Abstract
Objective
This study evaluates the impact of local and systemic administration of penicillin on the antimicrobial properties and growth factors of platelet-rich fibrin (PRF) under in vitro conditions.
Materials and methods
The study involved 12 volunteers. Four tubes of venous blood were collected before systemic antibiotic administration. Two tubes were centrifuged at 2700 RPM for 12 min to obtain PRF, while 0.2 ml of penicillin was locally added into other two tubes. After systemic administration, blood samples were again collected and subjected to centrifugation. The release of growth factors (IGF-1, PDGF, FGF-2, and TGFβ-1) was determined using the Enzyme-Linked Immunosorbent Assay (ELISA), and an antibiotic sensitivity test was performed for S. aureus and E. coli bacteria.
Results
Results showed that local antibiotic addition before PRF centrifugation had a significant antimicrobial effect without affecting growth factor releases. There was no statistically significant difference in antimicrobial properties between PRF prepared with systemic antibiotic administration and PRF prepared without antibiotics.
Materials and methods
The study suggests that incorporating localized antibiotics into PRF results in strong antimicrobial effects without compromise of growth factor release. However, the combination of PRF with systemic antibiotics did not significantly enhance its antimicrobial properties compared to PRF prepared without antibiotics.
Clinical relevance
Local addition of penicillin into PRF provides strong antimicrobial properties which may help reduce dependence on systemic antibiotic regimens, mitigating antibiotic resistance and minimizing associated side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical procedures commonly employed in dentistry, such as tooth extraction, periodontal surgery, and dental implant applications, often necessitate accelerated wound healing in both soft and hard tissues. In this context, the use of platelet-rich fibrin (PRF) has become routine to facilitate this process [1, 2]. PRF, derived from the patient’s own blood and prepared through a single centrifugation protocol, emerges as a biomaterial that can be directly applied to the surgical site.
Historically, thrombocyte concentrates have evolved from fibrin adhesives to various forms. Contemporary thrombocyte concentrates such as PRF exhibit greater success in terms of biological activity and preparation methods compared to earlier techniques [3]. These concentrates encompass a variety of leukocytes embedded within a dense fibrin matrix [4]. Host defense cells within the fibrin matrix contribute to preventing bacterial contamination within the surgical area by eliminating bacteria and pathogens present in the wound [5, 6].
Despite meticulous precautions, postoperative wound healing always carries the risk of infection [7, 8]. As a result, many practitioners lean towards prescribing antibiotics to prevent potential complications. However, unnecessary antibiotic usage can elevate the risk of antibiotic resistance and introduce adverse side effects [9]. Therefore, local and slow-release administration of antimicrobials via biological carriers directly in the wound may prevent this issue with minimal adverse side effects [10, 11]. However, the ideal agent for the local delivery of antibiotics and antiseptics is yet to be developed [11].
Autologous platelet concentrates demonstrated to enhance bone and soft tissue healing in periodontal regenerative procedures [12]. A recent comprehensive review concluded that PRF also exerts a significant antimicrobial activity and this effect is usually attributed to platelet proteins and reactive oxygen species [13]. Besides this natural antimicrobial activity, studies have explored the use of PRF as a drug delivery system [14]. PRF has been combined with drugs like metformin, statins, and bisphosphonates for evaluation [15,16,17,18]. In another study, silver nanoparticles were added to improve antibacterial activity, and their antibacterial, histological, and mechanical features were assessed [19]. In addition, certain antibiotics such as penicillin, clindamycin, metronidazole, gentamicin, linezolid, and vancomycin have been incorporated into various forms PRF with varying outcome levels of antimicrobial activity [7, 20, 21].
This study aims to evaluate the impact of local and systemic administration of antibiotic (penicillin) on the antimicrobial properties and growth factors of PRF under in vitro conditions.
Materials and methods
This study was conducted at the Department of Periodontology, Faculty of Dentistry, Cukurova University, with the participation of 6 male and 6 female volunteers (age between 22 and 26) with no previous history of periodontal disease. Prior to the study, all individuals were provided with information about the purpose, methodology, and procedures of the research, and written informed consent was obtained. The protocol of the study was approved by the Ethics Committee of Cukurova University Faculty of Medicine (Approval No: 86/59, Date: 08.03.2019).
The study included participants without systemic diseases and good cooperation. The individuals with systemic diseases, penicillin allergy, pregnancy or lactation, antibiotic treatment in the last 6 months, or medication causing bleeding disorders were excluded.
Study groups
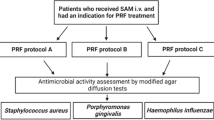
Blood samples were collected from a total of 6 male and 6 female participants, and 6 tubes were obtained for each participant, and three study groups were formed:
-
1.
Group (P-PRF): Two samples of pure PRF were prepared from 7 ml of blood obtained before systemic antibiotic administration. The PRF was obtained with centrifuging at 2700 rotations per minute (RPM) for 12 min.
-
2.
Group (LAB-PRF): Two samples of PRF prepared from 7 ml of blood were prepared before systemic antibiotic administration with the addition of 0.2 ml antibiotic (penicillin G, 1.000.000 IU solution, Pfezier, Istanbul, Turkey) in the blood tube before centrifuging with the same protocol.
-
3.
Group (SAB-PRF): Then, the patients received penicillin (2 g penicillin, 1.000.000 IU film-coated tablets, Pen-Os, Sandoz, Istanbul, Turkey) orally. PRF was prepared from 7 ml of blood obtained 1 h after systemic antibiotic administration with the same centrifuge protocol.
One PRF sample of each individual was used for microbiological tests, while the other one was analyzed for the growth factor release.
PRF preparation protocol
PRF membranes were produced using a protocol of 2700 RPM for 12 min (Relative Centrifugal Force- RCF-avg 708 g). PRF membranes were produced with 10-ml glass tubes using a Duo centrifugation device with a 40° rotor angulation with a radius of 88 mm at the clot and 110 mm at the max (Process for PRF, Nice, France).
Determination of local antibiotic dose
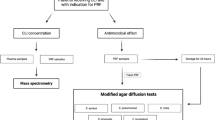
A pilot study was conducted prior to the main research to determine the appropriate local antibiotic dosage. Blood samples were collected from four volunteers, and before centrifugation, varying amounts of penicillin solution (0.5, 0.4, 0.3, and 0.2 ml) were added sequentially. After centrifugation at 2700 RPM for 12 min, the macroscopic physical characteristics of the resulting PRF were observed. It was observed that in groups where 0.5 ml, 0.4 ml, and 0.3 ml of penicillin were added, the PRF did not gel after centrifugation. In the group with 0.2 ml added, no noticeable physical changes were detected. Consequently, the local antibiotic dosage was determined as 0.2 ml of penicillin for our study.
Determination of systemic antibiotic dose
The commonly used systemic antibiotic in dentistry and periodontology, penicillin, was selected, and a prophylactic dose (1gr X 2 tablets) was administered according to the guidelines of the American Heart Association (AHA) [22]. After 60 min, venous blood was collected and subsequently centrifuged to obtain PRF.
Determination of growth factors
The obtained PRF samples from 12 volunteer participants were carefully separated from the red blood cell layer using sterile scissors and weighed on a precision balance. A volume of cell culture medium (HyClone™, RPMI-1640 MEDIUM) was added to each PRF sample in proportion to its weight. At the end of each time period (24, 48, and 72 h), small portions of the PRF samples containing the cell culture medium were collected from the top of the PRF and transferred to separate Eppendorf tubes for further use in the ELISA test. Throughout the incubation period, the samples were maintained on an orbital shaker under gentle conditions for 72 h after the addition of the cell culture medium. At the conclusion of each incubation period, the samples were subjected to ELISA assays (Fine Test®, Wuhan Fine Biotech Co., Ltd.) for the assessment of growth factors (IGF-1, PDGF, FGF-2, and TGFβ-1). All ELISA tests were performed according to the manufacturer’s instructions.
Microbiological analysis
Bacterial suspensions of S. aureus and E. coli were prepared. Platelet-rich plasma samples were mixed with bacterial suspensions, and dilutions were prepared for each group. The samples were incubated, and colony counts were determined after 18–24 h.
In accordance with reference studies, antimicrobial activity testing was conducted using strains of Gram-positive bacteria S. aureus and Gram-negative bacteria E. coli [125, 151]. Strains of S. aureus ATCC 29213 and E. coli ATCC 25922 were separately inoculated onto 5% sheep blood agar (Beckton Dickinson, Darmstadt, Germany) medium, followed by incubation at 37 °C in an incubator (Nüve EN 500, Turkey) overnight. After incubation, the cultured pure isolates of E. coli and S. aureus were subcultured onto Mueller Hinton Agar (MHA) and incubated overnight at 37 °C.
After incubating the samples in the incubator at 37 °C for 24, 48, and 72 h, 10 µl of each sample from the respective tubes of each group was taken and diluted in sterile broth to achieve a dilution ratio of 10^ − 8 (total dilution ratio of 10^ − 16). From the diluted suspension, 10 µl was further taken and inoculated onto three separate sheep blood agar plates for quantitative assessment. The cultures were incubated for 18–24 h, and colony counting was performed at the end of the incubation period. The arithmetic means of colonies from the three plates were calculated. The upper limit for countable colonies was set at 1000.
Statistical analysis
Numerical measurements were summarized as medians with minimum and maximum values, given the non-normal distribution of the data. The Kruskal–Wallis test was employed to compare more than two groups in terms of non-normally distributed numerical measurements, and the Dunn test was applied for pair-wise comparisons between groups where significant differences were detected. The Friedman test was used for comparison of non-normally distributed numerical measurements taken from the same samples at different time points. IBM SPSS Statistics Version 20.0 software package was utilized for statistical analysis. A significance level of 0.05 was considered.
Results
Evaluation of antimicrobial properties
Evaluation for S. aureus
Significant differences (p < 0.001) were observed in S. aureus growth ((× 100 × 106) cfu/ml) at 24, 48, and 72 h among the study groups. No S. aureus growth was observed at any time point in the LAB-PRF group, resulting in a bacterial count of zero (p < 0.001). In contrast, S. aureus growth was observed in the P-PRF and SAB-PRF groups throughout all 3 days. Although the SAB-PRF group exhibited less bacterial growth compared to the P-PRF group, no statistical significance was observed between these two groups (p > 0.05). Intra-group comparisons showed consistent S. aureus growth levels across all time periods within each group (p > 0.05) (Table 1).
Evaluation for E. coli
Statistically significant differences (p < 0.001) were found in E. coli growth ((× 100 × 106) cfu/ml) at 24, 48, and 72 h among the study groups. Similar to S. aureus, no E. coli growth was observed at any time point in the LAB-PRF group, resulting in a bacterial count of zero (p < 0.001). Conversely, E. coli growth was observed in the P-PRF and SAB-PRF groups throughout all 3 days. Similar to the S. aureus evaluation, no statistical significance was found between the P-PRF and SAB-PRF groups (p > 0.05). Intra-group comparisons indicated consistent E. coli growth levels across all time periods within each group (p > 0.05) (Table 1).
Evaluation of growth factors
TGF-β1
Inter-group comparisons showed no statistically significant differences in TGF-β1 measurements (pg/ml) at any time period among the study groups (p > 0.05) (Table 2). Intra-group comparisons revealed that TGF-β1 levels in the P-PRF group were consistent across all time points (p > 0.05), while in the LAB-PRF group, TGF-β1 level was statistically higher at 24 h compared to 72 h (p < 0.05). Similarly, in the SAB-PRF group, TGF-β1 level at 48 h was significantly higher than at 72 h (p < 0.05).
PDGF
Inter-group comparisons indicated no statistically significant differences in PDGF measurements (pg/ml) at 24 and 48 h (p > 0.05) (Table 2). However, at 72 h, PDGF level in the SAB-PRF group was statistically higher than in the LAB-PRF group (p < 0.05). Intra-group comparisons showed that PDGF level at 24 h was significantly higher in the P-PRF and LAB-PRF groups compared to 72 h (p < 0.05). Conversely, in the SAB-PRF group, no statistically significant difference was observed in PDGF levels at 24, 48, and 72 h (p > 0.05).
IGF-1
Inter-group comparisons revealed no statistically significant differences in IGF-1 measurements (pg/ml) at 24, 48, and 72 h among the study groups (p > 0.05) (Table 2). Intra-group comparisons indicated higher IGF-1 levels at 72 h in the P-PRF group compared to the other two time periods and in the LAB-PRF group at 72 h compared to 24 h (p < 0.05). Conversely, in the SAB-PRF group, higher IGF-1 levels were found at 48 h compared to the first day (p < 0.05).
FGF-2
Inter-group comparisons showed no statistically significant differences in FGF-2 measurements (pg/ml) at 24, 48, and 72 h among the study groups (p > 0.05) (Table 2). Intra-group comparisons revealed that the highest FGF-2 levels were observed at 72 h in all three groups, and this increase was statistically significant compared to the first 2 days (p < 0.05).
Discussion
In recent years, efforts to enhance wound healing in periodontal surgical procedures have led to the exploration of the antimicrobial properties of PRF and its potential synergies with local or systemic antibiotics. This study aimed to evaluate the impact of systemically administered and locally added penicillin before PRF centrifugation on the antimicrobial efficacy and growth factor release of the resulting tissue engineering scaffold. The results showed that the local addition of penicillin prior to PRF centrifugation exhibited robust antimicrobial activity without adversely affecting the release of growth factors, while systemic penicillin did not provide additional benefit.
Limited research exists focusing on the use of PRF in combination with antimicrobials. Polak et al. utilized PRF as a drug delivery system for antimicrobials (metronidazole, clindamycin, and penicillin solutions) in different volumes before centrifugation to evaluate their antibacterial activities [7]. It has been found that adding antibiotics locally to the tube before centrifugation causes changes in the physical properties of PRF. The authors reported that adding 2 ml and 1 ml penicillin solutions caused significant changes in the physical properties of PRF, while adding a 0.5 ml solution did not result in any macroscopic physical changes [7]. Before starting our study, a pilot study was conducted to determine the ideal antibiotic concentration. The results showed that PRF with 0.5 ml, 0.4 ml, and 0.3 ml penicillin solution displayed noticeable physical degradation, while PRF with 0.2 ml penicillin solution maintained its physical properties. Hence, our study utilized a 0.2 ml penicillin solution.
Polak et al. investigated the antimicrobial effectiveness of PRF using different forms (clot and membrane) and locally added solutions of metronidazole, clindamycin, and penicillin against F. nucleatum and S. aureus. The best antibacterial activity was observed with penicillin at 24, 48, 72, and 96 h [7]. Hence, in our study, we used penicillin, which is commonly used in dental and periodontal surgery for prophylactic, preventive, and therapeutic purposes.
In a similar study, vancomycin, clindamycin, and cefazidime were added to PRP, and their effectiveness against S. aureus, E. coli, and P. aeruginosa was assessed at 1, 4, 8, 24, 48, and 72 h. Ultimately, it was found that these combinations exhibited higher antimicrobial activity compared to the pure PRP group and continued to release above the MIC value even after 3 days, despite releasing the majority of antibiotics within the first 10 min [23].
Siawash and colleagues conducted a study where they systemically and locally added metronidazole solution before centrifugation to examine the antimicrobial effectiveness of PRF against P. gingivalis, P. intermedia, and F. nucleatum bacteria. It was found that locally added metronidazole significantly increased [20] the antibacterial capacity. In this study, the changes in growth factors for the locally added group were also assessed. The release of growth factors at 4 h, 1, 3, 7, and 14 days showed that the maximum release for PDGF-AB, VEGF, and TGF-β1 occurred at 4 h, 1st day, and 3rd day. When comparing the changes in growth factors with the antibiotic amounts, no significant differences were found [20].
There are limited articles exploring the contribution of systemically administered antibiotics to the antimicrobial activity of PRF. In our study, it was found that systemic antibiotic administration prior to the PRF preparation protocol did not result in additional antibacterial effects, aligning with previous research findings. Peck et al. assessed the antimicrobial activity of L-PRF against S. mutans following a single dose of systemic antibiotic. Inhibition was observed at 24 h, but not at 48 h and beyond [24]. Similarly, Saiwasch et al. investigated the antimicrobial properties of PRF prepared 2 h after systemic administration of 2 g amoxicillin and 500 mg metronidazole. In terms of pre- and post-antibiotic inhibition zones, amoxicillin exhibited the highest inhibition zone against P. gingivalis and the lowest against P. intermedia. Amoxicillin demonstrated significant effects against three pathogens. On the other hand, metronidazole was effective against P. gingivalis and F. nucleatum but showed no effect against P. intermedia [20].
Although extensive research has been performed on the clinical effectiveness of PRF, there are still controversies related to the preparation protocols which complicates the accurate interpretation of the research results. A consensus report of 2019 listed that the RCF values, dimensions of the centrifuge rotor and rotor angulation, RPM, time, and composition of the blood tubes are the main parameters of the production process of PRF [25]. In his detailed and authoritative review, Miron et al. [26] have described that the RCF should be included in all reports as this force significantly affects the outcome of PRF in terms of cell counts and growth factor release. In addition, the type of the blood tube may also cause substantial changes as tubes with silica and silicon coatings have shown to be cytotoxic leading to cell apoptosis [27].
The main disadvantage of the angle centrifugation system which is used in this study is that the blood cells are driven at the back walls of the tubes which causes difficulty during separation due to their different cell densities. In contrast, horizontal centrifugation systems cells are evenly distributed in the upper layers up to four times of concentration with minimal cell damage [26]. Other potential improvements of PRF preparation protocols include the concentrated PRF protocol in which the cells are specifically sent to the buffy coat region at higher RCF values [28], the development of PRF tubes hydrophobic inner surface to intentionally delay clotting, and the use of cooling devices to increase working time [29].
The main finding of the current study is that the local application of penicillin significantly enhanced the natural antibacterial effect of the PRF, preventing the growth of both tested microorganisms throughout all evaluation periods entirely. This observation may be attributed to the integration of penicillin into the PRF matrix structure, allowing for a slow and sustained release as the PRF is gradually resorbed. This characteristic may enable the antimicrobials to reach high concentrations locally, especially in contaminated wound areas such as periodontal defects. In both dentistry and medicine, fibrin-based matrices are commonly used in tissue engineering due to their receptor-binding capacity for various types of cells, proteins, and growth factors [30]. In addition, it was observed that the use of antibiotics in combination with PRF did not compromise the release of growth factors, a vital concern in tissue engineering applications. The preservation of growth factors’ integrity is crucial for successful tissue regeneration. Our study aligns with prior investigations that reported growth factor release patterns to be stable despite the incorporation of antibiotics into the scaffold. This concordance underscores the viability of our approach in maintaining tissue healing capabilities while augmenting antimicrobial effects.
The main limitations of the study include the lack of microbiological analysis for potent pathogens such as P. gingivalis, A. actinomycetemcommitans, and F. nucleatum and the use of only type of antibiotic without any analysis of the release of incorporated antibicrobial. In addition, PRF structure was assessed solely on a macroscopic level following local antibiotic application. Evaluating the mechanical properties histologically through techniques such as SEM would provide more comprehensive insights. Therefore, future studies with extended evaluation periods, along with more comprehensive microbial analyses and detailed mechanical property assessments, are needed to show the antimicrobial effects of PRF with local addition of different types and concentrations of antibiotics. Furthermore, certain individualistic characteristics (such as age, sex, systemic conditions) which may potentially influence the properties of PRF should also be investigated.
Conclusion
In conclusion, the results of the current study show the potential of incorporating antibiotics into PRF scaffolds to enhance their antimicrobial properties without compromising growth factor release. This localized approach offers a promising strategy for addressing oral infections and promoting tissue regeneration. Depending on the desired outcome, such as strong antimicrobial efficacy in cases of periodontal bone defects/tissue regeneration and immediate implant placement after extractions, the incorporation of antibiotics into PRF can be tailored accordingly. However, the complex interplay between antimicrobial effects, growth factor release, and potential antibiotic resistance necessitates further clinical investigations to refine and optimize this approach. The clinical extrapolation of the present in vitro findings necessitates a prudent approach, as the effectiveness of the formulation and any alterations in the properties of PRF must be substantiated through clinical investigations and animal trials. As the field of tissue engineering continues to evolve, the integration of antibiotics into PRF matrices holds promise for advancing both periodontal and broader regenerative therapies.
Conflict of interest
The authors declare no competing interests.
Data availability
The data of this study are available on request from the corresponding author.
References
Kumar VR, Gangadharan G (2015) Platelet rich fibrin in dentistry: a review of literature. Int J Med 3:72–76
Saini K, Chopra P, Sheokand V (2020) Journey of platelet concentrates: a review. Biomed Pharmacol J 13:185–191
Agrawal AA (2017) Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J Clin Cases 5:159
Varshney S, Dwivedi A, Pandey V (2019) Antimicrobial effects of various platelet rich concentrates-vibes from in-vitro studies-a systematic review. J Oral Biol Craniofac Res 9:299–305
Fabbro MD, Bortolin M, Taschieri S, Ceci C, Weinstein RL (2016) Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 27:276–285
Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang H-L (2017) Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Invest 21:1913–1927
Polak D, Clemer-Shamai N, Shapira L (2019) Incorporating antibiotics into platelet-rich fibrin: a novel antibiotics slow-release biological device. J Clin Periodontol 46:241–247
Yang LC, Hu SW, Yan M, Yang JJ, Tsou SH, Lin YY (2015) Antimicrobial activity of platelet-rich plasma and other plasma preparations against periodontal pathogens. J Periodontol 86:310–318
Kreutzer K, Storck K and Weitz J (2014) Current evidence regarding prophylactic antibiotics in head and neck and maxillofacial surgery. Biomed Res Int 879437:1–7
Yusri S, Elfana A, Elbattawy W, Fawzy El-Sayed KM (2021) Effect of locally delivered adjunctive antibiotics during surgical periodontal therapy: a systematic review and meta-analysis. Clin Oral Invest 25:5127–5138
Figuero E, Serrano J, Arweiler NB, Auschill TM, Gürkan A and Emingil G (2023) Supra and subgingival application of antiseptics or antibiotics during periodontal therapy. Periodontol 2000. 00:1–34
Quirynen M, Siawasch S, Temmerman A, Cortellini S, Dhondt R, Teughels W, Castro A (2023) Do autologous platelet concentrates (APCs) have a role in intra‐oral bone regeneration? A critical review of clinical guidelines on decision‐making process. Periodontol 2000. 00:1–16
Moraschini V, Miron RJ, Mourão CFdAB, Louro RS, Sculean A, da Fonseca LAM, Calasans Maia MD, Shibli JA (2023) Antimicrobial effect of platelet‐rich fibrin: a systematic review of in vitro evidence‐based studies. Periodontol 2000. 00:1–12
Miron RJ, Zhang Y (2018) Autologous liquid platelet rich fibrin: a novel drug delivery system. Acta Biomater 75:35–51
Li F, Jiang P, Pan J, Liu C and Zheng L (2019) Synergistic application of platelet-rich fibrin and 1% alendronate in periodontal bone regeneration: a meta-analysis. Biomed Res Int 18(9148183):1–12
Martande SS, Kumari M, Pradeep A, Singh SP, Suke DK, Guruprasad C (2016) Platelet-rich fibrin combined with 1.2% atorvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 87:1039–1046
Pradeep A, Karvekar S, Nagpal K, Patnaik K, Raju A, Singh P (2016) Rosuvastatin 1.2 mg in situ gel combined with 1: 1 mixture of autologous platelet-rich fibrin and porous hydroxyapatite bone graft in surgical treatment of mandibular class II furcation defects: a randomized clinical control trial. J Periodontol 87:5–13
Pradeep A, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad C (2015) Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 86:729–737
Khorshidi H, Haddadi P, Raoofi S, Badiee P and Dehghani Nazhvani A (2018) Does adding silver nanoparticles to leukocyte-and platelet-rich fibrin improve its properties? Biomed Res Int. 27(8515829):1–5
Siawasch S, Andrade C, Castro A, Teughels W, Temmerman A, Quirynen M (2022) Impact of local and systemic antimicrobials on leukocyte-and platelet rich fibrin: an in vitro study. Sci Rep 12:2710
Bennardo F, Gallelli L, Palleria C, Colosimo M, Fortunato L, De Sarro G, Giudice A (2023) Can platelet-rich fibrin act as a natural carrier for antibiotics delivery? A proof-of-concept study for oral surgical procedures. BMC Oral Health 23:1–10
Wilson W (2008) American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 118:887–896
Wang S, Li Y, Li S, Yang J, Tang R, Li X, Li L, Fei J (2021) Platelet-rich plasma loaded with antibiotics as an affiliated treatment for infected bone defect by combining wound healing property and antibacterial activity. Platelets 32:479–491
Peck M, Hiss D, Stephen L, Maboza E (2018) Antibiotic release from leukocyteand platelet-rich fibrin (L-PRF) an observational study. South Afr Dental J 73:268–270
Miron RJ, Pinto NR, Quirynen M, Ghanaati S (2019) Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. Book title, Wiley Online Library
Miron RJ, Fujioka‐Kobayashi M, Sculean A and Zhang Y (2023) Optimization of platelet‐rich fibrin. Periodontol 2000. 00:1–13
Miron RJ, Kawase T, Dham A, Zhang Y, Fujioka-Kobayashi M, Sculean A (2021) A technical note on contamination from PRF tubes containing silica and silicone. BMC Oral Health 21:1–11
Miron RJ, Chai J, Zhang P, Li Y, Wang Y, Mourão CFdAB, Sculean A, Fujioka Kobayashi M, Zhang Y (2020) A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin Oral Invest 24:2819–2828
Miron RJ, Horrocks NA, Zhang Y, Horrocks G, Pikos MA, Sculean A (2022) Extending the working properties of liquid platelet-rich fibrin using chemically modified PET tubes and the Bio-Cool device. Clin Oral Invest 26:2873–2878
Kram HB, Bansal M, Timberlake O, Shoemaker WC (1991) Antibacterial effects of fibrin glue-antibiotic mixtures. J Surg Res 50:175–178
Funding
This study was supported by the Cukurova University Research Fund, Project number TDH 2021–13751.
Author information
Authors and Affiliations
Contributions
M.O. and M.C.H. contributed to study conception and design. S.C.K. was responsible for obtaining and preparing the samples for data collection. H.O. performed ELISA analysis. B.A., O.U.T and S.C.I. performed the data analysis and prepared the manuscript. All authors critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The protocol of the study was approved by the Ethics Committee of Cukurova University Faculty of Medicine, Adana, Turkey (Approval No: 86/59, Date: 08.03.2019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozcan, M., Kabaklı, S.C., Alkaya, B. et al. The impact of local and systemic penicillin on antimicrobial properties and growth factor release in platelet-rich fibrin: In vitro study. Clin Oral Invest 28, 61 (2024). https://doi.org/10.1007/s00784-023-05428-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-023-05428-x