Abstract
Objectives
The aim of this study was to evaluate the clinical outcomes of diode laser as an adjunct to nonsurgical periodontal therapy (NSPT) for residual periodontal pockets in mandibular second molars.
Materials and methods
Sixty-seven mandibular second molars (154 residual periodontal pockets) were recruited into the study and randomly assigned to the Laser + NSPT group and the NSPT group. The Laser + NSPT group underwent NSPT adjunct with diode laser radiation (wavelength: 810 nm, power: 1.5 W, 40 s maximum), while the NSPT group underwent nonsurgical periodontal therapy alone. Clinical parameters were measured at baseline (T0) and 4(T1), 12(T2), and 24(T3), weeks after treatment.
Results
Periodontal pocket depth (PPD), clinical attachment loss (CAL), and bleeding on probing (BOP) in both groups showed significant improvements at the end of study compared to baseline. The reductions of PPD, CAL, and BOP in the Laser + NSPT group were significantly greater than NSPT group. At T3, the Laser + NSPT group had a mean PPD of 3.06 ± 0.86 mm, CAL of 2.58 ± 0.94 mm and BOP of 15.49%, while the NSPT group had a mean PPD of 4.46 ± 1.57 mm, CAL of 3.03 ± 1.25 mm and BOP of 64.29%.
Conclusions
The diode laser as an adjunct to nonsurgical periodontal therapy may contribute to clinical outcomes for residual periodontal pockets. However, the approach may cause reduction of keratinized tissue width.
Trial registration number
This study was registered in the Chinese Clinical Trial Registry ChiCTR2200061194.
Clinical relevance
Diode laser as an adjunct to nonsurgical periodontal therapy may contribute to the clinical outcomes for residual periodontal pockets in mandibular second molars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The success of periodontal treatment depends primarily on the effective removal of the pathogenic substances, such as supra- and subgingival pathogens and their endotoxins [1]. Removal of these pathogenic substances ensures biocompatibility between the root surface and the connective tissue attachment after treatment [2]. Nonsurgical periodontal therapy (NSPT) is mostly accomplished by manual instruments and ultrasonic scalers. Nevertheless, it is difficulty to thoroughly disinfect deep periodontal pockets and sites that are hard to access with these instruments [3]. These sites often include periodontal pockets with periodontal probing depth (PPD) ≥ 5 mm, root furcation, and root concavities [4]. The second mandibular molars are one of these challenging NSPT sites. Also, due to the potential existence of impacted mandibular third molars, mandibular second molars are prone to the recurrence of deep periodontal pockets [5]. Although there are several periodontal surgical approaches for this issue, these procedures have the same challenges as nonsurgical therapy.
Deep residual periodontal pockets may provide an environment for periodontal pathogens to colonize [6], leading to persistent or recurrent infections [7]. Ultimately, patients may have to undergo repeated mechanical treatment. However, such a protocol could not be ideal because it may lead to excessive removal of the dentin and cementum tissue. Similarly, frequent topical or systemic antibiotic use is not a viable choice since antibiotic usage can lead to complications such as bacterial resistance [8]. As a result of these limitations, a therapy that is both effective for the residual pockets and safe for the underlying tissue is required.
Diode laser is promising for applications in periodontal pocket disinfection due to its suitable wavelength for soft tissues, less thermal damage, and ease of use [9]. However, several past studies have reported contradictory results for the clinical outcomes of diode laser. Randomized controlled trials have found that diode laser as an adjunct for NSPT may have no or only minimal beneficial effects on periodontal healing [10, 11]. Conclusive recommendations from meta-analyses are also absent due to the heterogeneity of the studies or the limited sample size and disease severity descriptions [12, 13]. Nevertheless, a few recent studies have demonstrated that diode laser with NSPT produces promising results in patients who do not respond well to NSPT alone, such as those with deep periodontal pockets (PPD ≥ 6 mm), periodontitis patients with type 2 diabetes mellitus [14, 15], and aggressive periodontitis [16, 17]. There are also studies showing that diode laser with NSPT significantly improved the clinical outcomes of residual periodontal pockets in the maintenance phase [18]. Nd:YAG laser (wavelength: 1064 nm), which has a similar wavelength as the diode laser, has shown a beneficial PPD reducing effect [19]. Based on the above studies, the clinical outcomes of the diode laser as an adjunct to NSPT on residual periodontal pockets need to be evaluated further. The purpose of this study is to assess the clinical outcomes of using a diode laser as an adjunct to NSPT to treat residual periodontal pockets in mandibular second molars.
Materials and methods
Subject selection and study design
This study was a single-blind, randomized, controlled clinical trial. Subjects were patients who visited the Department of Periodontics, Stomatological Hospital of Tongji University, from August 2021 to August 2022. The study was conducted in accordance with the Declaration of Helsinki, which was enacted in 1975 and revised in 2000. The study protocol was approved by the Ethics Committee of the Affiliated Stomatology Hospital of Tongji University, Shanghai, China ([2022]-SR-069) and was registered in the Chinese Clinical Trial Registry (ChiCTR2200061194). All enrolled patients were thoroughly informed of the nature, potential risks, and benefits of their participation in the study and signed an informed consent form before participation.
The sample size was estimated using software PASS version11 (NCSS, LLC, UT, USA). The primary outcome “change of PPD after 6 months” was used to determine the sample size. A statistical power of 80% was used to detect a significant difference of 1.0 mm for PPD (α of 0.05, standard deviation [SD] of 1.5 mm) [18]. Based on this information, 24 patients were required in each group to have a power of 80%. To account for possible dropouts, at least 30 subjects should be recruited for each group.
Randomization was performed using a computer-generated random number table method (Microsoft Office Excel, Microsoft Corp., WA, USA). The randomization process was performed by an independent operator who was not subsequently involved in this study. The allocation sequence was concealed in opaque sealed envelopes, and the details of the series were unknown to the patients and the clinical parameters examiner. The NSPT and laser treatment were performed by an experienced periodontist. Patients were provided with opaque glasses during treatment, regardless of their allocation, to guarantee that they were unaware of their group. The examination and recording of clinical parameters were performed by another calibrated periodontist who was unaware of the treatment procedure and patient allocation at the first visit and the later follow-up visits.
The inclusion criteria were as follows: (1) residual periodontal pockets with probing depths ≥ 5 mm in the mandibular second molars after four weeks of NSPT; (2) mandibular third molars extracted for more than six months or congenitally absent; (3) less than 3 degrees of tooth movement; (4) no periodontal surgical treatment, antibacterial medication, or anti-inflammatory medication was administered in the past 6 months; and (5) no systemic disease related to the treatment. Exclusion criteria were (1) presence of caries, pulpal disease, and restorations in the mandibular second molar; (2) mandibular second molar inclination > 30° or severe malocclusion; (4) pregnant or lactating women; (5) ongoing orthodontic treatment or recent need for orthodontic treatment; and (6) smoking > 5 cigarettes/day.
Treatment protocols
At the first visit, the patients were given careful oral hygiene instructions (OHIs), which were repeated at every subsequent visit. Panoramic radiographs were taken to confirm that the mandibular second and third molars met the criteria. At the first three visits, supra- and subgingival scaling was accomplished using an ultrasonic device and manual instruments. All patients were re-evaluated four weeks after the completion of the initial therapy, and those who met the inclusion criteria were recruited for the study. As a baseline (T0), clinical parameters of the recruited residual pockets were recorded. After randomization, patients were assigned to the Laser + NSPT group or the NSPT group.
For the Laser + NSPT group, residual periodontal pockets were treated with laser as an adjunct to NSPT at T0. Laser therapy was performed by a diode laser device (Pioneer Pro Diode Laser, CAO Group, Inc., UT, USA). The laser parameters were set as follows: wavelength was 810 nm, power was 1.5 W, and radiation mode was continuous mode. During irradiation, the laser fiber was positioned parallel to the root surface and inserted into the periodontal pocket in contact with the soft tissue wall. The fiber was moved gently in a sweeping manner. The total irradiation time for each tooth did not exceed 40 s. Carbonized granulation tissue entangled around the fiber was promptly cleaned off using wet gauze containing 0.9% saline, and periodontal pockets were continuously rinsed with saline. The NSPT group underwent saline irrigation of the periodontal pockets. No sham laser irradiation was performed on the NSPT group to avoid unnecessary invasion of periodontal tissue, which might interrupt the healing of periodontal pocket.
Clinical measurements and outcomes
Clinical parameters were measured and recorded at baseline (T0) and at 4, 12, and 24 weeks after treatment (T1, T2, T3). A UNC-15 periodontal probe (Hu-Friedy, IL, USA) was used to record including PPD, clinical attachment loss (CAL), bleeding on probing (BOP), plaque index (PLI). These parameters were collected at six sites of the mandibular second molar: mesiobuccal, mesiolingual, buccal, lingual, distobuccal, and distolingual. Keratinized tissue width (KTW), tooth movement (TM) and furcation involvement of the buccal sites (BFI) and lingual sites (LFI) were also examined. The mucogingival junction was localized by direct visual inspection of the color and movability of the soft tissues, Keratinized tissue width was measured from the mucogingival junction to the gingival margin with a UNC-15 periodontal probe, at the mesiobuccal, buccal and distobuccal sites, and the mean values were used to evaluate the KTW of the teeth. Furcation involvement was defined and graded by Hamp’s index [20].
The primary outcome of this study was changes in PPD. The secondary outcomes were changes in CAL, KTW, TM, BFI, LFI, and percentage of sites with BOP and PLI,
Examiner calibration
A calibrated examiner who was uninformed of the patient’s grouping performed all measurements. Five patients were recruited for examiner calibration. At two separate sessions 48 h apart, duplicate measurements of PPD, KTW and CAL were obtained at the same tooth. Calibration was accepted if the percentage agreement between measurements was more than 90%. The procedure was repeated every month throughout the period of the study.
Statistical analysis
The statistical analysis of this study was performed using SPSS version 27.0, (IBM, IL, USA). The significance level α was set at 0.05. Normality of the data was tested using the Shapiro‒Wilk test. Clinical parameters were analyzed using the generalized estimating equations method (GEE), and post hoc comparisons were made using least significant differences (LSD). Continuous variables are expressed as the mean ± SD, while categorical variables are expressed as percentages.
Results
General information
A total of 70 mandibular second molars were examined and received initial therapy during the first three visits. 3 teeth were excluded for not meeting inclusion criteria. Sixty-seven teeth were randomized and allocated to Laser + NSPT group or NSPT group. Four teeth at T1 (6 sites) and 4 teeth at T2 (7 sites) were lost to follow-up due to personal or schedule issues. A total of 59 teeth (141 sites) eventually completed the study, including 29 teeth (71 sites) in the Laser + NSPT group and 30 teeth (70 sites) in the NSPT group (Fig. 1). There was no significant difference in age, gender, number of smokers, or sites distribution between the two groups. PPD and BOP showed no significant differences between two groups at baseline, while CAL and KTW were significant greater in the Laser + NSPT group than in the NSPT group.
Clinical outcomes
The PD, CAL, BOP, PLI, and KTW for the Laser + NSPT group and the NSPT group are shown in Table 1. In the intragroup comparison, all sites, regardless of grouping, showed significant improvements in PPD, CAL, and BOP at T1, T2, and T3 compared with T0. The primary outcome PPD in the Laser + NSPT group was 3.06 ± 0.86 mm at T3, which was significantly better than the previous follow-up (T1 or T2). PPD in the NSPT group was 4.46 ± 1.76 mm at T3, which was not significantly different with T1 or T2. Both the Laser + NSPT and NSPT groups showed a significant reduction in CAL at T3 compared to T1 and T2. The BOP was 15.49% in the Laser + NSPT group and 64.29% in the NSPT group. When compared to T0, the BOP of the laser + NSPT group significantly improved at T1, and it further significantly decreased at T3. The BOP of NSPT group also significantly improved at T1 compared to T0, but there was no subsequent improvement. The KTW of laser + NSPT group significantly reduced at T3 compared to T0, while the NSPT group showed no significant change in KTW over the trial. Statistical analysis did not reveal significant differences in PLI, TM, BFI and LFI between follow-ups.
In the intergroup comparison, the Laser + NSPT groups showed significantly better clinical outcomes at T1, T2, and T3 for PPD, CAL, and BOP than the NSPT group. KTW at T0 was significantly wider in the Laser + NSPT group (5.07 ± 0.91 mm) than in the NSPT group (4.17 ± 1.14 mm). At T3, the Laser + NSPT group was 4.32 ± 1.06 mm, whereas the NSPT group measured 3.99 ± 0.99 mm, with no significant difference between the two groups. At all follow-ups, PLI, TM, BFI and LFI were not significantly different between the two groups.
Discussion
The mandibular second molar is susceptible to developing deep residual periodontal pockets due to impacted third molars or poor plaque management [21]. Several approaches, including repeated mechanical therapy and topical or systemic antibiotics, have been proposed for the treatment of residual periodontal pockets in patients. However, studies demonstrated that repeated scaling and root plaining during the maintenance phase did not improve the effectiveness of subgingival debridement of periodontal pockets that had been treated during initial periodontal therapy [22].
The applications of laser in the treatment of periodontitis or peri-implantitis are quite extensive, including laser treatment alone, laser as an adjunct to NSPT [23], photodynamic therapy [24] and photothermal therapy with outside irradiation [23]. Because of the variety of laser devices and the diversity in application and treatment protocols, an effective standard treatment protocol has been absent thus far. As a result, the outcomes of laser treatment reported in meta-analyses remain controversial [25]. However, a recent meta-analysis reported beneficial outcomes of diode laser as an adjunct to the treatment of deep periodontal pockets and recommended it as a first-line adjunct for clinical use [26]. The present study investigated the clinical benefits of the diode laser as an adjunct to nonsurgical therapy for residual periodontal pockets of mandibular second molars, with the hope of providing evidence of additional nonsurgical treatment approaches in such challenging sites for conventional nonsurgical therapy.
The laser parameters adopted in this study were 1.5 W and continuous mode, which remained consistent with most laser experiments [13]. However, timing of laser irradiation was performed four weeks after NSPT rather than immediately. The rationale for this was to give the periodontal soft tissue sufficient healing time after NSPT to reduce bleeding during laser irradiation. Energy of the diode laser is much readily and selectively absorbed by blood components [24]. It is assumed that if periodontal pockets are still bleeding during the irradiation, it would result in an excessive amount of energy being absorbed by the blood, not pockets, diminishing its disinfection effect on the soft tissues of the periodontal pocket wall. Additionally, it would cause unwanted thermal side effects and discomfort. Although bleeding during laser irradiation of inflaming periodontal pockets is inevitable, the accumulation of blood in the pockets can be reduced by continuous saline irrigation. Some former studies also recommend delayed laser irradiation and rinsing with saline during irradiation to flush away blood in the periodontal pocket [27, 28].
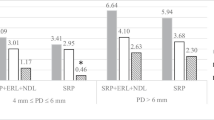
Table 2 shows the changes of clinical parameters at every visit. In our study, despite a considerable reduction in PPD at T1, T2, and T3 compared to T0, many patients still have periodontal pockets (PPD ≥ 4 mm), indicating that repeated nonsurgical therapy was less effective for residual periodontal pockets. PPD in the Laser + NSPT group reduce to 3.85 ± 1.34 mm at the first month follow-up, and further to 3.06 ± 0.86 mm at T3. Figure 2 shows the changes of PPD, CAL, BOP, and KTW during the study. The PPD reduction of Laser + NSPT group was significantly greater than that of the NSPT group throughout the study (Fig. 2). This may suggest that laser as an adjunct to NSPT, performing with the protocol described above, may be an effective approach for reducing PPD for the residual periodontal pockets.
As the Laser + NSPT group had a greater CAL than the NSPT group at baseline, the comparability of the control group was undermined. However, the following clinical results demonstrated a significant reduction in CAL in the Laser + NSPT group since T1 and showed consistent improvement in CAL during follow-ups. The outcome of CAL reduction in the Laser + NSPT group was significantly greater than the NSPT group at the end of the study (Fig. 2). CAL in the NSPT group also improved significantly after treatment but is still approximately 3 mm at T3. The mean value for the Laser + NSPT group was 2.58 ± 0.94 mm, about 1.5 mm reduction from baseline, indicating greater attachment gain, which improves long-term tooth retention.
BOP is one of the most key parameters of ongoing inflammation in gingival tissue. The major cause of persistent BOP is the existence of bacterial infections in residual periodontal pockets. According to our findings, NSPT significantly decreased BOP, but was still detected in 64.29% of sites at T3, suggesting that the gingival tissue was still in an inflammatory condition at the end of the study. This also implies, as the results above, that NSPT was less effective in removing the bacterial biofilm of the residual periodontal pocket, which is consistent with earlier studies [22]. As an adjunctive treatment, the laser was proven to be beneficial in reducing BOP within the present study. At T1, the BOP of the Laser + NSPT group had already decreased to below 30%. At T3, the BOP was 15.49%, representing a stable clinical condition (Fig. 2).
It is controversial whether laser as an adjunctive treatment has significant clinical benefits. Almost all previous studies [14, 16,17,18,19, 27,28,29,30] reported significant intragroup improvements in clinical parameters at the end of treatment compared to baseline, both in the experimental groups and control groups. However, when it comes to intergroup comparison, some studies [29, 30] reported that laser as an adjunct had no beneficial effects compared to NSPT. Nevertheless, Saglam et al. [27] reported a significant beneficial effect of laser as an adjunct in PD, CAL, and BOP reduction, using a similar laser setting to the present study, and with saline rinses applied during laser irradiation. Kamma et al. [16] and Matarese et al. [17] reported significant reduction of PD, CAL with laser as an adjunct on aggressive periodontitis but no differences were detected for BOP. Studies [14, 15] of chronic periodontitis in patients with type 2 diabetes mellitus also reported that laser as an adjunct could significantly reduce PD and CAL and found that the NSPT with laser has better outcomes in deep periodontal pockets. Tabari et al. [18] also applied the laser to recurrent periodontal pockets during maintenance phase and reported that the laser as an adjunct significantly enhanced PD, CAL, and bleeding index reduction in the short term, but only enhanced bleeding index reduction in the long term. Dortaj et al. [19] reported better PD reduction and better attachment gain in NSPT with laser but concluded that the PPD reduction was more from gingival recession. Different allocations, treatment protocols, and study populations may have contributed to the discrepancies in these studies.
Beyond these potential causes, it is also possible that, for the majority of mild periodontitis, NSPT alone already provides significant clinical improvement and therefore shows no additional effect by laser as an adjunct. When NSPT alone fails to achieve favorable clinical improvement, the clinical benefits of the laser as an adjunct are shown. Based on the results of the present study, for sites such as residual periodontal pockets in mandibular second molars, NSPT is less effective but Laser + NSPT may significantly improve clinical outcomes that last for six months of follow up. This may reduce the need for further surgical treatment for patients with residual periodontal pockets, offering it a more efficient and acceptable approach both for practitioner and patient.
At T0, KTW was significantly wider in the Laser + NSPT group than in the NSPT group. However, there was no longer a significant difference between the two groups since T2 (Fig. 2). The KTW of the Laser + NSPT group was reduced by about 1 mm at T3 compared to T0. No significant KTW reduction was found in the NSPT group throughout treatment. A recent study, using Nd:YAG laser with a similar wavelength, also found a reduction in KTW when applying laser as an adjunct [19]. Nevertheless, the mean KTW of Laser + NSPT group was 4.32 ± 1.06 mm at the end of the study, which was adequate for long-term periodontal health. Yet, caution should be maintained when considering lasers as an adjunct for teeth with narrow KTW (≤ 2 mm).
Figure 3 and Supplementary Table S1 show changes in PLI, TM, and furcation involvement (BFI and LFI) at each follow-up. Although there were consistent improvements in these parameters over the study (Fig. 3), statistical analysis did not reveal significant differences in these parameters between follow-up visits or groups. Due to the anatomy and position, it is challenging to achieve thorough and lasting plaque control for the mandibular second molar. The present study demonstrated that despite careful and repeated OHIs were exerted at each visit, approximately half of the sites still had poor plaque control (PLI > 2) at the end of trial (Fig. 3). There was no significant difference between the groups, implying that patients still generally struggle to maintain long-term plaque control in the mandibular second molar when relying simply on OHIs. Regular mechanical plaque removal treatment is recommended in some cases when long-term good plaque control is indispensable.
Clinical changes in PLI (a) as percentage of sites, clinical changes in TM (b), BFI (c) and LFI (d) at T0 (baseline) and T1, T2, and T3 between the Laser + NSPT group and NSPT group. NSPT, nonsurgical periodontal therapy; PLI, plaque index; TM, tooth movement; BFI, buccal furcation involvement; LFI, lingual furcation involvement
There are still some limitations in this study. First, there was a significant difference in CAL and KTW between the two groups at baseline, which may cause bias and led to poor comparability of the two groups. However, the primary outcome PPD showed no significant difference at baseline, limiting the bias induced by baseline comparison. Moreover, CAL was worse in the trial group than in the control group at baseline, yet the trial group still had a better outcome. Second, a small percentage of teeth (approximately 8% of sites) were dropped during follow-ups. Although telephone review shows that these patients stopped follow-up due to personal and scheduling problems, it is also possible that they may have stopped since their self-consciousness symptoms had improved. Third, the maximum follow-up was set at six months, and further long-term (> 12 months) follow-up studies may be necessary to observe longer-term clinical outcomes.
Conclusion
NSPT alone is less effective in maintaining the long-term periodontal health of periodontally compromised mandibular second molars. Diode laser as an adjunct to NSPT may contribute to clinical outcomes for residual periodontal pockets and maintain for 6 months. The approach could, however, result in KTW reduction.
Data Availability
The data are available from the corresponding author on reasonable request.
References
Cugini MA, Haffajee AD, Smith C, Kent RL Jr, Socransky SS (2000) The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol 27:30–36. https://doi.org/10.1034/j.1600-051x.2000.027001030.x
Chace R (1975) Subgingival curettage in periodontal therapy. J Periodontol 45:107–109. https://doi.org/10.1902/jop.1974.45.2.107
Sherman PR, Hutchens LH Jr, Jewson LG (1990) The effectiveness of subgingival scaling and root planing. II. Clinical responses related to residual calculus. J Periodontol 61(1):9–15. https://doi.org/10.1902/jop.1990.61.1.9
Umeda M, Takeuchi Y, Noguchi K, Huang Y, Koshy G (2000) Ishikawa I (2004) Effects of nonsurgical periodontal therapy on the microbiota. Periodontol 36:98–120. https://doi.org/10.1111/j.1600-0757.2004.03675.x
Kan KW, Liu JK, Lo EC, Corbet EF, Leung WK (2002) Residual periodontal defects distal to the mandibular second molar 6–36 months after impacted third molar extraction. J Clin Periodontol 29:1004–1011. https://doi.org/10.1034/j.1600-051x,2002.291105.x
Sbordone L, Ramaglia L, Gulletta E, Iacono V (1990) Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol 61:579–584. https://doi.org/10.1902/jop.1990.61.9.579
Mombelli A, Schmid B, Rutar A, Lang NP (2000) Persistence patterns of Porphyromonas gingivalis, Prevotella intermedia/nigrescens, and Actinobacillus actinomyetemcomitans after mechanical therapy of periodontal disease. J Periodontol 71:14–21. https://doi.org/10.1902/jop.2000.71.1.14
Cerceo E, Deitelzweig SB, Sherman BM, Amin AN (2016) Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb Drug Resist 22:412–431. https://doi.org/10.1089/mdr.2015.0220
Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22(5):302–311. https://doi.org/10.1002/(sici)1096-9101(1998)22:5%3c302::aid-lsm7%3e3.0.co;2-t
De Micheli G, de Andrade AK, Alves VT, Seto M, Pannuti CM, Cai S (2011) Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: a randomized controlled trial. Lasers Med Sci 26:43–48. https://doi.org/10.1007/s10103-009-0753-5
Dukić W, Bago I, Aurer A (2013) Roguljić M (2013) Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol 84:1111–1117. https://doi.org/10.1902/jop.2012.110708
Karlsson MR, Diogo Löfgren CI, Jansson HM (2008) The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol 200(79):2021–2028. https://doi.org/10.1902/jop.2008.080197
Pawelczyk-Madalińska M, Benedicenti S, Sălăgean T, Bordea IR, Hanna R (2021) Impact of Adjunctive Diode Laser Application to Non-Surgical Periodontal Therapy on Clinical, Microbiological and Immunological Outcomes in Management of Chronic Periodontitis: A Systematic Review of Human Randomized Controlled Clinical Trials. J Inflamm Res 15(14):2515–2545. https://doi.org/10.2147/JIR.S304946
Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Loos BG, Hakkı SS (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci 31:343–353. https://doi.org/10.1007/s10103-016-1868-0
Dengizek Eltas S, Gursel M, Eltas A, Alptekin NO, Ataoglu T (2019) Evaluation of long-term effects of diode laser application in periodontal treatment of poorly controlled type 2 diabetic patients with chronic periodontitis. Int J Dent Hyg 17:292–299. https://doi.org/10.1111/idh.12384
Kamma JJ, Vasdekis VG, Romanos GE (2009) The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters. Photomed Laser Surg 27:11–19. https://doi.org/10.1089/pho.2007.2233
Matarese G, Ramaglia L, Cicciù M, Cordasco G, Isola G (2017) The Effects of Diode Laser Therapy as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis: A 1-Year Randomized Controlled Clinical Trial. Photomed Laser Surg 35:702–709. https://doi.org/10.1089/pho.2017.4288
Alizadeh Tabari Z, Pournasir L, Mohammadreza S, Anbari F (2021) Clinical Efficacy of the 940-nm Diode Laser in the Treatment of Recurrent Pockets in the Periodontal Maintenance Phase. J Lasers Med Sci 12:e68. https://doi.org/10.34172/jlms.2021.68
Dortaj D, Bassir SH, Hakimiha N, Hong H, Aslroosta H, Fekrazad R, Moslemi N (2022) Efficacy of Nd:YAG laser-assisted periodontal therapy for the management of periodontitis: A double-blind split-mouth randomized controlled clinical trial. J Periodontol 93:662–672. https://doi.org/10.1002/JPER.21-0242
Hamp SE, Nyman S, Lindhe J (1975) Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol 2(3):126–35. https://doi.org/10.1111/j.1600-051x.1975.tb01734.x
Kan KW, Liu JK, Lo EC, Corbet EF, Leung WK (2002) Residual periodontal defects distal to the mandibular second molar 6–36 months after impacted third molar extraction. J Clin Periodontol 29:1004–1011. https://doi.org/10.1034/j.1600-051x.2002.291105.x
Anderson GB, Palmer JA, Bye FL, Smith BA, Caffesse RG (1996) Effectiveness of subgingival scaling and root planing: single versus multiple episodes of instrumentation. J Periodontol 67:367–373. https://doi.org/10.1902/jop.1996.67.4.367
de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, Scombatti de Souza SL, Ribeiro FJ (2009) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol 80:98–105. https://doi.org/10.1902/jop.2009.070465
Mizutani K, Aoki A, Coluzzi D, Yukna R, Wang CY, Pavlic V, Izumi Y (2016) Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000 71:185–212. https://doi.org/10.1111/prd.12123
Salvi GE, Stähli A, Schmidt JC, Ramseier CA, Sculean A, Walter C (2020) Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J Clin Periodontol 47(Suppl 22):176–198. https://doi.org/10.1111/jcpe.13236
Jia L, Jia J, Xie M, Zhang X, Li T, Shi L, Shi H, Zhang X (2020) Clinical attachment level gain of lasers in scaling and root planing of chronic periodontitis: a network meta-analysis of randomized controlled clinical trials. Lasers Med Sci 35:473–485. https://doi.org/10.1007/s10103-019-02875-5
Saglam M, Kantarci A, Dundar N, Hakki SS (2014) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29:37–46. https://doi.org/10.1007/s10103-012-1230-0
Crispino A, Figliuzzi MM, Iovane C, Del Giudice T, Lomanno S, Pacifico D, Fortunato L, Del Giudice R (2015) Effectiveness of a diode laser in addition to non-surgical periodontal therapy: study of intervention. Ann Stomatol (Roma) 6(1):15–20 (https://www.ncbi.nlm.nih.gov/pm c/articles/PMC4475909/)
Euzebio Alves VT, de Andrade AK, Toaliar JM, Conde MC, Zezell DM, Cai S, Pannuti CM, De Micheli G (2013) Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial. Clin Oral Investig 17(1):87–95. https://doi.org/10.1007/s00784-012-0703-7
Nguyen NT, Byarlay MR, Reinhardt RA, Marx DB, Meinberg TA, Kaldahl WB (2015) Adjunctive Non-Surgical Therapy of Inflamed Periodontal Pockets During Maintenance Therapy Using Diode Laser: A Randomized Clinical Trial. J Periodontol 86(10):1133–1140. https://doi.org/10.1902/jop.2015.150152
Acknowledgements
This study was supported by Foundation of Shanghai Science and Technology Committee (project 20Y11904200). The authors report no conflict of interest.
Funding
This study was supported by Foundation of Shanghai Science and Technology Committee (project 20Y11904200).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to conception and design of the study. Jia-wei Lu, Shi-hui Huang, Li-jun Luo have been involved in data analysis, data interpretation, drafting the manuscript and revising it critically. Jia-wei Lu have been involved in treatment performing. Shi-hui Huang, Xiao-xiao Lei have been involved in data collection and statistical analysis, Li Deng have been involved in randomization and all authors have given final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, which was enacted in 1975 and revised in 2000. The study protocol was approved by the Ethics Committee of the Affiliated Stomatology Hospital of Tongji University, Shanghai, China ([2022]-SR-069) and was registered in the Chinese Clinical Trial Registry (ChiCTR2200061194). All enrolled patients were thoroughly informed of the nature, potential risks, and benefits of their participation in the study and signed an informed consent form before participation.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Jw., Huang, Sh., Lei, Xx. et al. Clinical outcomes of diode laser as an adjunct to nonsurgical periodontal therapy for residual periodontal pockets in mandibular second molars—a randomized controlled clinical trial. Clin Oral Invest 27, 4493–4501 (2023). https://doi.org/10.1007/s00784-023-05071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05071-6