM), osteogenic medium (OM), and after the addition of varying concentrations of IGF2BP1 detected by Western blot. (E) ALP staining images and quantitative analysis of PDLSCs undergoing osteogenic induction for 7 days. (F) ARS staining images and quantitative analysis of PDLSCs after 14 days of osteogenic induction. The cell experiments were repeated three times; Scale bar, 200 um; Data were indicated as mean ± SD. P < 0.05 was marked as *, P < 0.01 was marked as **
Inhibition of lncRNA MALAT1 suppresses in vivo osteogenic potential of PDLSCs
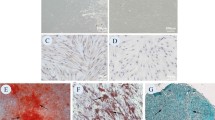
Micro-CT findings demonstrated substantial new bone formation in the calvarial defect area of the control group (NC), whereas the si-MALAT1 inhibition group exhibited diminished new bone formation. The bone volume fraction (BV/TV) in the control group (NC) presented that in the si-MALAT1 group by 55.76% addressing lncRNA MALAT1’s potential in promoting bone formation, which proved by the reduced new bone formation upon its inhibition (Fig. 7A). Histological analyses achieved HE and Masson staining further expressing the inhibited bone formation within the si-MALAT1 group. These stains also revealed reduced collagen fibers and osteoblasts in the same group, showing the detrimental impact of lncRNA MALAT1 inhibition on bone formation and its associated cellular constituents. Quantitative analysis of CVF showed that the percentage of CVF in the NC group was higher than that in the si-MALAT1 group (Fig. 7B).
Silence of lncRNA MALAT1 inhibits the osteogenic differentiation ability of PDLSCs in vivo. (A) The impact of lncRNA MALAT1 on bone regeneration. Left: Three-dimensional reconstructed images of rat calvaria reveal suppressed bone regeneration in the si-MALAT1 group. Right: Bone volume fraction (BV/TV) significantly decreased in the si-MALAT1 group. (B) Left: HE staining and Masson staining of the NC group and si-lncRNA MALAT1 group. Right: Quantitative analysis of CVF showed that the percentage of CVF in the NC group was higher than that in the si-MALAT1 group. (n = 12) NC: negative control; The cell experiments were repeated three times. Scale bar, 500 um; Data were indicated as mean ± SD. P < 0.05 was marked as *, P < 0.01 was marked as **
Discussion
The tooth loss resulting from slow degeneration of periodontal supporting tissue is a characterization of both periodontal disease and chronic inflammatory disorder. The restoration of injured periodontal tissues is therefore the first objective of periodontal treatment, for this purpose, the regeneration promoted by using beneficial seed cells has been demonstrated as an effective strategy [48,49,50]. Among these cells, PDLSCs stand out as the essential contributors in periodontal regeneration, due to their considerable osteogenic capacity, which greatly aids alveolar bone repair and the periodontal healing process [51]. PDLSCs consist of various periodontal tissue-derived cells that have can further develop into fibroblasts, osteoblasts, and cementoblasts. This diverse differentiation is considered highly relevant to the periodontal defect repair and regeneration mechanism.
In our study, the expression of stem cell surface markers from the cultivated cells was verified via flow cytometry analysis, which is consistent with previous findings and hereby enhances the robustness of this study. CD146, STRO-1, and Vimentin are early stem cell markers for PDLSCs with positivity rates as high as 98.2%, 99%, and 98.6% in this experiment. Upon 7 and 14 days of osteogenic induction, increased expression of osteogenesis-related genes namely ALPL, SPP1, and RUNX2 demonstrate the osteogenic potential of PDLSCs. Increased ALPL activity indicates early regulation in osteogenesis, while the expression of SPP1 indicates its function in bone remodeling and periodontal regeneration. The RUNX2, a marker for osteoblast development and chondrocyte maturation, is overexpressed for effective osteogenic differentiation of PDLSCs [52,53,54]. This study takes a closer look at the lncRNA MALAT1 with in-vitro overexpression and knockdown plasmids. The effect of lncRNA MALAT1 on PDLSCs’ osteogenic differentiation was further determined. Notably, lncRNA MALAT1 overexpression significantly increased the expression of osteogenesis-associated markers (ALPL, SPP1, and RUNX2) at both the mRNA and protein levels, which confirms the findings of earlier studies [55]. ALP and ARS staining, on the other hand, showed reduced osteogenic differentiation caused by lncRNA MALAT1 suppression, which further highlighted the importance of this molecule in promoting osteogenesis in PDLSCs. A potential mechanism is proposed that lncRNA MALAT1 acts as ceRNA by sequestering miR-124-3p. The antagonism between lncRNA MALAT1 and miR-124-3p, as well as their suppressive effects on gene expression, was confirmed by the dual-luciferase test. The miR-124-3p overexpression in PDLSCs prevented osteogenic differentiation, whereas miR-124-3p inhibition resulted in osteogenesis promotion. miR-124-3 was highly expressed in osteoclasts, and reduction of miR-214-3p inhibited RANKL-induced osteoclastogenesis and bone destruction [56].
Given the proposed mechanism, the functionality of IGF2BP1 was subsequently investigated. The addition of 50 ng/ml IGF2BP1 restored the osteogenic capacity of PDLSCs after si-MALAT1 intervention, confirming the up-regulatory effect on osteogenic differentiation. The promotion effect of lncRNA MALAT1 towards PDLSC osteogenesis was further validated in vivo via a rat skull defect model. Moreover, Masson and HE staining revealed reduced collagen fibers and osteoblasts in the si-MALAT1 group. This study enhances our understanding of lncRNAs’ regulatory processes in the context of osteogenic differentiation and highlights their therapeutic potential as targets for maxillofacial bone abnormalities.
The central role of PDLSCs in periodontal regeneration is well-recognized, owing to their ability to differentiate into various lineages required for tissue repair. Our results underscore the robust osteogenic potential of PDLSCs, as a significant factor in the restoration of alveolar bone defects. This observation highlights the clinical relevance of harnessing PDLSCs for regenerative strategies targeting periodontal defects. The up-regulation of lncRNA MALAT1 in osteogenic differentiation of PDLSCs reveals its significance as a potential therapeutic target. The mechanistic exploration of its interaction with miR-124-3p as a ceRNA provides valuable insights into the complexity of gene regulation during osteogenesis. These findings open the horizon for further investigations into the potential roles of lncRNA MALAT1 in stem cell differentiation and tissue regeneration. The dual role of miR-124-3p observed in this study as both a suppressor of osteogenesis and a mediator of lncRNA MALAT1 adds insights into the regulatory networks. The ability to adjust miR-124-3p levels could be a key to fine-tuning the osteogenic differentiation processes, not only in PDLSCs but also in other stem cell populations. Moreover, the functional impact of IGF2BP1 as a downstream target of miR-124-3p adds another pathway to the regulatory network. IGF2BP1’s role in enhancing osteogenic differentiation emphasizes the potential for modulating its expression to promote tissue repair. Exploring IGF2BP1’s broader functions in stem cell biology and its interactions with other regulatory elements could be the next direction to enhance its therapeutic potential. The in vivo validation of the observed promotion effect of lncRNA MALAT1 adds evidence of its function in a physiological context. This highlights the potential of lncRNA-based strategies for periodontal tissue engineering and bone defect therapies. The schematic diagram illustrating the mechanisms is shown in the Fig. 8.
Conclusions
Our study contributes to the emerging understanding of lncRNA-mediated regulation of osteogenic differentiation in PDLSCs. The identified lncRNA MALAT1/miR-124-3p/IGF2BP1 axis offers a potential avenue for therapeutic interventions in periodontal regeneration. The insights gained from this research pave the way for further investigations into the broader roles of these regulatory elements in stem cell biology and regenerative medicine.
Data availability
No datasets were generated or analysed during the current study.
References
Monsarrat P, Vergnes JN, Nabet C, Sixou M, Snead ML, Planat-Bénard V, Casteilla L, Kémoun P (2014) Concise review: mesenchymal stromal cells used for periodontal regeneration: a systematic review. Stem Cells Transl Med 3:768–774. https://doi.org/10.5966/sctm.2013-0183
Qi L, Zhang Y (2014) The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell Physiol Biochem 33:433–445. https://doi.org/10.1159/000358624
Ha SH, Choung PH (2020) MSM promotes human periodontal ligament stem cells differentiation to osteoblast and bone regeneration. Biochem Biophys Res Commun 528:160–167. https://doi.org/10.1016/j.bbrc.2020.05.097
Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Prim 3:17038. https://doi.org/10.1038/nrdp.2017.38
Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, Gronthos S (2014) Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev 23:1001–1011. https://doi.org/10.1089/scd.2013.0490
Yu B, Hu J, Li Q, Wang F (2021) CircMAP3K11 contributes to proliferation, apoptosis and migration of human periodontal ligament stem cells in inflammatory microenvironment by regulating TLR4 via miR-511 sponging. Front Pharmacol 12:633353. https://doi.org/10.3389/fphar.2021.633353
Li X, Zhang B, Wang H, Zhao X, Zhang Z, Ding G, Wei F (2020) The effect of aging on the biological and immunological characteristics of periodontal ligament stem cells. Stem Cell Res Ther 11:326. https://doi.org/10.1186/s13287-020-01846-w
Zhou Y, Fan W, Xiao Y (2014) The effect of hypoxia on the stemness and differentiation capacity of PDLC and DPC. Biomed Res Int 2014:890675. https://doi.org/10.1155/2014/890675
ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. https://doi.org/10.1038/nature11247
Chen LL (2016) Linking long noncoding RNA localization and function. Trends Biochem Sci 41:761–772. https://doi.org/10.1016/j.tibs.2016.07.003
Lin C, Yang L (2018) Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol 28:287–301. https://doi.org/10.1016/j.tcb.2017.11.008
Quinn JJ, Chang HY (2016) Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17:47–62. https://doi.org/10.1038/nrg.2015.10
Struhl K (2007) Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol 14:103–105. https://doi.org/10.1038/nsmb0207-103
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15:7–21. https://doi.org/10.1038/nrg3606
Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, Wang Y, Kong B, Langerød A, Børresen-Dale AL, Kim SK, van de Vijver M, Sukumar S, Whitfield ML, Kellis M, Xiong Y, Wong DJ, Chang HY (2011) Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43:621–629. https://doi.org/10.1038/ng.848
Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi G, Zhichen W, Zirui W, Shengwang W (2020) The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res 53:40. https://doi.org/10.1186/s40659-020-00309-z
Bellavia D, Salamanna F, Raimondi L, De Luca A, Carina V, Costa V, Alessandro R, Fini M, Giavaresi G (2019) Deregulated miRNAs in osteoporosis: effects in bone metastasis. Cell Mol Life Sci 76:3723–3744. https://doi.org/10.1007/s00018-019-03162-w
Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y, Ren J (2019) Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol Life Sci 76:441–451. https://doi.org/10.1007/s00018-018-2940-7
Zheng Y, Li X, Huang Y, Jia L, Li W (2018) Time series clustering of mRNA and lncRNA expression during osteogenic differentiation of periodontal ligament stem cells. PeerJ 6:e5214. https://doi.org/10.7717/peerj.5214
Chen J, Wang Y, Wang C, Hu JF, Li W (2020) LncRNA functions as a new emerging epigenetic factor in determining the fate of stem cells. Front Genet 11:277. https://doi.org/10.3389/fgene.2020.00277
Tello-Flores VA, Beltrán-Anaya FO, Ramírez-Vargas MA, Esteban-Casales BE, Navarro-Tito N, Alarcón-Romero LDC, Luciano-Villa CA, Ramírez M, Del Moral-Hernández Ó, Flores-Alfaro E (2021) Role of long non-coding RNAs and the molecular mechanisms involved in insulin resistance. Int J Mol Sci 22. https://doi.org/10.3390/ijms22147256
Yan C, Chen J, Chen N (2016) Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep 6:22640. https://doi.org/10.1038/srep22640
Vimalraj S, Subramanian R, Dhanasekaran A (2021) LncRNA MALAT1 promotes tumor angiogenesis by regulating microRNA-150-5p/VEGFA signaling in osteosarcoma: in-vitro and in-vivo analyses. Front Oncol 11:742789. https://doi.org/10.3389/fonc.2021.742789
Lu Y, Gong Z, Jin X, Zhao P, Zhang Y, Wang Z (2020) LncRNA MALAT1 targeting miR-124-3p regulates DAPK1 expression contributes to cell apoptosis in Parkinson’s Disease. J Cell Biochem 121:4838–4848. https://doi.org/10.1002/jcb.29711
Li X (2022) LncRNA metastasis-associated lung adenocarcinoma transcript-1 promotes osteogenic differentiation of bone marrow stem cells and inhibits osteoclastic differentiation of Mø in osteoporosis via the miR-124-3p/IGF2BP1/Wnt/β-catenin axis. J Tissue Eng Regen Med 16:311–329. https://doi.org/10.1002/term.3279
Younes N, Syed N, Yadav SK, Haris M, Abdallah AM, Abu-Madi M (2021) A whole-genome sequencing association study of low bone mineral density identifies new susceptibility loci in the Phase I Qatar Biobank Cohort. J Pers Med 11. https://doi.org/10.3390/jpm11010034
Song C, Guo Y, Chen F, Liu W (2022) lncRNA MALAT1 promotes osteogenic differentiation through the miR-217/AKT3 axis: a possible strategy to alleviate osteoporosis. J Gene Med 24:e3409. https://doi.org/10.1002/jgm.3409
Zhou Y, Xu Z, Wang Y, Song Q, Yin R (2022) LncRNA MALAT1 mediates osteogenic differentiation in osteoporosis by regulating the miR-485-5p/WNT7B axis. Front Endocrinol (Lausanne) 13:922560. https://doi.org/10.3389/fendo.2022.922560
Li J, Zhuang H, Wang Z, Cai J, Ma X, Chen W, Jiang X, Zhao D, Hou W, Tao Y (2022) lncRNAs MALAT1 and LINC00657 upstream to miR-214-3p/BMP2 regulate osteogenic differentiation of human mesenchymal stem cells. Mol Biol Rep 49:6847–6857. https://doi.org/10.1007/s11033-022-07136-3
Xiao X, Zhou T, Guo S, Guo C, Zhang Q, Dong N, Wang Y (2017) LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol 243:404–412. https://doi.org/10.1016/j.ijcard.2017.05.037
Bao M, Liu G, Song J, Gao Y (2020) Long non-coding RNA MALAT1 promotes odontogenic differentiation of human dental pulp stem cells by impairing microRNA-140-5p-dependent downregulation of GIT2. Cell Tissue Res 382:487–498. https://doi.org/10.1007/s00441-020-03246-1
Hua L, Zhang X (2021) MALAT1 regulates osteogenic differentiation of human periodontal ligament stem cells through mediating miR-155-5p/ETS1 axis. Tissue Cell 73:101619. https://doi.org/10.1016/j.tice.2021.101619
Zhang L, Sheng M, Cao H, Zhang L, Shao W (2023) Decoding the role of long non-coding RNAs in periodontitis: a comprehensive review. Biomed Pharmacother 166:115357. https://doi.org/10.1016/j.biopha.2023.115357
Xia F, Xu Y, Zhang X, Lyu J, Zhao P (2021) Competing endogenous RNA network associated with oxygen-induced retinopathy: expression of the network and identification of the MALAT1/miR-124-3p/EGR1 regulatory axis. Exp Cell Res 408:112783. https://doi.org/10.1016/j.yexcr.2021.112783
Gan L, Leng Y, Min J, Luo XM, Wang F, Zhao J (2022) Kaempferol promotes the osteogenesis in rBMSCs via mediation of SOX2/miR-124-3p/PI3K/Akt/mTOR axis. Eur J Pharmacol 927:174954. https://doi.org/10.1016/j.ejphar.2022.174954
Qadir AS, Um S, Lee H, Baek K, Seo BM, Lee G, Kim GS, Woo KM, Ryoo HM, Baek JH (2015) miR-124 negatively regulates osteogenic differentiation and in vivo bone formation of mesenchymal stem cells. J Cell Biochem 116:730–742. https://doi.org/10.1002/jcb.25026
Cao Y, Lv Q, Li Y (2021) Astragaloside IV improves tibial defect in rats and promotes proliferation and osteogenic differentiation of hBMSCs through MiR-124-3p.1/STAT3 Axis. J Nat Prod 84:287–297. https://doi.org/10.1021/acs.jnatprod.0c00975
Scalia P, Williams SJ, Fujita-Yamaguchi Y (2023) Human IGF2 gene epigenetic and transcriptional regulation: at the core of developmental growth and tumorigenic behavior. Biomedicines 11. https://doi.org/10.3390/biomedicines11061655
Wang P, Zhang L, Zhang J, Xu G (2022) MicroRNA-124-3p inhibits cell growth and metastasis in cervical cancer by targeting IGF2BP1. Exp Ther Med 23:233. https://doi.org/10.3892/etm.2022.11157
Chen W, Chen M, Xu Y, Chen X, Zhou P, Zhao X, Pang F, Liang W (2018) Long non-coding RNA THOR promotes human osteosarcoma cell growth in vitro and in vivo. Biochem Biophys Res Commun 499:913–919. https://doi.org/10.1016/j.bbrc.2018.04.019
Qu Y, Pan S, Kang M, Dong R, Zhao J (2016) MicroRNA-150 functions as a tumor suppressor in osteosarcoma by targeting IGF2BP1. Tumour Biol 37:5275–5284. https://doi.org/10.1007/s13277-015-4389-8
Kang H, Sung J, Jung HM, Woo KM, Hong SD, Roh S (2012) Insulin-like growth factor 2 promotes osteogenic cell differentiation in the parthenogenetic murine embryonic stem cells. Tissue Eng A 18:331–341. https://doi.org/10.1089/ten.TEA.2011.0074
Hardouin SN, Guo R, Romeo PH, Nagy A, Aubin JE (2011) Impaired mesenchymal stem cell differentiation and osteoclastogenesis in mice deficient for Igf2-P2 transcripts. Development 138:203–213. https://doi.org/10.1242/dev.054916
Yakar S, Werner H, Rosen CJ (2018) Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol 61:T115–T137. https://doi.org/10.1530/jme-17-0298
Diao S, Yang H, Cao Y, Yang D, Fan Z (2020) IGF2 enhanced the osteo-/dentinogenic and neurogenic differentiation potentials of stem cells from apical papilla. J Oral Rehabil 47(Suppl 1):55–65. https://doi.org/10.1111/joor.12859
Zheng Y, Li X, Huang Y, Jia L, Li W (2017) The circular RNA landscape of periodontal ligament stem cells during osteogenesis. J Periodontol 88:906–914. https://doi.org/10.1902/jop.2017.170078
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Wang Y, Li J, Tang M, Peng C, Wang G, Wang J, Wang X, Chang X, Guo J, Gui S (2023) Smart stimuli-responsive hydrogels for drug delivery in periodontitis treatment. Biomed Pharmacother 162:114688. https://doi.org/10.1016/j.biopha.2023.114688
Sedghi LM, Bacino M, Kapila YL (2021) Periodontal disease: the good, the bad, and the unknown. Front Cell Infect Microbiol 11:766944. https://doi.org/10.3389/fcimb.2021.766944
Chen Q, Liu X, Wang D, Zheng J, Chen L, Xie Q, Liu X, Niu S, Qu G, Lan J, Li J, Yang C, Zou D (2021) Periodontal inflammation-triggered by periodontal ligament stem cell pyroptosis exacerbates periodontitis. Front Cell Dev Biol 9:663037. https://doi.org/10.3389/fcell.2021.663037
Chen L, Zhu S, Guo S, Tian W (2023) Mechanisms and clinical application potential of mesenchymal stem cells-derived extracellular vesicles in periodontal regeneration. Stem Cell Res Ther 14:26. https://doi.org/10.1186/s13287-023-03242-6
Jiang Z, Hua Y (2016) Hydrogen sulfide promotes osteogenic differentiation of human periodontal ligament cells via p38-MAPK signaling pathway under proper tension stimulation. Arch Oral Biol 72:8–13. https://doi.org/10.1016/j.archoralbio.2016.08.008
Singh A, Gill G, Kaur H, Amhmed M, Jakhu H (2018) Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog Orthod 19:18. https://doi.org/10.1186/s40510-018-0216-2
Komori T (2018) Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol 149:313–323. https://doi.org/10.1007/s00418-018-1640-6
Liu JL, Liu YS, Zheng MJ, He HY (2022) The management of bone defect using long non-coding RNA as a potential biomarker for regulating the osteogenic differentiation process. Mol Biol Rep 49:2443–2453. https://doi.org/10.1007/s11033-021-07013-5
Musolino C, Oteri G, Allegra A, Mania M, D’Ascola A, Avenoso A, Innao V, Allegra AG, Campo S (2018) Altered microRNA expression profile in the peripheral lymphoid compartment of multiple myeloma patients with bisphosphonate-induced osteonecrosis of the jaw. Ann Hematol 97:1259–1269. https://doi.org/10.1007/s00277-018-3296-7
Funding
This study was financially supported by grants from the National Natural Science Foundation of China, Changchun, China (No.82370934).
Author information
Authors and Affiliations
Contributions
Nan Gu: Conducted Experiments and Data Analysis.
Yao Wang: Conducted Literature Review and Manuscript Writing.
Lingfeng Li: Picture Production.
Xin Sui:Formal Analysis.
Zhihui Liu: Oversaw Project Design and Supervision.
Corresponding author
Ethics declarations
Ethics approval
The experiment was conducted under the supervision of the Institutional Animal care and Use Committee of Jilin University. The number of permit was SY202104016.
Consent to participate
Not applicable.
Conflict of interest
The authors declare that there is no conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. MALAT1 Enhances Osteogenesis: lncRNA MALAT1 positively regulates osteogenic differentiation in PDLSCs, highlighting its role in bone regeneration.
2. Unveiling the Regulatory Axis: miR-124-3p targets IGF2BP1 to modulate osteogenic potential, unveiling a novel regulatory axis in periodontal tissue repair.
3. Therapeutic Insights for Bone Defects: Insights into lncRNA-mediated osteogenesis provide potential avenues for innovative therapies in periodontal regeneration and bone defect management.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, N., Wang, Y., Li, L. et al. The mechanism of lncRNA MALAT1 targeting the miR-124-3p/IGF2BP1 axis to regulate osteogenic differentiation of periodontal ligament stem cells. Clin Oral Invest 28, 219 (2024). https://doi.org/10.1007/s00784-024-05616-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05616-3