Abstract
Objectives
This study investigated the cytotoxicity, the residual monomer release, degree of conversion (DC), calcium ion (Ca2+) release, and crystal structure of TheraCal PT (ThPT) by comparison with TheraCal LC (ThLC) and mineral trioxide aggregate (MTA).
Materials and methods
The cytotoxicity of the cured materials was evaluated on human dental pulp stem cells (hDPSCs) isolated from third molars by the water-soluble tetrazolium salt (WST-1) method. The monomer release and DC of the resin-containing materials were analyzed by high-performance liquid chromatography (HPLC) and Fourier transform infrared (FTIR), respectively. The chemical composition and Ca2+ release of the materials were determined by scanning electronic microscopy–energy-dispersive spectroscopy (SEM–EDS), X-ray diffractometry (XRD), and inductively coupled plasma-optical emission spectroscopy (ICP-OES), respectively. Statistical differences were evaluated with one-way ANOVA, repeated measure ANOVA, and the Tukey test (p < 0.05).
Results
MTA showed significantly lower cytotoxicity than either ThLC or ThPT after 1, 3, and 7 days (p < 0.05). TEGDMA release of ThPT is significantly higher than ThLC (p < 0.05). All materials showed calcium Ca2+ release, with MTA significantly higher than the others (p < 0.05).
Conclusions
MTA showed low cytotoxicity and high Ca2+ release compared to ThLC and ThPT.
Clinical relevance.
The cytotoxicity and residual monomer release of ThLC and ThPT may raise concerns about the viability of hDPSCs. Further investigations with the use of in vivo research models are required to validate in vitro bioactivity properties and the potential adverse biological effects of ThLC and ThPT on hDPSCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vital pulp therapies, including indirect, direct capping, and pulpotomy procedures, manage the treatment of reversible pulpitis. Thus, it is aimed to preserve the vitality of the pulp, which has important roles in the nutrition and formation of dentin, as well as functions such as tooth innervation and defense [1]. In the case of pulp exposure, human dental pulp stem cells (hDPSCs) in pulp tissue can differentiate into odontoblast-like cells, stimulating hard tissue regeneration [2]. In vitro demonstration of the expression of odontogenic genes and in vivo measurement of dentin bridge formation demonstrate the ability of hDPSCs to regenerate dentin during dentinogenesis [3, 4]. In addition to the potential for hDPSCs to form a hard tissue barrier, pulp capping materials also promote hard tissue regeneration and prevent bacterial invasion [5].
Vital pulp therapies include the preservation of exposed pulp health and a bio-stimulus effect by the inducement of the repair mechanism, following the placement of a biocompatible material on the pulpal exposure area [6, 7]. Thus, pulpal tissue is protected from further stress which can contain operative procedures, the toxicity of restorative materials, and bacterial penetration due to microleakage. The toxicity of the pulp capping materials is an essential factor since the applied materials and pulp tissue are in contact with each other in the treatment method which aims to maintain its vitality. Therefore, the biocompatibility, antibacterial and anti-inflammatory properties, and hDPSC activity of pulp capping materials should be evaluated on the basis of chemical features and bioactivity [8, 9].
The use of calcium hydroxide-based materials has been accepted as a part of the standard treatment method for a long time in vital pulp therapies [10]. In addition, mineral trioxide aggregate (MTA; MTA Angelus, Londrina, PR, Brazil), a calcium silicate–based material, is a pulp capping material containing tricalcium silicate, tricalcium aluminate, calcium oxide, and some minerals. In comparative studies on biocompatibility, histological and clinical applications in the pulp capping applications of calcium hydroxide and MTA materials, MTA material was found to be more successful than calcium hydroxide [11, 12]. It was observed that MTA produced a less inflammatory response and pulp necrosis in the pulp exposure area and formed more homogeneous and high-thickness hard tissue [13]. MTA is currently accepted as the gold standard due to its high antibacterial performance and biocompatibility [14]. However, the major disadvantages of MTA are its long curing time, difficulty of application, long preparation time, and low resistance to discoloration [11, 15]. Also, another major disadvantage of MTA and similar hydraulic calcium silicate–based materials is that they exhibit a water-based chemical structure and therefore create an inadequate micromechanical adhesion to the overlying resin restoration [16]. It has been suggested to delay tooth restoration so as not to affect the setting reaction [17]. To overcome these limitations, new light-cured resin-modified tricalcium silicate materials are being formulated for pulp capping procedures. TheraCal LC (ThLC; Bisco Inc., Schaumburg, IL, USA) was introduced as a pulp capping material resembling the properties of MTA and containing resin monomers and calcium silicate. Among its various advantages, it can be cured immediately by exposure to light, exhibits low solubility and high mechanical strength, is easy to use, and can be fully applied to exposed pulp [18]. ThLC is transported and placed via a syringe, which, unlike conventional MTA products, eliminates the need for manual mixing procedures, thanks to its viscous nature that can flow over an exposure surface before light-curing. Therefore, a tight seal can be created, which can be easily placed on the teeth and also clinically shortened the operation time, which reduces the risk of contamination [19]. Recently, TheraCal PT (ThPT; Bisco Inc.), a new dual-cured resin-modified calcium silicate material designed for pulp capping, has been introduced for clinical use. According to the manufacturer, ThPT protects the vitality of pulp tissue by acting as a barrier to protect the dentin-pulp complex, is primarily indicated for pulpotomies, and can also be used for indirect and direct pulp capping [20, 21]. The purpose of dual-cure materials is to combine chemical polymerization and photo-polymerization to achieve complete curing in the absence of a visible light source. ThLC insertion thickness is limited to 1 mm, while ThPT is dual-cure so it can be safely placed in a single layer. However, the fact that ThLC and ThPT contain resin monomers gains these materials’ positive properties such as high bond strength, whereas it raises concerns about its detrimental effect on hDPSCs [22].
The materials used in vital pulp treatment remain in direct contact with the pulp tissue for a long time. Therefore, it should exhibit not only adequate cytocompatibility but also bioactive properties. Calcium silicate–based cements are bioactive materials with the ability to release hydroxyl and calcium ions, thereby creating an adequate environment to promote healing and repair, and providing odontoblastic cell differentiation responsible for the production of the reparative dentin bridge [23]. Calcium silicate–based materials tend to exhibit bioactivity by releasing Ca2+ and precipitation of apatite crystals when exposed to physiological fluids containing phosphate. Resin-modified calcium silicate cements are promising dental materials for pulp protection [24]. However, the release of unpolymerized monomers is believed to have a major impact on cytotoxicity in pulp cells, both directly by diffusion of monomers into pulp tissue and indirectly via intra-tubular and peritubular tubular dentin [25,26,27]. Considering that these materials are used in contact with the pulp, they should have the ability to structurally protect the pulp, as well as have a low release of monomers and a high degree of conversion to reduce the possibility of diffusion of monomers [28].
There was limited knowledge of the biochemical properties of this new material, ThPT, and its comparison with ThLC and MTA. Although there were previous studies investigating the cytotoxicity of ThPT versus ThLC using the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) on hDPSCs [20, 21], a study investigating the cytotoxicity of ThPT using the water-soluble tetrazolium salt (WST-1) assay, which does not show cytotoxic effects on cells compared to the MTT assay and providing a cell viability monitoring period longer than 3 days, has not been found in the literature. Furthermore, performing the crystal phase analysis of the ThPT material with X-ray diffractometry (XRD), comparing the similarities and differences between ThLC and MTA, and supporting this microstructural analysis with the analyzes of residual monomer, degree of conversion (DC), Ca2+ release, and energy-dispersive spectroscopy (EDS) will contribute to the literature. Therefore, this study aimed to compare the cytotoxicity, the residual monomer release, DC, release of Ca2+, and crystal structure of ThPT with ThLC and MTA. The null hypothesis of the study was that there would be no difference among the tested materials with regard to their cytotoxicity, residual monomer release, DC, release of Ca2+, and crystal structure.
Material and methods
Preparation of samples
The manufacturer, production location, composition, and lot numbers of the materials used in the study are shown in Table 1. The tested materials were prepared by placing them in sterilized cylindrical Teflon molds with a diameter of 4 mm and a height of 2 mm in accordance with the manufacturer’s instructions. ThLC and ThPT samples were polymerized at a distance of 2 mm using an LED light source (Freelight Elipar II, 3 M, MN, USA) with a light output of 1200 mW/cm2 for 20 s. MTA was prepared by mixing 1:1 MTA powder and distilled water with a metal spatula according to the manufacturer’s instructions. The prepared samples were stored in an incubator (CB 160; Binder GmbH, Tuttlingen, Germany) at 37 °C and 95% relative humidity for 24 h to ensure a complete setting.
Isolation, culture, and characterization of human dental pulp stem cells
The present study and the human dental pulp isolation protocol were found medically appropriate with the ethics committee report numbered 2021/117.65 of Cukurova University Faculty of Medicine Clinical Research Ethics Committee. The healthy adult donors with permanently impacted third mandibular molars and planned orthodontic extraction were included in the study. Of the 14 contacted patients, 10 (6 women and 4 men) agreed to participate in the study, and written informed consent was obtained from each adult patient in accordance with the Declaration of Helsinki (n = 10). The donors aged between 18 and 30 years, with no systemic disease, no pregnancy, and no routine drug use, were determined as eligibility criteria for this study. The impacted teeth were extracted from the donors in the Clinic of Oral, Dental, and Maxillofacial Surgery of Dentistry Faculty of Cukurova University under sterile operating room conditions.
The obtained teeth were immersed in a physiological solution containing 5% penicillin/streptomycin/amphotericin (Gibco, Grand Island, NY, USA) to avoid contamination. Shortly thereafter, under sterile conditions, the dental crown was divided into several pieces to reveal dental pulp tissue and was washed in Hanks Balanced Salt Solution (HBSS; Gibco). Collagenase type I (Sigma-Aldrich, St. Louis MO, USA) was used to generate single-cell suspensions from pulp tissue. The cells were cultured in the modified Eagle’s medium-alpha (MEM; Gibco) containing 10% fetal bovine serum (FBS; Gibco) and 100 IU/mL penicillin–100 µg/mL streptomycin at 37 °C in 5% CO2 conditions, and the medium was freshened every 3 days [29]. Stem cell characterization was performed within the scope of the International Society of Cellular Therapy guidelines to confirm their mesenchymal phenotype [30, 31]. The morphological characteristics of the cultured cells by sticking to the surface of the cell dish were followed by contrasting phase microscopy throughout the study. Flow cytometric analysis and immunofluorescence labeling were performed for mesenchymal stem cell markers to determine the immunophenotypic characteristics. Surface antigens of hDPSCs were analyzed in a flow cytometer (Becton Dickinson FACSCalibur; Becton Dickinson, San Jose, CA, USA) using fluorescent dye–conjugated CD45 FITC (BD 55,482), HLA-DR FITC (BD 340,688), CD 44 FITC (BD 555,478), CD34 PE (BD 345,802), CD29 PE (BD 555,443), CD73 PE (BD 550,257), CD140b PE (BD 558,821), CD146 PE (BD 550,315), CD 105 PE (BD 560,839), and Ig G1G2A FITC-PE (BD 342,409) antibodies. Mouse IgG1 FITC and IgG2a PE were used as isotype controls.
In addition, adipogenic, osteogenic, and chondrogenic differentiation protocols were applied to determine the in vitro differentiation capacity of mesenchymal stem cells. In adipogenic differentiation, after inoculation of 3000 cells/cm2 cells in the culture dish, the culture medium was replaced with a differentiation medium (DMEM culture medium with 10% FBS, 0.5 mM isobutyl-methylxanthine, 1 µM dexamethasone, 10 µg/mL insulin, and 200 µM indomethacin) when it reached 70% cell compaction. In osteogenic differentiation, after inoculating 3000 cells/cm2 cells in the culture dish, the culture medium was replaced with a differentiation medium (DMEM culture medium containing 10% FBS, 100 nM dexamethasone, 0.05 µM ascorbic acid, and 10 mM β-glycerophosphate) when the cells reached 70% cell density. In chondrogenic differentiation, cell differentiation was performed using the micro-mass technique. Cell suspension with a density of 107 cells in 1 mL was dropped into the plastic culture dish, and the cells were adhered to in a dense environment. After waiting for 24 h at 37 °C for adhesion, the cells were cultured for 2 weeks with high glucose DMEM culture medium containing 10 ng/mL TGF-β1, 50 µg/mL ascorbic acid, 0.1 µM dexamethasone, 100 µg/mL sodium pyruvate, 40 µg/mL proline, and 50 mg/mL insulin.
Cytotoxicity assay
Cell viability with water-soluble tetrazolium salt (WST-1) was used to determine the cytotoxic effects of pulp capping materials on hDPSCs. The analysis performed is based on the principle of conversion of tetrazolium salt to formazan, which gives color by cellular mitochondrial dehydrogenase. The amount of dye produced by dehydrogenase enzyme activity is directly proportional to the number of viable cells. Twenty-four-well culture dishes were used and 25,000 cells were seeded in all wells of the experimental setup. After the cells adhered to the surface, material samples were added to the wells, and the group without any material was designed as the control group. For all experimental groups, cells were incubated in Dulbecco’s modified Eagle’s medium (L-DMEM; Gibco) culture medium containing 1% penicillin/streptomycin, 10% FBS at 5% CO2, and 37 °C. Cell viability was established in separate culture setups at 3 different times on the 1st, 3rd, and 7th days. At the end of the measurement times, the medium in the wells was replaced with L-DMEM containing 10% WST-1 reagent, and the cells were incubated for 2 h in the dark at 5% CO2 and 37 °C. L-DMEM containing only 10% WST-1 reagent, without both cells and any sample, was used as blank. At the end of the incubation, viable cell count measurements were completed with a spectrophotometer (Versamax microplate reader; Molecular Devices, CA, USA) at a wavelength of 450 nm. In all cell culture wells, the standards were performed as 3 replicates, and the controls and experimental groups were performed as 5 replicates.
Residual monomer release
The resin-based materials, ThLC and ThPT, were prepared as stated above (n = 10). The polymerized samples were placed in amber bottles containing 5 mL of 75% ethanol/water solution and kept them in a 37 °C incubator. The samples taken from these solutions at 1, 3, and 7 days were analyzed by high-performance liquid chromatography-ultraviolet (HPLC–UV; Shimadzu Nexera X2, Kyoto, Japan). A water/acetonitrile solution ratio of 30/70 was used as a mobile phase in the analysis of the monomers (BisGMA, UDMA, and TEGDMA). The flow rate of the mobile phase is 1.0 mL/min, the column temperature was 25 °C, and the absorbance readings were taken at 210 nm. Inertsil ODS-4 C18 (250 mm × 4.6, 5 μm) was used as the column. The volume of the liquid injected into the column from each sample was determined as 20 µL. The residual monomer concentrations were determined by defining the calibration points (1–5-10–50 µg/mL) for each monomer and creating the calibration curves.
Degree of Conversion (DC)
The Fourier transform infrared (FTIR) spectrometer (JASCO, FT/IR-6800, Japan) was used to measure the immediate DC of ThLC and ThPT samples (n = 10). The ATR crystal was placed in close contact with the bottom surface, and FTIR spectra ranging from 400 to 4000 cm−1 were documented by 16 scans at a resolution of 4 cm−1. The peak height of around 1638 cm−1, indicating the absorbance intensities of aliphatic C = C, was calibrated according to Rueggeberg et al. [32]. The peak of aromatic C = C around 1608 cm−1 was taken as the internal standard and was also calibrated. The values of DC were calculated according to the formula shown below:
Calcium ion (Ca 2+ ) release
The samples were prepared from each pulp capping material and were placed in 10 mL deionized water at 37 °C in clean plastic 25-cm3 centrifuge tubes (n = 5). Three hours, 24 h, 3 days, 7 days, and 14 days were collected from the tubes, and after each measurement time, the samples were transferred to new tubes containing fresh deionized water. The storage water samples were then analyzed using an inductively coupled plasma-optical emission spectroscopy (ICP-OES; Perkin Elmer Optima 7000DV, Waltham, MA, USA) at an inductively coupled wavelength of 317.933 nm and plotting a calibration curve between 0.1 and 15 ppm. The rate of calcium Ca2+ released from each material was analyzed at five measurement times determined for 14 days.
Scanning electronic microscopy and energy-dispersive spectroscopy (SEM–EDS)
The disk-shaped samples with the same dimensions were prepared from each material (n = 3). After the samples were prepared, they were subjected to a gold plating process (Quorum, Q150R ES, UK) to obtain high-quality images.
Then, the elemental quantitative analysis of the investigated material was performed from 3 randomly selected spots of each sample of each material using SEM–EDS (FEI, Quanta 650 FEG, Eindhoven, The Netherlands).
Three areas of 20 × 20 µm of each sample were scanned at × 500 magnification. A spectrum was obtained showing the elements in the basic composition of the material as a peak. In addition, the SEM images of each material were taken at × 5000 magnification.
X-ray diffractometry (XRD)
The phase analysis of the materials was performed using XRD. The samples were dried in a vacuum desiccator and crushed to a very fine powder using an agate mortar and pestle. The diffraction meter (Empyrean XRD, PANalytical, UK) used Cu Kα radiation (λ = 0.15406 nm) at 40 mA and 45 kV, and the detector was set to rotate between 10 and 90° with a sampling width of 0.05 and a scan rate of 1°/min at 15 cycles. Phase identification was performed using a search matching software and using the ICDD database (International Center for Diffraction Data, Newtown Square, PA, USA).
Statistical analysis
Statistical analysis was performed by using the SPSS program for Mac version 26 (IBM SPSS Inc., Chicago, IL, USA). The homogeneity of variance and normal distribution of the data was confirmed by the Shapiro–Wilk test. The statistical differences were analyzed by using one-way ANOVA followed by Tukey’s post hoc test for pair-wise comparison. Time-dependent changes were evaluated by repeated measure ANOVA. All data were evaluated with a 95% of confidence interval (p = 0.05).
Results
Characterization of human dental pulp stem cells
The particles such as dead cells and debris were eliminated using the R1 gate in flow cytometry. All markers of all cells scattering around the R1 gate were shown in the histograms (Fig. 1a).
The flow cytometry analysis of the hDPSCs isolated was shown to be positive for the markers of CD44 FITC (96%), CD29 PE (99.7%), anti-human CD90 (99.76%), CD73 PE (99.62%), CD140 PE (99.49%), CD146 PE (91.97%), and CD105 PE (99.84%) and negative for the markers CD45 FITC (0.01%), HLA DR FITC (0.01%), and CD34 PE (0.24%) (Fig. 1b). Mouse IgG1 FITC and IgG2a PE were used as isotype controls. The percentages are represented relative to the proper expression of mesenchymal stem cell surface markers.
The multilineage differentiation ability of stem cells in terms of adipogenic, osteogenic, and chondrogenic was observed in the culture medium. As a result of adipogenic differentiation, lipid vesicles were observed in the cytoplasm of the cells after 20 days; as a result of osteogenic differentiation, mineral deposits in the cytoplasm of cells after 4 weeks; and as a result of chondrogenic differentiation, phosphate proteoglycans in the cartilage matrix after 2 weeks.
Cytotoxicity
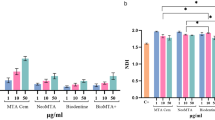
The absorbance in all cell culture wells prepared for standards, controls, and experimental groups was measured at 450 nm after incubation. Then, from the data obtained with the standards, an equation was found which gives the number of viable cells and was shown as followed: \(y=21171x+794.27\), \({R}^{2}=0.9953\). The number of viable cells in the experimental groups and in the control was determined according to this equation, and the normalization of the experimental groups was compared with the number of cells in the control group and is shown in Fig. 2.
The number of viable cells in MTA (4177.4), ThLC (1031.4), and ThPT (2183.1) groups decreased significantly compared to the control group (8902.8) for day 1 (p < 0.0001), and MTA was significantly higher than in ThLC and ThPT (p < 0.002). There was no difference between ThLC and ThPT (p = 0.078).
The number of viable cells was further reduced in the MTA (3376), ThLC (726.4), and ThPT (670.4) groups compared to the control group (8304.2) for day 3 (p < 0.0001). There was no difference between the ThLC and ThPT materials in terms of the number of viable cells (p = 0.999), while these two groups contained significantly fewer viable pulp cells than the MTA (p < 0.0001).
When the differences in the measurement times were compared, there was no difference in the number of viable cells among the day 1 (4177.4), the day 3 (3376), and the day 7 (3357.3) measurements of the MTA group (p = 0.221). The number of viable cells of ThLC and ThPT from day 1 to day 3 was significantly reduced, and by day 7, there were no viable cells in either material group (p < 0.0001). The control group had similar viable pulpal cells in terms of measurement times (p = 0.811).
Residual monomer release
The time-dependent monomer releases of the materials are presented in Fig. 3. BisGMA release was greater in ThLC than ThPT at all measurement times (p < 0.0001). TEGDMA release was significantly lower for ThLC on day 3 and day 7 compared to ThPT (p < 0.0001). BisGMA release from ThLC was higher than TEGDMA for all measurement times (p < 0.0001). While ThPT showed similar releases of the monomers on day 1, TEGDMA released from ThPT on days 3 and 7 was considerably higher than BisGMA (p < 0.0001). UDMA monomer was not found in the monomer release concentration curve of the tested materials.
Degree of conversion
The representative graphic of the DC of ThLC and ThPT is presented in Fig. 4. As a result of statistical analysis of homogeneously distributed the data of DC by independent t-test, the DC of ThLC and ThPT was found to be significantly higher than ThLC (p < 0.0001).
Ca +2 release
The time-dependent change of Ca2+ released from the materials and the differences between them is presented in Fig. 5 with a line chart and table. At all measurement times except day 14, the Ca2+ release of MTA was significantly higher than that of ThLC and ThPT (p ≤ 0.02). There is no difference in Ca2+ release between ThLC and ThPT at hour 3, day 1, and day 7 (p ≥ 0.340), but the Ca2+ released from ThLC on days 3 and 14 was higher than ThPT (p ≤ 0.03).
There is a statistically significant difference among the five measurement times of the Ca2+ release for all materials (p < 0.0001). MTA showed the highest Ca2+ release on day 1. MTA exhibited similar ion release at hour 3 and day 3, and the release significantly decreased for days 7 and 14 compared to other measurement times (p ≤ 0.02). ThLC showed the highest Ca2+ release on days 3, 7, and 14, and it was observed similar values while ThPT showed the highest Ca2+ release on days 7 and 14.
SEM/EDS
The representative SEM images of the investigated materials at × 5000 magnification are presented in Fig. 6. MTA exhibited a surface containing interspersed particles in similar proportions and a dense microstructure with a porosity under 10 µm. MTA surface also showed mixtures of relatively large and small particles composed of aggregate deposition. ThLC and ThPT that showed a relatively more homogeneous and less rough surface than MTA exhibited mineral particles dispersed in the organic resin matrix, similar to the resin composite. Compared to ThLC, globular-shaped structures including elements with relatively higher atomic numbers were intensely observed on the resin matrix surface of ThPT.
A representative EDS spectrum was obtained from a randomly scanned region for each material examined, and the average weight and atomic percentage of the main elements in the composition of the material obtained from 3 different regions of 3 samples for each investigated material are presented in Fig. 7. Carbon (C), oxygen (O), aluminum (Al), silicon (Si), and Ca were found in all materials. Also, MTA contained ytterbium (Yb). There are significant differences among the materials in terms of percentage weights of C, Al, and Ca (p < 0.0001). The ratio of C (5.07%) in MTA is lower than that in ThLC and ThPT materials (p = 0.001). The ratio of Ca in MTA (37.59%) is significantly higher than in other materials (p < 0.0001). The ratio of Ca in ThLC (9.55%) was significantly higher than that in ThPT (5.94%) (p = 0.034). ThPT has a higher ratio of Al than the other materials (p < 0.0001).
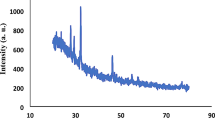
XRD
The chemical composition and the crystal structure of the tested materials are represented in Fig. 8. The components of MTA, tricalcium silicate (ICDD:98–009-4742), calcium silicate (ICDD:98–024-0448), calcium oxide (ICDD:98–002-6959), and silicon oxide (ICDD:98–019-3155), showed the highest peaks at 29.478, 36.648, 37.621, and 29.029 2Ɵ, respectively. The chemical compositions of ThLC and ThPT were similar. Barium peaks with zirconium and the particles of Si, strontium aluminum, and Ca were observed in the matrix of these materials. ThLC showed the highest peak of tricalcium silicate (ICDD: 98–008-1100) at 32.193 2Ɵ, while ThPT indicated only calcium oxide (98–018-0198) at 37.153 2Ɵ. ThLC displayed the highest peaks at 30.073, 38.593, and 30.117 2Ɵ for barium zirconate (98–009-7462), aluminum oxide (98–015-1589), and silicon oxide (ICDD:98–015-8531), respectively. ThPT showed the peaks of barium zirconate (ICDD: 98–009-7461), aluminum oxide (ICDD: 98–017-3713), and silicon oxide (ICDD: 98–007-7457) at 30.095, 33.215, and 21.909 2Ɵ, respectively.
Discussion
The present study compared the current vital pulp capping materials in terms of the cytotoxicity on hDPSCs, the release of residual monomer, the DC, the release of Ca+2, and the crystal structure. Biocompatibility and bioactivity are considered essential features due to the direct contact of the material with vital tissues in choosing a material for the vital pulp procedure [33]. This study compared ThPT, a recent-developed material for vital pulp therapy, with currently used ThLC and MTA materials in terms of cytotoxicity on days 1, 3, and 7. At the end of 7 days, it was found that there were no viable cells in ThPT and ThLC, while cell viability continued in MTA. In addition, the materials differed from each other in terms of chemical properties and ion release. Therefore, the null hypothesis of the study was rejected.
A number of quantitative and qualitative in vitro methods are available to evaluate the cytotoxicity of biomaterials and their potential adverse effects on cell behavior [34]. Among the various cytotoxicity tests, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) is widely used as an indicator of cell viability. MTT is reduced by a mitochondrial enzyme in metabolically active cells to form insoluble purple formazan crystals, and then, the crystals are dissolved by the addition of a detergent. However, insoluble purple formazan crystals may be cytotoxic, and inevitably added detergents may also affect cell viability which may complicate the analysis [35]. The WST-1 assay works similarly to the MTT method by reacting with mitochondrial succinate-tetrazolium reductase and forming the formazan dye, but the WST-1 reagent produces a water-soluble formazan that is stable in solution for more than 24 h and non-cytotoxic. In addition, the WST-1 assay is three times more sensitive than the MTT test [36]; hence, the WST-1 assay was preferred to detect cytotoxicity in this study.
There are two studies evaluating the cytotoxicity of the new ThPT in the literature. They reported an improved in vitro cytocompatibility of hDPSCs compared to ThLC, the precursor of ThPT [20, 21]. In those studies, cell viability was evaluated at 24, 48, and 72 h using MTT as a cytotoxicity test method. Those results, which are inconsistent with our results, may be due to our use of a more sensitive method, WST-1, and our evaluation on days 1, 3, and 7. The cell viability of ThPT and ThLC observed on day 3 ceased when followed up to day 7. Moreover, the materials were not evaluated in different concentration media which considers as being one of the limitations of the present study. In studies, it was revealed that ThLC negatively affected hDPSC viability in all extraction medium concentrations, whereas this effect was lower in ThPT and the concentration ratios had no effect [20, 21]. In previous studies comparing the cytotoxicity of ThLC, MTA, and a similar material (Biodentine), they reported that ThLC had a cytotoxic effect on hDPSC viability [9, 22, 37]. It was not surprising that MTA showed high cell viability for 7 days which has often been proven in previous studies [20, 21, 37]. However, in this study, the loss of cell viability by half in MTA when compared to the control group is likely due to the high alkaline pH observed in the initial setting. Bortoluzzi et al. [38] specified that all freshly set calcium silicate cements (resin-modified and resin-free) are initially cytotoxic, which may be due to their high alkalinity.
One of the methods used to determine the cytotoxicity of resin-structured materials is the measurement of the concentration of unreacted monomers released from the polymerized resin material [39]. The main reason for the cell cytotoxicity found in the TheraCal materials is that the main component (45%) contains resin monomers which can be toxic to dental pulp cells [40]. The safety data sheet shows that ThLC contains only BisGMA monomer, but UDMA and TEGDMA content was observed in studies and patients [41,42,43]. BisGMA, UDMA, and TEGDMA were tested by methods of cell culture, and it was reported that they might cause cytotoxic, genotoxic, mutagenic, and allergenic effects [44, 45]. Unreacted monomers and other degradation products can be analyzed using HPLC, gas chromatography, electrospray ionization, and mass spectrometry [46, 47]. HPLC is the most common analytical method for determining the quality and quantity of eluted monomers [46,47,48]. Studies on the release of monomers from resin materials are carried out using various extraction media. Solvent media are divided into two groups as aqueous mixtures (distilled water, cell culture media, artificial saliva, human saliva, and water-based buffer solutions) and different organic extraction media (ethanol, methanol, acetone, acetonitrile, tetrahydrofuran, and chloroform) [48,49,50]. These solvent environments are important as they penetrate the resin matrix and cause the release of unreacted monomers [48]. Ferracane reported that fluids in the oral cavity exhibited extraction properties intermediate between aggressive organic solvent and water [51]. Therefore, the US Food and Drug Administration recommends using a 75% ethanol/water solution as a liquid with properties corresponding to oral cavity conditions [39]. In this study, residual monomer releases of ThLC and ThPT were investigated by HPLC using 75% ethanol/water solution, and BisGMA and TEGDMA releases were observed while UDMA peaks were not observed. ThLC showed predominantly BisGMA release and tended to decrease after day 3, but TEGDMA releases for both materials revealed an increasing curve from day 1 to day 7. The decrease and loss of cell viability in the TheraCal materials may be due to the dramatic increase of TEGDMA release on day 7. Previous studies have suggested that TEGDMA may be more toxic than other monomers [35, 36]. This argument is in line with the results of this study.
Resin-based materials should be highly polymerized to minimize the amount of residual monomer elution [52]. The DC of monomers in resin materials is a measure of the percentage of carbon double bonds converted into single-carbon bonds to form a polymer chain, depending on the polymerization process. In a material, relatively 70% of the average monomers are converted to polymers, and a relative 30% remain unpolymerized. While most of these unconverted monomers remain in the polymer matrix, 9% of them remain free and may diffuse into the dentinal tubules and reach the pulp [9]. Various methods are used to determine the DC of resin materials. FTIR spectroscopy is a powerful and reliable method based on molecular vibrations that able to be used to directly detect the amount of unreacted C = C bond in the resin matrix, as opposed to indirect techniques that rely on measuring changes in the mechanical performance of the material to assess relative DC [53, 54]. In this study, DC values of ThLC and ThPT were compared using the FTIR spectroscopy. It was determined that ThPT showed higher DC. ThPT was introduced to the market as a dual-cure with the improved formulation of ThLC. The purpose of dual-cure materials is able to provide complete curing in the absence of a visible light source by combining chemical polymerization and photo-polymerization. DC is generally considered to correlate with the release of unreacted monomers; however, a specific DC limit that occurs proportionally to the DC of a material for a significant amount of monomer release is unknown [55]. However, this result contradicts the data of the highest TEGDMA monomer release observed in ThPT. Similar to our study, there are studies reporting no correlation between the DC and monomer release [56,57,58]. This was attributed to the imperfect match of the data of HPLC analysis with the data of FTIR analysis, the different sensitivity of these two analytical techniques, and the possibility that unpolymerized monomers may not be released into the surrounding environment by remaining trapped in the polymeric network. Therefore, the highest DC values may not always correlate with the release of the lowest monomer amounts [56].
The ability to release biologically active ions is a prerequisite for a material to be bioactive and induce apatite formation. Ca2+ is considered a bioactive component in pulp capping materials [59]. Ca2+ is essential for the differentiation and mineralization of pulp cells, and a Ca-rich environment stimulates both proliferation and differentiation into odontoblast-like cells [60]. The released Ca2+ increases the growth of hDPSCs in a dose-dependent manner and induces the activity of pyrophosphatase, which helps maintain dentin mineralization and develop a dentin bridge [38, 61]. In light of this knowledge, the sustained release of Ca2+ from a pulp capping material is probably the main cause of material-induced proliferation and differentiation of hDPSCs.
ICP-OES, XRD, and SEM–EDS results of all pulp capping materials tested in this study proved to be formulations showing calcium content and ion release in all materials as previously reported [62, 63]. In the EDS analysis, MTA was found to have higher Ca content than ThLC and ThPT. The ICP-OES analysis showed that the highest release of MTA was observed on day 1, and Ca2+ release tended to decrease until day 14. This steep Ca peak observed on day 1 confirms the decrease in cell viability with high alkalinization. The EDS analysis revealed a higher ratio of Ca of ThLC than ThPT, which supports ThLC with more Ca2+ release than ThPT. Also, these results are supported by previous studies showing that MTA releases higher Ca2+ than TheraCal materials [62, 63].
MTA particularly showed Ca and calcium silicate peaks in XRD analysis. Calcium silicate peak was not observed in ThPT compared to MTA and ThLC. This result is similar to the study reporting that the calcium silicate phase is weak in ThPT [62]. Also, ThLC and ThPT both showed peaks for barium strontium zirconium oxide, similar to a previous study [63]. This study has limitations of the in vitro test setup. Further studies are recommended, including in vivo animal tests and clinical studies, as the behavior of tested materials can be potentially affected by a number of external factors in the clinical setting.
Conclusion
MTA emerged as a superior material with low cytotoxicity compared to ThLC and ThPT. After 7 days, no viable hDPSCs were detected in the ThLC and ThPT groups. All materials had Ca content in the certain ratios and exhibited time-dependent Ca2+ release. Although the ThPT had a better DC with its dual-cure feature, the residual monomer release of ThPT was higher than ThLC.
References
Chan C, Lan W, Chang M, Chen Y, Lan W, Chang H, Jeng J (2005) Effects of TGF-βs on the growth, collagen synthesis and collagen lattice contraction of human dental pulp fibroblasts in vitro. Arch Oral Biol 50:469–479. https://doi.org/10.1016/j.archoralbio.2004.10.005
Huang G-J, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806. https://doi.org/10.1177/0022034509340867
Rosa V, Zhang Z, Grande R, Nör J (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970–975. https://doi.org/10.1177/0022034513505772
Tatullo M, Codispoti B, Sied J, Makeeva I, Paduano F, Marrelli M, Spagnuolo G (2019) Stem cells-based and molecular-based approaches in regenerative dentistry: a topical review. Curr Stem Cell Res Ther 14:607–616. https://doi.org/10.2174/1574888X14666190626111154
Kim J-H, Kim S-Y, Woo S-M, Jeong H-N, Jung J-Y, Kim S-M, Lim H-S (2019) Combination of mineral trioxide aggregate and propolis promotes odontoblastic differentiation of human dental pulp stem cells through ERK signaling pathway. Food Science and Biotechnology 28:1801–1809. https://doi.org/10.1007/s10068-019-00609-5
Maria de Lourdes RA, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E Jr, Souza V, Alessandro LD (2008) Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod 34:1–6. https://doi.org/10.1016/j.joen.2007.09.012
Camargo S, Camargo C, Hiller KA, Rode S, Schweikl H, Schmalz G (2009) Cytotoxicity and genotoxicity of pulp capping materials in two cell lines. Int Endod J 42:227–237. https://doi.org/10.1111/j.1365-2591.2008.01506.x
Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, Ito K (2006) Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J 39:415–422. https://doi.org/10.1111/j.1365-2591.2008.01506.x
Collado-González M, García-Bernal D, Oñate-Sánchez R, Ortolani-Seltenerich P, Álvarez-Muro T, Lozano A, Forner L, Llena C, Moraleda J, Rodríguez-Lozano F (2017) Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int Endod J 50:19–30. https://doi.org/10.1111/iej.12751
Da Rosa W, Piva E, Da Silva A (2018) Disclosing the physiology of pulp tissue for vital pulp therapy. Int Endod J 51:829–846. https://doi.org/10.1111/iej.12906
Poggio C, Arciola CR, Beltrami R, Monaco A, Dagna A, Lombardini M, Visai L (2014) Cytocompatibility and antibacterial properties of capping materials. Scientific World Journal 2014:1–10. https://doi.org/10.1155/2014/181945
Sawicki L, Pameijer CH, Emerich K, Adamowicz-Klepalska B (2008) Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am J Dent 21:262–266
Moghaddame-Jafari S, Mantellini MG, Botero TM, McDonald NJ, Nör JE (2005) Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod 31:387–391. https://doi.org/10.1097/01.don.0000145423.89539.d7
Roberts HW, Toth JM, Berzins DW, Charlton DG (2008) Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater 24:149–164. https://doi.org/10.1016/j.dental.2007.04.007
Yoldaş SE, Bani M, Atabek D, Bodur H (2016) Comparison of the potential discoloration effect of bioaggregate, biodentine, and white mineral trioxide aggregate on bovine teeth: in vitro research. J Endod 42:1815–1818. https://doi.org/10.1016/j.joen.2016.08.020
Ajami AA, Navimipour EJ, Oskoe SS, Kahnamoui MA, Lotfi M, Daneshpooy M (2013) Comparison of shear bond strength of resin-modified glass ionomer and composite resin to three pulp capping agents. J Dent Res Dent Clin Dent Prospects 7(3):164. https://doi.org/10.5681/joddd.2013.026
Hashem DF, Foxton R, Manoharan A, Watson TF, Banerjee A (2014) The physical characteristics of resin composite–calcium silicate interface as part of a layered/laminate adhesive restoration. Dent Mater 30(3):343–349. https://doi.org/10.1016/j.dental.2013.12.010
Nielsen MJ, Casey JA, VanderWeele RA, Vandewalle KS (2016) Mechanical properties of new dental pulp-capping materials. Gen Dent 64(1):44–48
Kim Y, Lee D, Kim HM, Kye M, Kim SY (2021) Biological characteristics and odontogenic differentiation effects of calcium silicate-based pulp capping materials. Materials 14(16):4661. https://doi.org/10.3390/ma14164661
Rodríguez-Lozano FJ, López-García S, Garcia-Bernal D, Sanz J, Lozano A, Pecci-Lloret M, Melo M, López-Ginés C, Forner L (2021) Cytocompatibility and bioactive properties of the new dual-curing resin-modified calcium silicate-based material for vital pulp therapy. Clin Oral Investig 25:5009–5024. https://doi.org/10.1007/s00784-021-03811-0
Sanz JL, Soler-Doria A, López-García S, García-Bernal D, Rodríguez-Lozano FJ, Lozano A, Llena C, Forner L, Guerrero-Gironés J, Melo M (2021) Comparative biological properties and mineralization potential of 3 endodontic materials for vital pulp therapy: Theracal PT, Theracal LC, and Biodentine on human dental pulp stem cells. J Endod 47:1896–1906. https://doi.org/10.1016/j.joen.2021.08.001
Jeanneau C, Laurent P, Rombouts C, Giraud T, About I (2017) Light-cured tricalcium silicate toxicity to the dental pulp. J Endod 43(12):2074–2080. https://doi.org/10.1016/j.joen.2017.07.010
Arias-Moliz FC, Lung CYK, Wismayer PS, Camilleri J (2017) Antimicrobial and biological activity of leachate from light curable pulp capping materials. J Dent 64:45–51. https://doi.org/10.1016/j.jdent.2017.06.006
Chen L, Suh BI (2017) Cytotoxicity and biocompatibility of resin-free and resin-modified direct pulp capping materials: a state-of-the-art review. Dent Mater J 36(1):1–7. https://doi.org/10.4012/dmj.2016-107
Klein-Júnior CA, Zimmer R, Hentschke GS, Machado DC, Santos RBD, Reston EG (2018) Effect of heat treatment on cytotoxicity of self-adhesive resin cements: cell viability analysis. Eur J Dent 12(02):281–286. https://doi.org/10.4103/ejd.ejd_34_18
Klein-Junior CA, Zimmer R, Borghetti DLB, Portell FF, Abich FC, Marinowic DR, Hosaka K, Reston EG (2020) Hot air stream reduces cytotoxicity of light-cured calcium hydroxide based cements. J Clin Exp Dent 12(3):e215. https://doi.org/10.4317/jced.56590
Leite MLdA, Costa CAdS, Duarte RM, Andrade AKMd, Soares DG (2018) Bond strength and cytotoxicity of a universal adhesive according to the hybridization strategies to dentin. Braz Dent J 29:68–75. https://doi.org/10.1590/0103-6440201801698
Salehi S, Gwinner F, Mitchell JC, Pfeifer C (2015) Ferracane JL (2015) Cytotoxicity of resin composites containing bioactive glass fillers. Dent Mater 31(2):195–203. https://doi.org/10.1016/j.dental.2014.12.004
Karaöz E, Doğan BN, Aksoy A, Gacar G, Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PÇ (2010) Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 133:95–112. https://doi.org/10.1007/s00418-009-0646-5
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Rodríguez-Lozano F, Collado-González M, Tomás-Catalá C, García-Bernal D, López S, Onate-Sanchez R, Moraleda J, Murcia L (2019) GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent Mater 35:114–124. https://doi.org/10.1016/j.dental.2018.11.003
Rueggeberg F, Hashinger D, Fairhurst C (1990) Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent Mater 6:241–249. https://doi.org/10.1016/S0109-5641(05)80005-3
Lee B-N, Lee K-N, Koh J-T, Min K-S, Chang H-S, Hwang I-N, Hwang Y-C, Oh W-M (2014) Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J Endod 40:1217–1222. https://doi.org/10.1016/j.joen.2014.01.036
Buttke TM, McCubrey JA, Owen TC (1993) Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods 157:233–240. https://doi.org/10.1016/0022-1759(93)90092-L
Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K (1996) A combined assay of cell vability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull 19:1518–1520. https://doi.org/10.1248/bpb.19.1518
Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, P-g HE (1993) A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem Pharm Bull 41:1118–1122. https://doi.org/10.1248/cpb.41.1118
Nam OH, Kim J-H, Choi SC, Kim Y (2020) Time-dependent response of human deciduous tooth-derived dental pulp cells treated with TheraCal LC: functional analysis of gene interactions compared to MTA. J Clin Med 9:531. https://doi.org/10.3390/jcm9020531
Bortoluzzi EA, Niu L-n, Palani CD, El-Awady AR, Hammond BD, Pei D-d, Tian F-c, Cutler CW, Pashley DH, Tay FR (2015) Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dent Mater 31:1510–1522. https://doi.org/10.1016/j.dental.2015.09.020
Łagocka R, Jakubowska K, Chlubek D, Buczkowska-Radlińska J (2015) Elution study of unreacted TEGDMA from bulk-fill composite (SDR™ Dentsply) using HPLC. Adv Med Sci 60(2):191–198. https://doi.org/10.1016/j.advms.2015.02.003
Schneider TR, Hakami-Tafreshi R, Tomasino-Perez A, Tayebi L, Lobner D (2019) Effects of dental composite resin monomers on dental pulp cells. Dent Mater J 2018-163. https://doi.org/10.4012/dmj.2018-163
Nilsen BW, Jensen E, Örtengren U, Michelsen VB (2017) Analysis of organic components in resin-modified pulp capping materials: critical considerations. Eur J Oral Sci 125:183–194. https://doi.org/10.1111/eos.12347
Di Foggia M, Prati C, Gandolfi MG, Taddei P (2019) Spectroscopic and morphological data assessing the apatite forming ability of calcium hydroxide-releasing materials for pulp capping. Data Brief 23:103719. https://doi.org/10.1016/j.dib.2019.103719
Suh B, Yin R, Cannon M, Martin DE, Polymerizable dental pulp healing, capping, and lining material and method for use. 2008, Google Patents.
Geurtsen W (2000) Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med 11:333–355. https://doi.org/10.1177/10454411000110030401
Issa Y, Watts D, Brunton P, Waters C, Duxbury A (2004) Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater 20:12–20. https://doi.org/10.1016/S0109-5641(03)00053-8
Janani K, Teja KV, Sandhya R, Alam MK, Al-Qaisi RK, Shrivastava D, Alnusayri MO, Alkhalaf ZA, Sghaireen MG, Srivastava KC (2021) Monomer elution from three resin composites at two different time interval using high performance liquid chromatography—an in-vitro study. Polymers 13(24):4395. https://doi.org/10.3390/polym13244395
Gul P, Miloglu FD, Akgul N (2014) HPLC analysis of eluted monomers from dental composite using different immersion media. J Liq Chromatogr Relat Technol 37(2):155–170. https://doi.org/10.1080/10826076.2012.738619
Väkiparta M, Puska M, Vallittu PK (2006) Residual monomers and degree of conversion of partially bioresorbable fiber-reinforced composite. Acta Biomater 2(1):29–37. https://doi.org/10.1016/j.actbio.2005.08.009
Moharamzadeh K, Noort RV, Brook IM, Scutt AM (2007) HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med 18(1):133–137. https://doi.org/10.1007/s10856-006-0671-z
Goldberg M (2008) In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig 12(1):1–8. https://doi.org/10.1007/s00784-007-0162-8
Ferracane J (1994) (1994) Elution of leachable components from composites. J Oral Rehabil 21(4):441–452. https://doi.org/10.1111/j.1365-2842.1994.tb01158.x
Munksgaard EC, Peutzfeldt A, Asmussen E (2000) (2000) Elution of TEGDMA and BisGMA from a resin and a resin composite cured with halogen or plasma light. Eur J Oral Sci 108(4):341–345. https://doi.org/10.1034/j.1600-0722.2000.108004341.x
Imazato S, McCabe JF, Tarumi H, Ehara A, Ebisu S (2001) Degree of conversion of composites measured by DTA and FTIR. Dent Mater 17(2):178–183. https://doi.org/10.1016/s0109-5641(00)00066-x
Stansbury J, Dickens SH (2001) Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater 17(1):71–79. https://doi.org/10.1016/s0109-5641(00)00062-2
Lempel E, Czibulya Z, Kunsági-Máté S, Szalma J, Sümegi B, Böddi K (2014) Quantification of conversion degree and monomer elution from dental composite using HPLC and micro-Raman spectroscopy. Chromatographia 77(17):1137–1144. https://doi.org/10.1007/s10337-014-2647-3
Nocca G, Iori A, Rossini C, Martorana GE, Ciasca G, Arcovito A, Cordaro M, Lupi A, Marigo L (2015) Effects of barriers on chemical and biological properties of two dual resin cements. Eur J Oral Sci 123:208–214. https://doi.org/10.1111/eos.12178
Miletic V, Santini A, Trkulja I (2009) Quantification of monomer elution and carbon–carbon double bonds in dental adhesive systems using HPLC and micro-Raman spectroscopy. J Dent 37:177–184. https://doi.org/10.1016/j.jdent.2008.11.006
Pongprueksa P, De Munck J, Duca RC, Poels K, Covaci A, Hoet P, Godderis L, Van Meerbeek B, Van Landuyt KL (2015) Monomer elution in relation to degree of conversion for different types of composite. J Dent 43:1448–1455. https://doi.org/10.1016/j.jdent.2015.10.013
Manaspon C, Jongwannasiri C, Chumprasert S, Sa-Ard-Iam N, Mahanonda R, Pavasant P, Porntaveetus T, Osathanon T (2021) Human dental pulp stem cell responses to different dental pulp capping materials. BMC Oral Health 21:1–13. https://doi.org/10.1186/s12903-021-01544-w
Lopez-Cazaux S, Bluteau G, Magne D, Lieubeau-Teillet B, Guicheux J, Alliot-Licht B (2006) Culture medium modulates the behaviour of human dental pulp-derived cells. eClls and Mater J 11:35–42
Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C (2015) Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater 13:43–60. https://doi.org/10.5301/jabfm.5000201
Elbanna A, Atta D, Sherief DI (2022) In vitro bioactivity of newly introduced dual-cured resin-modified calcium silicate cement. Dent Res J 19. https://doi.org/10.4103/1735-3327.33668
Camilleri J (2014) Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent Mater 30:709–715. https://doi.org/10.1016/j.dental.2014.03.012
Acknowledgements
The authors thank Ahmet Öztürk and Kocaeli University, Stem Cell and Gene Therapies Research, and Application Center (KÖGEM) for the support of this study.
Funding
This research was supported by the Scientific Research Projects of Cukurova University (project number TSA-2022–14441).
Author information
Authors and Affiliations
Contributions
Conceptualization: C.K, S.N.K., and S.G.B.; methodology: C.K, S.N.K., and S.G.B.; formal analysis and investigation: S.N.K. and S.G.B.; writing—original draft preparation: C.K, S.N.K., and S.G.B.; writing—review and editing: S.N.K. and C.K.; funding acquisition: S.N.K.; resources: C.K, S.N.K., and S.G.B; supervision: S.N.K. and C.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All methods performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later modifications or comparable ethical standards. The present study and the protocol were found medically appropriate with the ethics committee report numbered 2021/117.65 of Cukurova University Faculty of Medicine Clinical Research Ethics Committee.
Consent to participate
The written informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Küden, C., Karakaş, S.N. & Batmaz, S.G. Comparative chemical properties, bioactivity, and cytotoxicity of resin-modified calcium silicate–based pulp capping materials on human dental pulp stem cells. Clin Oral Invest 26, 6839–6853 (2022). https://doi.org/10.1007/s00784-022-04713-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04713-5