Abstract
Objectives
To assess color change efficacy and the adverse effects of varied over-the-counter (OTC) bleaching protocols.
Methodology
The study included randomized clinical trials evaluating color changes from OTC bleaching agents. Nine databases were searched, including the partial capture of the grey literature. The RoB2 tool analyzed the individual risk of bias in the studies. Frequentist network meta-analyses compared treatments through common comparators (∆Eab* and ∆SGU color changes, and tooth sensitivity), integrating direct and indirect estimates and using the mean and risk differences as effect measures with respective 95% confidence intervals. The GRADE approach assessed the certainty of the evidence.
Results
Overall, 37 remaining studies constituted the qualitative analysis, and ten composed the meta-analyses. The total sample included 1,932 individuals. ∆Eab* was significantly higher in groups 6% hydrogen peroxide (HP) strips (≥ 14 h). ∆SGU was significantly higher in groups at-home 10% carbamide peroxide (CP) (≥ 14 h), followed by 6% HP strips (≥ 14 h) and 3% HP strips (≥ 14 h). At-home 10% CP (7-13 h) and placebo showed lower risks of tooth sensitivity without significant differences between these treatments.
Conclusion
Considering the low level of evidence, OTC products presented satisfactory short-term effects on tooth bleaching compared to the placebo, with little to no impact on dentin hypersensitivity and gingival irritation.
Clinical Relevance.
OTC products are proving to be practical alternatives for tooth whitening. However, patients should be advised about the possible risks of carrying out such procedures without professional supervision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over-the-counter (OTC) products appeared in the early 2000s and represent bleaching agents that are available in drugstores, supermarkets, and general stores [1, 2]. These products, encompassing strips, dentifrices, paint-on gels, mouthwashes, chewing gum, and varnishes, are self-applicable and commonly marketed without the need for dentist supervision [3]. They contain hydrogen or carbamide peroxide in their composition, although the concentration may vary according to the regulatory agency of each country [4]. While some regions, such as Europe, prohibit the commercialization of whitening products with hydrogen peroxide concentrations exceeding 0.1% without dentist supervision [5], OTC products are classified as “cosmetics” in other locations. This categorization facilitates their worldwide purchase through online sales without necessitating a prescription [4, 5].
Although several clinical studies have used methodologies with OTC products demonstrating significant color changes, the delivery methods present various application protocols [6,7,8,9,10]. Such a scenario opens comparison possibilities that are unfeasible for conventional randomized clinical trials because of the need for numerous groups and samples [11, 12].
Network meta-analyses (NMA) create simultaneous direct and indirect estimates and are applied to these cases to integrate several groups [13]. Moreover, systematic reviews promote the data survey of possible adverse effects in tooth bleaching procedures with OTC products. Considering that these products are not individualized, the occurrence and intensity of dentin hypersensitivity and gingival irritation are concerning [14, 15].
Considering the variety of OTC products available and their easy purchase and use, the primary objective of this systematic review was to map the global scientific literature to assess the color change efficacy from different OTC bleaching protocols. The secondary goal was to evaluate the adverse effects of the various techniques.
Methodology
Protocol registration
The protocol of this systematic review was reported according to the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) guidelines [16] and registered in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO) under CRD42021276125. The systematic review was produced according to the JBI Manual for Evidence Synthesis [17] and reported following the PRISMA-NMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [18]. There were no deviations from the registered original protocol.
Research question and eligibility criteria
This systematic review aimed to answer the following guiding question based on the PICOS acronym (Population, Intervention, Comparator, Outcome, and Study design): "In adult patients undergoing vital tooth whitening, does the application of over-the-counter whitening protocols result in a superior effect on color change when compared to placebo or dentist-supervised protocols?". To compare their effectiveness, we included comparisons between dentist-supervised treatment to evaluate if OTC treatment can reach the same results in color change as the gold standard treatments. Additionally, we also included comparisons between OTC products and placebo treatments to analyze if these products would provide real effects for color change, and comparisons between different OTC bleaching protocols.
Inclusion criteria
-
Population: Adult individuals subjected to tooth bleaching in vital teeth;
-
Intervention: At least one group treated with OTC products composed of hydrogen peroxide (HP) or carbamide peroxide (CP), regardless of the concentration or application method;
-
Comparator: OTC products based on HP or CP, placebo (negative control), or conventional at-home and/or in-office tooth bleaching methods, regardless of the bleaching agent (positive control);
-
Outcomes: Color changes after tooth bleaching (∆Eab* / ΔSGU) as the primary outcome, and adverse effects, such as dentin sensitivity or gingival irritation, as a secondary outcomes;
-
Study design: Parallel or split-mouth randomized clinical trials without restricting publication year or language.
Exclusion criteria
-
Studies with participants subjected to tetracycline staining;
-
Studies with participants undergoing orthodontic treatment;
-
Studies that did not clearly describe the used bleaching technique.
Information sources and search
The electronic searches were performed until December 2021 in the Cochrane Library, Embase, LILACS, MedLine (via PubMed), SciELO, Scopus, and Web of Science databases. Google Scholar and ProQuest partially captured the "grey literature" to reduce selection bias. An update was performed up to January 2023 in the MedLine (via PubMed) database. The search strategies in each database agreed to their respective syntax rules (Table 1).
Study selection
The results obtained in the primary databases were initially exported to the EndNote Web™ software (Thomson Reuters, Toronto, Canada) for cataloging and deduplicating. The "grey literature" results were exported to Microsoft Word (Microsoft™, Ltd, Washington, USA) for manually extracting duplicates.
The results were exported to Rayyan QCRI software (Qatar Computing Research Institute, Doha, Qatar) [19] for study selection. Two reviewers performed all phases independently, and in case of disagreements, a third reviewer (LRP) was consulted for a final decision. Examiners were considered eligible for the subsequent phase only after reaching an agreement of Kappa ≥ 0.81, and this procedure was applied for all steps of the systematic review. In the first phase, the titles were read and those unrelated to the topic were excluded. In the second phase, the abstracts were evaluated with the initial application of the eligibility criteria. The titles that met the study objectives but did not have abstracts available were fully analyzed in the next phase. In the third phase, the full texts of eligible articles so far were read to verify whether they met the eligibility criteria. If the full texts were not found, a bibliographic request was made to a library database, and e-mails were sent to the corresponding author up to three times within 15 days to obtain the texts. Full texts published in languages other than English or Portuguese were translated for applying the eligibility criteria.
Data collection process
A calibration exercise was performed before data extraction, in which the reviewer’s extracted information from three eligible studies jointly to ensure consistency. Next, the full texts of the selected articles were reviewed, and the following data were systematically extracted: (a) study identification (author, year, location, and funding sources); (b) sample characteristics (the number of participants, distribution by sex, and mean age); (c) bleaching protocol (OTC product and application method); (d) contact time between the bleaching agent and the tooth surface; (e) assessment methods for color changes (spectrophotometry / Vita scale) and sensitivity; (f) outcomes of color changes (ΔEab* / ΔSGU), tooth sensitivity, and gingival irritation; (g) follow-up time for post-bleaching color assessment. The corresponding author was contacted via e-mail in the case of incomplete or insufficient information.
The color change estimate could be extracted using two metrics. The first is the Commission Internationale de L’Eclairage (CIE) LAB coordinates system, an objective method to evaluate color change using a spectrophotometer. This system is based on the luminosity (L* coordinate) and the a* (red-green axis) and b* (yellow-blue axis) chromaticity coordinates. The result is calculated using the following formula: \(\mathrm\Delta\mathrm E\mathrm a\mathrm b\ast=\sqrt{\left(L1-L2\right)^2+\left(a1-a2\right)^2+\left(b1-b2\right)^2}\) [20]. The second is a subjective method based on the Vita Shade Guide (Vita Zahnfabrik, Sackingen, Germany). Initially, the shade units are ranked by their value, according to the manufacturer, and the operator can use a spectrophotometer or visually evaluates the initial and final color using the Shade Guide. The difference is showed as the ΔSGU [21].
Only studies that reported mean values and respective standard deviations of ∆Eab* or ∆SGU were included in this NMA.
Risk of bias within individual studies
Two reviewers (MNO and MTCV) independently assessed the individual risk of bias in the eligible studies with the Risk of Bias Tool of the Cochrane Collaboration (version 2.0) (RoB2) for randomized clinical trials. This tool consists of five domains: bias from the randomization process, bias due to deviations from the intended interventions, bias from missing outcome data, bias from outcome measurements, and bias from the selection of the reported result. Each domain was assessed according to the algorithms proposed in the RoB2 manual and included signaling questions with "yes," "probably yes," "probably not," "no," or "no information" as potential answers. These answers showed the occurrence and provided the base to judge the risk of bias at the domain level, which could be "high risk," "some concerns," or "low risk." The article had a "low risk" of bias if all domains had a low risk, "some concerns" if at least one domain showed some concerns, and a "high risk" of bias if at least one domain presented a high risk, or several domains showed some concerns. Reviewer disagreements were solved by discussing and consulting with a third reviewer (LRP).
Data synthesis
Three review outcomes were quantitatively analyzed: ∆Eab*, ΔSGU, and tooth sensitivity. Firstly, we performed pairwise comparisons with available head-to-head data. Treatments were grouped into common nodes based on each OTC bleaching product and respective use protocol. The treatments were grouped according to the delivery method (i.e., strips, gel), bleaching agent (HP or CP) and its respective concentrations, and the duration of the contact between the bleaching agent and the tooth structure. For example, in Kim et al., 2018 [10], the treatments were performed twice daily for 30 min each, for four weeks, totalizing 28 h of contact between the bleaching agent and the teeth. Although classification decisions were arbitrary and may compromise the outcomes, the lack of grouping would merge several OTC products and contribute to network incoherence. Subsequently, a random-effects frequentist NMA compared multiple OTC bleaching protocols through common comparators by integrating direct and indirect estimates [13]. Transitivity was evaluated by comparing the distribution of important covariates across comparisons [22]: sex, age, and follow-up assessment time point for ∆Eab*; age and follow-up assessment time point for ∆SGU; and only follow-up assessment time point for tooth sensitivity [23]. We must anticipate that all analyses were conducted using data from the second week of follow-up; the low number of comparisons precluded analyses using other time points. Random-effects models with the Der-Simonian and Laird variance estimator [24] were preferred over fixed-effects models based on the deviance information criterion (DIC). Gingival irritation was narratively described due to the very low density in the network.
The effect estimate was the mean difference (MD) instead of standardized MD (SMD), as studies used comparable scales for the assessed outcomes and to prevent the standard deviation (SD) effect on SMD estimates. For the tooth sensitivity outcome, we used the risk difference (RD). The 95% confidence intervals (CI) were calculated for all estimates. Direct, indirect, and network estimate (0.05 significance level) comparisons evaluated local incoherence.
League tables presented the outcomes, and each treatment was ordered from best to worst according to the ranking probabilities of treatment effects. The MetaInsight, version 5.1.2, hosted all analyses.
Geometry of the network
The geometry of the networks was explored using conventional measurements of number of nodes and edges as well as additional metrics of density (the ratio between real and possible edges) and number of strong edges (edges with more than one trial) [25]. Edges proportional to the number of arms in the corresponding pairwise meta-analysis represented direct comparisons among the various OTC bleaching protocols.
Assessment of inconsistency
The presence of inconsistency in NMAs was assessed by examining the agreement between direct and indirect effect estimates. When applicable, the difference between head-to-head and indirect estimates was calculated, along with the respective 95% confidence interval.
Certainty of evidence
The GRADE tool classified the certainty of evidence of treatment effect estimates for the network meta-analysis [26, 27]. First, the certainty of evidence evaluation of each direct comparison verified the risk of bias, inconsistency, indirectness, and publication bias. Indirect comparisons considered the first-order loop with the lowest certainty and evaluated intransitivity. Finally, concerns of imprecision or incoherence in the network meta-analysis caused certainty of evidence downgrading. The certainty of evidence could be high, moderate, low, or very low [26].
Results
Study selection
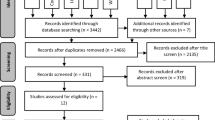
The first study selection phase yielded 18,564 results distributed in nine electronic databases, including the "grey literature." After removing duplicates, 12,614 results remained for analysis. A careful reading of titles and abstracts excluded 12,535 articles. Five of the 79 remaining studies were not found, and 74 were fully read, of which 37 were excluded. Appendix S1 describes the reasons for exclusions. The 37 remaining articles constituted the qualitative analysis, and ten composed the meta-analyses (Fig. 1).
Characteristics of eligible studies
The articles were published between 2001 and 2022 and performed in 12 countries, with 20 studies in America [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47], 12 in Europe [1, 6, 7, 9, 48,49,50,51,52,53,54,55], and five in Asia [10, 56,57,58,59]. Two studies [9, 41] had a split-mouth design, and the others were parallel. 27 articles declared funding sources [10, 28, 30, 31, 33,34,35,36,37,38,39,40,41,42,43, 48,49,50,51,52,53,54,55,56,57].
The total sample included 1,932 participants, with 548 men and 1,177 women in studies reporting the sex of participants. The age group ranged between 15 and 79 years. Among the products used, 25 studies tested the whitestrips (HP concentration ranging from 5,3% to 14%) [1, 10, 28,29,30, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47, 51, 52, 54, 56, 57], 13 studies evaluated paint-on products (HP concentration ranging from 3 to 9%) [7, 9, 10, 31, 32, 37, 38, 49, 50, 53, 56,57,58], three studies tested OTC bleaching gels (HP concentration ranging from 6 to 10%) [45,46,47] and dentifrices (HP concentration ranging from 0.75% to 2.8%) [6, 55, 59]. In 14 studies, a placebo group (negative control) was present [10, 32, 33, 36, 38,39,40, 44, 45, 49, 55,56,57,58] and 12 studies [1, 9, 10, 28, 30, 35, 41, 43, 46, 47, 51, 54] had a positive control group using a dentist supervised bleaching protocol – 10 of them, with an at-home treatment [1, 10, 28, 30, 35, 41, 43, 46, 47, 54] and three with an in-office bleaching group [1, 9, 43]. More details about the bleaching protocols and the duration of the treatments are shown in (Table 2). Spectrophotometry (ΔEab*) and the Vita scale (ΔSGU) analyzed tooth bleaching. The occurrence of tooth sensitivity and gingival irritation was assessed by self-perception with a categorical evaluation (yes or no), and intensities were examined with visual analog scales (VAS) (Table 3). Moreover, color change assessments had different intervals, from immediately after, one day, seven days, and several others up to360 days. The most common evaluation interval was 14 days, reported in nine studies [29, 30, 32, 34, 49, 51, 56,57,58].
Risk of individual bias in the studies
Only four studies had an overall low risk of bias [6, 46, 47, 55] (Fig. 2). Three studies showed an overall high risk of bias due to missing outcome data [49], deviations from the intended interventions [37], and outcome measurements [36, 49].
Syntheses of results and meta-analyses
Color changes (∆Eab*)
Among the 13 studies that provided the ∆Eab* values, three [27, 33, 57] were excluded because they did not present the values of standard deviation, one [35] because used a different method of color assessment (colorimeter), one [8] did not provide the concentration of HP in the intervention group, four [28, 41, 42, 46] due to the lack of a common comparator group, and one [58] due to violating the transitivity assumption (the study sample exclusively comprised female individuals.). Hence, three studies [10, 30, 40] with five treatments and eight pairwise comparisons were included, totaling 169 participants. Density was 0.8 and each edge was composed by only one trial; that is, there was no strong edge. Figure 3 demonstrates the network and Appendix S2 presents direct evidence findings.
Figure 4 shows estimates from NMA. ∆Eab* was significantly higher for 6% HP strips (≥ 14 h) (MD: 3.07; 95% CI: 0.63 – 5.50) compared to placebo two weeks after treatment. No other significant differences were observed. The differences among direct, indirect, and NMA evidence suggested no inconsistency (Appendix S3).
Color changes (∆SGU)
Among the 17 studies that presented ∆SGU evaluation, two [1, 37] were excluded due to not providing the exact contact time between the bleaching agent and tooth surface, and one [45] showed the results as visual graphs, without specifying the mean and standard deviation values. Moreover, this NMA excluded ten studies [7, 9, 31, 41, 46,47,48,49,50, 53] due to the lack of a common comparator group and one [36] due to violating the transitivity assumption (the study only provided the color assessment at the third week). Hence, three studies [10, 32, 51] with six treatments and eight pairwise comparisons were included, totaling 239 participants. Figure 5 demonstrates the network, and Appendix S4 presents direct evidence findings. This geometry had a density of 0.53 and no strong edge.
Figure 6 shows estimates from NMA. The ranking probability showed that the most effective treatment was at-home 10% CP (≥ 14 h), followed by 6% HP strips (≥ 14 h) and 3% HP strips (≥ 14 h). The at home 10% CP (≥ 14 h) protocol had significantly higher ∆SGU than all other treatments, except for 6% HP strips (≥ 14 h) (MD: 0.41; 95% CI: -1.01 – 1.83). Moreover, ∆SGU was significantly higher for 6% HP strips (≥ 14 h) compared to 6% HP paint-on gel (7-13 h) (MD: 1.93; 95% CI: 0.27 – 3.59) and placebo (MD: 2.60; 95% CI: 1.01 – 4.19). The assessment of inconsistency was not possible due to the absence of mixed (both direct and indirect) evidence (Appendix S5).
Adverse effects
Among the 30 studies that evaluated tooth sensitivity, four [28, 30, 50, 51] employed desensitizing agents in at least one group (which could introduce bias in pooled estimates), three [42, 45, 52] were excluded due to providing only total sample estimates of tooth sensitivity occurrence (without specifying it according to comparison group), one [1] due to not providing the exact contact time between the bleaching agent and tooth surface, other [53] did not reported the assessment method for tooth sensitivity, and another [45] showed the results as visual graphs, without specifying the occurrence of tooth sensitivity according to each group. Furthermore, this NMA excluded ten studies [7, 9, 34, 35, 38, 41, 47, 48, 51, 57] due to the lack of a common comparator group and five [33, 35, 40, 42, 43] due to violating the transitivity assumption. The tooth sensitivity NMA included five studies [28, 39, 45, 46, 56] with 9 treatments and 11 pairwise comparisons, totaling 216 participants. Figure 7 demonstrates the network and Appendix S6 presents direct evidence findings. The geometry had very low density (0.31) and no strong edge.
Figure 8 shows estimates from NMA. The ranking probability showed that at-home 10% CP (7-13 h) exhibited lower risk of tooth sensitivity, followed by placebo, and 6% HP paint-on gel (≥ 14 h). Placebo (RD: -0.21; 95% CI: -0.39 – -0.04) and 6% HP paint-on gel (≥ 14 h) (RD: -0.21; 95% CI: -0.42 – -0.01) had significantly lower risk of tooth sensitivity than 10% HP strips (≥ 14 h). The assessment of inconsistency was not possible due to the absence of mixed (both direct and indirect) evidence (Appendix S7).
Certainty of evidence
Overall, this study analyzed 61 direct and indirect comparisons considering three outcomes (color changes (∆E*), color changes (∆SGU), and tooth sensitivity). The certainty of evidence varied from very low to low. The main reasons for evidence downgrading were the risk of bias and imprecision (Appendix S8).
Discussion
The ∆Eab* analysis did not show statistical differences between OTC protocols and the positive control. Considering meta-analysis limitations, only one supervised protocol could be compared (at-home 10%CP ≥ 14 h), and the similar time of bleaching agent application among all groups (≥ 14 h) and the close HP concentration justify the results. However, the positive control in the ∆SGU analysis presented more color changes than all four evaluated OTC protocols. One analyzed study [10] used paint-on gel (3% ≥ 14 h) and reported participants with difficulties using the bleaching agent. That may explain the difference in results because OTC protocols are not personalized, and the absence of professional support may cause complications in product use.
The ∆Eab* analysis showed that two OTC protocols did not differ from the placebo group: 3%HP strips ≥ 14 h [10] and 3%HP paint-on gel ≥ 14 h [10]. That may be due to the low bleaching agent concentration, reinforced by the higher color change achieved with 6%HP strips ≥ 14 h in two studies [20, 30]. The ∆Eab* indices of one positive control also did not differ from the placebo treatment: at-home 10%CP > 14 h [30]. The low product concentration in these cases associated with short application times (30 min) potentially influenced the findings. Carbamide peroxide takes longer to react and release hydroxyl radicals [60]. Therefore, at-home bleaching with carbamide peroxide should use an impression tray as the bleaching agent for longer.
It is worth noting that although ∆Eab* and ∆SGU provide two different analyses, both methods generally evaluate and identify color changes [61]. Therefore, the findings should be complementary.
Gingival irritation is an adverse effect of bleaching procedures caused by the direct contact of hydrogen peroxide with the gingival mucosa. The bleaching agent is highly toxic when working on fibroblasts in the gingival tissue, reducing cell survival [14]. OTC products are not customized for everyone, potentially promoting contact between the bleaching agent and the gingival tissue and consequent irritation [4]. The data from our search did not allow a meta-analysis comparing gingival irritation between OTC products and positive controls. Further clinical studies should perform this comparison.
An important point to emphasize is that the application of products containing HP should always be supervised by a professional. Indiscriminate use of bleaching products can potentially cause oral lesions. Additionally, the lack of a personalized reservoir for at-home use of these products may result in the ingestion of HP [4], leading to irritation of the gastrointestinal tract, nausea, and vomiting [62].
Tooth sensitivity is the most common adverse effect of bleaching treatments. Although its biological mechanism has not been established, it might occur from the permeability of oxygen ions, which are cytotoxic in odontoblastic extensions close to pulp cells [63]. Considering that tooth permeability is higher in exposed dentin due to dentinal tubules, OTC products may promote the direct contact of bleaching agents with the exposed dentin in patients with gingival recessions, or non-carious cervical lesions may lead to severe inflammatory reactions in pulp cells [15, 63]. Different standardizations in visual analog scales limited the sensitivity intensity assessment.
Despite our extensive search, none of the direct comparisons (∆Eab, ∆SGU, or sensitivity) included more than one study comparing similar treatments, restricting the application of the findings to clinical conditions, considering the high number of indirect comparisons. Moreover, the study limitations excluded other delivery methods from the meta-analysis, such as dentifrices and mouthwashes. Thus, we suggest that further studies standardize the outcomes, providing means and standard deviations of ∆Eab and/or ∆SGU in each group and assessing more bleaching products.
Another factor worth noting is that 25 eligible articles declared funding sources by private companies related to dental material production and all presented satisfactory results for bleaching with OTC products. Bradley et al. [64] advocate that conflict reporting or interest statements should become more open, thus establishing reliability in study objectivity. More studies unattached to company funding should be conducted.
The body of evidence in this review presents noteworthy limitations. First, only four studies showed a low risk of bias [6, 46, 47, 55]. The primary source of bias referred to randomization, which is common in dental randomized clinical trials and significantly implicates the internal validity of studies. Second, the low number of studies in each comparison and the small sample sizes directly impacted estimate precision, contributing to the uncertainty of findings. Lastly, the low density (indicating low graph connectedness) and the lack of strong edges (meaning a low weight of evidence on each pair) impacted the certainty of NMA estimates. Nevertheless, these metrics indicate potential evidence gaps that should be addressed in further RCTs.
Conclusion
Over-the-counter products achieved satisfactory effects on tooth bleaching compared to the placebo, with little to no impact on dentin hypersensitivity and gingival irritation but with very uncertain evidence. Lower risks of bias and larger study samples are required to draw more conclusive directions.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Auschill TM, Hellwig E, Schmidale S, Sculean A, Arweiler NB (2005) Efficacy, side-effects and patients’ acceptance of different bleaching techniques (OTC, in-office, at-home). Oper Dent 30:156–163
Ghalili KM, Khawaled K, Rozen D, Afsahi V (2014) Clinical study of the safety and effectiveness of a novel over-the-counter bleaching tray system. Clin Cosmet Investig Dent 6:15–19
Naidu AS, Bennani V, Brunton JMAP, Brunton P (2020) Over-the-counter tooth whitening agents: a review of literature. Braz Dent J 31:221–235
Demarco FF, Meireles SS, Masotti AS (2009) Over-the-counter whitening agents: a concise review. Braz Oral Res 23:64–70
European Commission on Consumer Products (2005) Scientific committee on consumer products. Preliminary opinion on hydrogen peroxide in tooth whitening products 0844(4):1–50
Vladislavic NZ, Tadin A, Gavic L, Jerkovic D, Franic I, Verzak Z (2022) In vivo evaluation of whitening toothpaste efficiency and patient treatment satisfaction: a randomized controlled trial. Clin Oral Investig 26:739–750
Zantner C, Derdilopoulou F, Martus P, Kielbassa AM (2006) Randomized clinical trial on the efficacy of 2 over-the-counter whitening systems. Quintessence Int 37:695–706
Bizhang M, Chun YH, Damerau K, Singh P, Raab WH, Zimmer S (2009) Comparative clinical study of the effectiveness of three different bleaching methods. Oper Dent 34:635–641
Calatayud J, Mateos de la Varga P, Oteo Calatayud C, Calvo Box MJ (2009) Comparative clinical study of two tooth bleaching protocols with 6% hydrogen peroxide. Int J Dent 2009:928306
Kim YM, Ha AN, Kim JW, Kim SJ (2018) Double-blind Randomized Study to Evaluate the Safety and Efficacy of Over-the-counter Tooth-whitening Agents Containing 2.9% Hydrogen Peroxide. Oper Dent 43:272–281
Sanson-Fisher RW, Bonevski B, Green LW, D’Este C (2007) Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med 33:155–161
Wang X, Ji X (2020) Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies. Chest 158:S12–S20
Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ (2018) Network meta-analysis for comparative effectiveness research. Hoboken, New Jersey: John Wiley & Sons Ltd
Furukawa M, K-Kaneyama JR, Yamada M, Senda A, Manabe A, Miyazaki A (2015) Cytotoxic Effects of Hydrogen Peroxide on Human Gingival Fibroblasts In Vitro. Oper Dent. 40:430–9
Silva-Costa RSGD, Ribeiro AEL, Assunção IV et al (2018) In-office tooth bleaching with 38% hydrogen peroxide promotes moderate/severe pulp inflammation and production of ll-1β, TNF-β, GPX, FGF-2 and osteocalcin in rats. J Appl Oral Sci 26:e20170367
Shamseer L, Moher D, Clarke M et al (2015) Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647
Aromataris E, Munn Z (2020) JBI Manual for Evidence Synthesis. JBI. https://doi.org/10.46658/JBIMES-20-01>. Access in: 18 Oct 2023
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Clin Exp Metastasis 5:210
CIE Publication 15: Colorimetry Technical Report (2004) Commission Internationale de l’Eclairage; 3:18
Browning WD (2003) Use of shade guides for color measurement in tooth-bleaching studies. J Esthet Restor Dent 15(Suppl 1):S13–S20
Chaimani A, Caldwell DM, Li T et al. (2023) Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Charpter 11, Cochrane
Rezende M, Loguercio AD, Kossatz S, Reis A (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: A multi regression and logistic analysis. J Dent 45:1–6
DerSimonian R (2023) Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2.Access.In:18Oct
Tonin FS, Borba HH, Mendes AM et al (2019) Description of network meta-analysis geometry: A metrics design study. PLoS ONE 14(2):e0212650
Puhan MA, Schünemann HJ, Murad MH et al (2014) A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349:g5630
Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B (2018) Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 93:36–44
Gerlach RW, Barker ML, Sagel PA (2001) Comparative efficacy and tolerability of two direct-to-consumer tooth whitening systems. Am J Dent 14:267–272
Gerlach RW, Sagel PA, Jeffers ME, Zhou X (2002) Effect of peroxide concentration and brushing on whitening clinical response. Compend Contin Educ Dent 23:16–49
Karpinia K, Magnusson I, Barker ML, Gerlach RW (2003) Clinical comparison of two self-directed bleaching systems. J Prosthodont 12:242–248
Nathoo S, Stewart B, Petrone ME et al (2003) Comparative clinical investigation of the tooth whitening efficacy of two tooth whitening gels. J Clin Dent 14:64–69
Collins LZ, Maggio B, Liebman J et al (2004) Clinical evaluation of a novel whitening gel, containing 6% hydrogen peroxide and a standard fluoride toothpaste. J Dent 32:13–17
García-Godoy F, Villalta P, Barker ML, Gerlach RW (2004) Placebo-controlled, 6-week clinical trial on the safety and efficacy of a low-gel, 14% hydrogen-peroxide whitening strip. Compend Contin Educ Dent 25:21–26
Gerlach RW, Sagel PA (2004) Vital bleaching with a thin peroxide gel: the safety and efficacy of a professional-strength hydrogen peroxide whitening. J Am Dent Assoc 135:98–100
Gerlach RW, Zhou X (2004) Clinical trial comparing two daytime hydrogen-peroxide professional vital-bleaching systems. Compend Contin Educ Dent 25:33–40
Swift EJ Jr, Miguez PA, Barker ML, Gerlach RW (2004) Three-week clinical trial of a 14% hydrogen-peroxide, strip-based bleaching system. Compend Contin Educ Dent 25:27–32
Cronin MJ, Charles CA, Zhao Q, Dembling WZ (2005) Comparison of two over-the-counter tooth whitening products using a novel system. Compend Contin Educ Dent 26:140–148
Guerrero JC, Jiménez-Farfán MD, Lopez-Salgado A, Barker ML, Gerlach RW (2007) Professional whitening strips in a university population. Am J Dent. 20 Spec No A:15A-18A
Papas AS, Kugel G, Singh M, Barker ML, Gerlach RW (2009) Placebo-controlled clinical trial of use of 10% hydrogen peroxide whitening strips for medication-induced xerostomia. Gerontology 55:511–516
Swift EJ Jr, Heymann HO, Wilder AD Jr, Barker ML, Gerlach RW (2009) Effects of duration of whitening strip treatment on tooth color: a randomized, placebo-controlled clinical trial. J Dent 37:e51–e56
Costa JB, McPharlin R, Hilton T, Ferracane JI, Wang M (2012) Comparison of two at-home whitening products of similar peroxide concentration and different delivery methods. Oper Dent 37:333–339
Oliveira GM, Miguez PA, Oliveira GB et al (2013) Safety and efficacy of a high-adhesion whitening strip under extended wear regimen. J Dent 41:e46–e52
Perry R, Conde E, Farrell S, Gerlach RW, Towers J (2013) Comparative performance of two whitening systems in a dental practice. Compend Contin Educ Dent. 34(8):15–18
Simon JF, Powell L, Hollis S, Anastasia MK, Gerlach RW, Farrell S (2014) Placebo-controlled clinical trial evaluating 9.5% hydrogen peroxide high-adhesion whitening strips. J Clin Dent 25:49–52
Pinto MM, Gonçalves ML, Mota AC et al (2017) Controlled clinical trial addressing teeth whitening with hydrogen peroxide in adolescents: a 12-month follow-up. Clinics (Sao Paulo) 72:161–170
Cordeiro D, Toda C, Hanan S et al (2019) Clinical Evaluation of Different Delivery Methods of At-Home Bleaching Gels Composed of 10% Hydrogen Peroxide. Oper Dent 44:13–23
Monteiro MJF, Lindoso JBC, de Oliveira Conde NC, da Silva LM, Loguercio AD, Pereira JV (2019) Evaluation of the genotoxic potential of different delivery methods of at-home bleaching gels: a single-blind, randomized clinical trial. Clin Oral Investig 23:2199–2206
Brunton PA, Ellwood R, Davies R (2004) A six-month study of two self-applied tooth whitening products containing carbamide peroxide. Oper Dent 29:623–626
Gambarini G, Testarelli L, De Luca M, Dolci G (2004) Efficacy and safety assessment of a new liquid tooth whitening gel containing 5.9% hydrogen peroxide. Am J Dent 17:75–79
Zantner C, Derdilopoulou F, Martus P, Kielbassa AM (2006) Randomized clinical trial on the efficacy of a new bleaching lacquer for self-application. Oper Dent 31:308–316
Hannig C, Lindner D, Attin T (2007) Efficacy and tolerability of two home bleaching systems having different peroxide delivery. Clin Oral Investig 11:321–329
Yudhira R, Peumans M, Barker ML, Gerlach RW (2007) Clinical trial of tooth whitening with 6% hydrogen peroxide whitening strips and two whitening dentifrices. Am J Dent. 20 Spec NoA:32A-36A
Ziebolz D, Hannig C, Attin T (2008) Influence of a desensitizing agent on efficacy of a paint-on bleaching agent. Am J Dent 21:77–82
Ferrari M, Cagidiaco MC, Monticelli F, Kugel G, Barker ML, Gerlach RW (2007) Daytime use of a custom bleaching tray or whitening strips: initial and sustained color improvement. Am J Dent. 20 Spec No A:19A-22A
Llena C, Oteo C, Oteo J, Amengual J, Forner L (2016) Clinical efficacy of a bleaching enzyme-based toothpaste. A double-blind controlled clinical trial. J Dent 44:8–12
Xu X, Zhu L, Tang Y, et al. (2007) Randomized clinical trial comparing whitening strips, paint-on gel and negative control. Am J Dent. 20 Spec No A:28A-31A
Lo EC, Wong AH, McGrath C (2007) A randomized controlled trial of home tooth-whitening products. Am J Dent 20:315–318
Jung YS, Jo HY, Ahn JH et al (2019) In vivo and in vitro assessment of the bleaching effectiveness of a brush-off patch containing 3.0% hydrogen peroxide. Clin Oral Investig 23:2667–2673
Kim HJ, Jang JH, Choi D, Kim J, Shim JH, Kim DS (2020) Bleaching toothpaste with two different concentrations of hydrogen peroxide: A randomized double-blinded clinical trial. Int J Dent 103:103508
Peixoto AC, Vaez SC, Pereira NAR (2018) High-concentration carbamide peroxide can reduce the sensitivity caused by in-office tooth bleaching: a single-blinded randomized controlled trial. J Appl Oral Sci 26:e20170573
Guan YH, Lath DL, Lilley TH, Willmot DR, Marlow I, Brook AH (2005) The measurement of tooth whiteness by image analysis and spectrophotometry: a comparison. J Oral Rehabil 32(1):7–15
Ito A, Naito M, Naito Y et al (1982) Induction and characterization of gastro-duodenal lesions in mice given continuous oral administration of hydrogen peroxide. Gan. 73(2):315–22
Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74:835–840
Bradley SH, DeVito NJ, Lloyd KE et al (2020) Reducing bias and improving transparency in medical research: a critical overview of the problems, progress and suggested next steps. J R Soc Med 113:433–443
Funding
This study was partially funded by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES) – Finance Code 001. We also appreciate the support of CNPq (National Counsel of Technological and Scientific Development—Brazil) and the Minas Gerais Research Foundation—Brazil (FAPEMIG).
Author information
Authors and Affiliations
Contributions
MNO: Conception and design, analysis and interpretation of data, drafting the manuscript, final approval.
MTCV: Conception and design, analysis and interpretation of data, drafting the manuscript, final approval.
WAV: Analysis and interpretation of data, drafting the manuscript, final approval.
CLLC: Analysis and interpretation of data, drafting the manuscript, final approval.
LMO: Analysis and interpretation of data, drafting the manuscript, final approval.
GGN: Interpretation of data, drafting the manuscript (review), final approval.
GRS: Interpretation of data, drafting the manuscript (review), final approval.
LRP: Drafting the manuscript (review), final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no commercial or associative interest representing a conflict of interest regarding the manuscript.
The authors do not have any financial interests or commercial associations to disclose.
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, M.N., Vidigal, M.T.C., Vieira, W. et al. Assessment of color changes and adverse effects of over-the-counter bleaching protocols: a systematic review and network meta-analysis. Clin Oral Invest 28, 189 (2024). https://doi.org/10.1007/s00784-024-05595-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05595-5