Abstract
Objective
The study aims to evaluate the effect of bone morphogenetic protein-2 (BMP-2) and transforming growth factor-beta 1 (TGF-β1) co-stimulation on odontogenic differentiation of human dental pulp stem cells (hDPSCs).
Materials and methods
The viability/proliferation of hDPSCs treated with BMP-2 (group B), TGF-β1 (group T), or BMP-2/TGF-β1 (group BT) were evaluated. The experiments on odontogenic differentiation were done for 14 days. The following subgroups were added to investigate the effect of co-stimulation with different timing: subgroup B1, TGF-β1 co-stimulation in the first week; subgroup B2, TGF-β1 co-stimulation in the second week; subgroup T1, BMP-2 co-stimulation in the first week; and subgroup T2, BMP-2 co-stimulation in the second week. The mineralization was assessed using alizarin red staining. The expression of following genes was assessed using quantitative real-time polymerase chain reaction: dentin sialophosphoprotein (DSPP), dentin matrix protein-1 (DMP1), osteopontin (OPN), and alkaline phosphatase.
Results
All groups showed viability similar to the control group (P > .05). The greater mineralization was detected in B groups on day 14. The expressions of DSPP, DMP-1, and OPN increased on day 14 (P < .05). In the combination groups, the higher expressions of DSPP and DMP-1 were observed in subgroups B1 and B2 than groups B and T (P < .05).
Conclusions
BMP-2 was the key in odontogenic differentiation of hDPSCs, which was further enhanced by co-stimulation with TGF-β1. Continuous stimulation with TGFβ-1 did not improve the differentiation of hDPSCs.
Clinical relevance
Combined use of the BMP-2 and TGFβ-1 at the specific sequence can provide a tissue engineering approach for the future guided dentin regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the basics of tooth development and odontoblast differentiation is a fundamental basis for regenerative endodontics. The goal in regenerative endodontic treatments is to regenerate the pulp-dentin complex [1]. Tooth development is driven through sequential and reciprocal interactions between dental epithelium and mesenchyme in specific spatial–temporal patterns, in which several growth and transcription factors are expressed in a time-specific manner [2]. Transforming growth factor-beta (TGF-β) superfamily, including TGF-β1–3, bone morphogenic protein (BMP) 2–7, and their transducers (Smad 1–7), mediate biological functions in embryonic development [3]. Identifying the specific role of growth factors during odontoblast differentiation has been challenging due to their overlapping expression patterns and functional redundancy.
BMP-2 promotes the differentiation of dental pulp stem cells into odontoblastic lineage [4], with canonical BMP signaling (i.e., Smad1/5 [5] or Smad4 [6]) and non-canonical BMP signaling (i.e., Jun N-terminal kinase (JNK) pathway [7]). During odontogenic differentiation, BMP-2 expression is detectable in dental epithelia through the initial, bud, and cap stages. Its expression is also detected in both epithelia and mesenchyme at the late bell and differentiation stages [3]. TGF-β1 has been identified as a promotor of odontoblast differentiation. During mouse tooth development, the expression of TGF-β1 starts to increase at bud and cap stages [8].
The individual application of BMP-2 or TGF-β1 was shown to be effective in enhancing odontogenic differentiation [9, 10]. However, the effect of combined stimulation by BMP-2 and TGF-β1 on odontogenic differentiation on human dental pulp stem cells (hDPSCs) as well as the optimum timing of their delivery has not been studied. Thus, the current study was aimed to investigate the effect of combined delivery of BMP-2 and TGF-β1 at different time sequences on odontogenic differentiation of hDPSCs.
Materials and methods
Isolation and characterization of hDPSCs
hDPSCs were isolated from intact/sound third molars extracted from patients at the Oral and Maxillofacial Surgery Department at Shahid Beheshti Dental School. The approval was obtained by the Ethics Committee at National Institutes for Medical Research Development, Tehran, Iran (NIMAD) (IR.NIMAD.REC.1399.262). The pulp tissue was digested in 3 mg/mL of collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37 °C. The cell suspension was cultured in Dulbecco modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Australia) and 1% penicillin–streptomycin (Sigma-Aldrich, St Louis, MO). The culture medium was changed every 3 days. After reaching 80–90% confluency, cells were collected and passaged. Cells from third to fourth passage were used for the current experiments.
Flow cytometric analysis was used to characterize the immunophenotype of hDPSCs by assessing the expression of mesenchymal stem cell markers (CD90, CD 105, and CD73), and lack of expression of hematopoietic markers (CD 31, CD34, and CD45).
hDPSCs (~ 2 × 105 cells) were washed and resuspended in phosphate-buffer saline (PBS; Sigma-Aldrich, St Louis, MO, USA) + 0.1% FBS, containing saturating concentrations (1:100 dilution) of the following fluorescein isothiocyanate (FITC)-conjugated anti-human monoclonal antibodies: CD 90 (BD Biosciences, San Jose, CA, Cat# 740,786, RRID: AB_2740449), CD 105 (BD Biosciences, San Jose, CA, Cat# 562,380, RRID: AB_11154054), CD 73 (BD Biosciences, San Jose, CA, Cat# 550,256, RRID: AB_393560), CD 31 (BD Biosciences, San Jose, CA, Cat# 550,274, RRID: AB_393571), CD 34 (BD Biosciences, San Jose, CA, Cat# 340,862, RRID: AB_400150), and CD 45 (BD Biosciences, San Jose, CA, Cat# 610,265, RRID: AB_397660) for 1 h on dry ice in the dark. Cell suspensions were washed twice and resuspended in 0.1% FBS/PBS for analysis on a flow cytometer (FACS Calibur, BD Biosciences, San Jose, CA,) using the Cell Quest software (BD Biosciences, San Jose, CA).
Viability/proliferation assay
Cell viability and proliferation were measured using an MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay (Sigma-Aldrich Chemicals, Germany). The experiments were done in triplicates. hDPSCs were seeded into 96-well plates (3 × 103/well) in growth medium, which was replaced with osteogenic medium (DMEM supplemented with 1% anti-biotics/anti-mycotics, 10% FBS, 10 nmol/L dexamethasone, 50 μg/mL ascorbate phosphate, and 10 mmol/L b-glycerophosphate) after 24 h. Based on the previous reports, the set concentrations for BMP-2 and TGF-ß1 were 10 ng/mL [11] and 5 ng/mL [12], respectively. The experimental groups were as follow:
-
Group B: hDPSCs treated with BMP-2 (10 ng/mL) (Cat# GF166, Sigma-Aldrich Chemicals, Germany);
-
Group T: hDPSCs treated with TGF-β1 (5 ng/mL) (Cat# T7039, Sigma-Aldrich Chemicals, Germany);
-
Group BT: hDPSCs treated with BMP-2 (10 ng/mL) and TGF-β1 (5 ng/mL).
hDPSCs cultured in osteogenic medium were considered as controls. Every 3 days, the culture medium was replaced with a fresh medium containing the aforementioned concentrations of growth factors.
At days 1, 3, 7, and 14, the cells were treated with MTT reagent for 3 h at 37 °C. The medium was then replaced with 100 µL dimethyl sulfoxide solvent (DMSO; Sigma-Aldrich Chemicals, Germany) to dissolve formazan crystals. The optical density was measured at 570 nm using an Elisa Reader (Anthos 2020, Austria) [13].
Odontogenic induction of hDPSCs
hDPSCs (5 × 103/well) were seeded in 24-well plates. To assess the time-dependent effects of BMP-2 (10 ng/mL), TGF-β1 (5 ng/mL), or their combinations, the following subgroups were added to the experiments (Fig. 1):
-
Subgroup B1: TGF-β1 present in the first week.
-
Subgroup B2: TGF-β1 present in the second week.
-
Subgroup T1: BMP-2 present in the first week.
-
Subgroup T2: BMP-2 present in the second week.
hDPSC in osteogenic medium was considered as control group.
Alizarin red staining (ARS)
At day 14, ARS was used to evaluate the mineral deposition. The cell cultures were fixed with 4% formaldehyde and stained with ARS solution (Sigma-Aldrich; 2% w/v, pH = 4.2) as previously described [14]. Transmitted light images of the morphology of mineralized matrices were recorded in a NIKON ECLIPSE TS100 microscope (Nikon, Tokyo, Japan) with a 10 × objective lens using color camera Nikon DXM-1200 (Nikon, Tokyo, Japan) and Nikon ACT-1 software (version 2.63).
qRT-PCR
qRT-PCR was used to evaluate the relative gene expression of dentin sialophosphoprotein (DSPP), dentin matrix acidic phosphoprotein 1 (DMP1), osteopontin (OPN), and alkaline phosphatase (ALP). After 7 and 14 days of culture, total RNA was isolated using TRIzol reagent (Takara Bio Inc., Shiga, Japan). Complementary DNA (cDNA) was synthesized using the cDNA synthesis kit (Yekta Tajhiz Azma, Tehran, Iran). Sequences of primers were determined as previously described [14] and were verified online using Gene Runner version 3.05 (Hastings software, Inc., Hastings, NY, USA) (Table 1).
qRT-PCR was performed using an ABI 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The following thermal cycling condition was applied: step 1, 50 °C/2 min; step 2, 95 °C/10 min; and step 3, 40 cycles of 95 °C/15 s followed by 65 °C/1 min. The fold change of the expression of each marker normalized against a housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) was calculated using the 2−ΔΔCT method. qRT-PCR experiments were done in two rounds of triplicates.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL). The statistical differences between experimental groups at two time points were performed using Mann–Whitney U test. The multiple comparisons of the experimental groups were assessed using Kruskal–Wallis test followed by Dunn post hoc test with significance level set at < 0.05.
Results
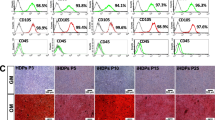
hDPSCs were isolated from dental pulp tissue of impacted third molars. The primary cells appeared as a heterogeneous population of stem/progenitor cells having typical mesenchymal stem cell-like features (i.e., spindle-shape fibroblast-like morphology) (Fig. 2a and b). Flow cytometry analyses showed that hDPSCs were uniformly positive for mesenchymal stem cell markers (i.e., CD73, CD105, and CD90), and negative for hematopoietic stem cell markers (i.e., CD45, CD31, and CD 34) (Fig. 2c).
Isolation and characterization of hDPSCs. a Morphology of hDPSCs at passage 0. b Morphology of hDPSCs at passage 3. c Flow cytometry histograms of the expression of cell surface markers for hDPSCs. Top row: positive for mesenchymal stem cell markers (CD90, CD105, CD73). Bottom row: negative for hematopoietic stem cell markers (CD31, CD34, CD45)
Viability/proliferation assay
All groups showed viability similar to the control groups, with no significant differences on days 1, 3, 7, and 14 (P > 0.05). Cell proliferation in group B was higher than control group on day 14 (P < 0.05). Cell proliferation in groups T and BT was not different from control group at any time points (P > 0.05) (Table 2).
ARS
ARS assay showed that groups B and subgroups B1 and B2 had the greater formation of mineralized nodules on day 14 (Fig. 3).
qRT-PCR
The expression levels of DSPP, DMP-1, OPN, and ALP markers were evaluated on days 7 and 14 to examine the effect of time. The overall expression levels of DSPP, DMP1, and OPN increased by day 14 (P < 0.05), whereas ALP expression increased by day 7 (P < 0.05), and then decreased by 14 (P < 0.05). On day 14, expression levels of DSPP, DMP1, OPN, and ALP markers were upregulated in all experimental groups compared with the control group (P < 0.05).
For data obtained on 7th day, we combined data from groups that have been received the same treatment as follow: group B (i.e., combined data from group B and subgroup B2), group T (i.e., combined data from group T and subgroup T2), and group BT (i.e., combined data from group BT, subgroups B1 and T1). For DSPP and ALP expressions, groups B and T had significantly greater levels compared with group BT (P < 0.05). In addition, group B showed significantly higher levels than group T (P < 0.05). For DMP1 expression, groups B and T expressed a significantly higher level than group BT (P > 0.05). OPN marker was not upregulated in the experimental groups on day 7.
On day 14, first we compared the single groups with each combination group (Fig. 4).
qRT-PCR analysis of odonto/osteogenic gene expression of the experimental groups on day 14. The expression levels of DSPP, DMP1, OPN, and ALP markers are presented as mean fold change and standard deviation (n = 6). a Comparison of groups B and T with subgroup B1. b Comparison of groups B and T with subgroup B2. c Comparison of groups B and T with subgroup T1. d Comparison of groups B and T with subgroup T2. e Comparison of groups B and T with subgroup BT. f Comparison of groups B and T with subgroups B1 and B2. The columns under the same bracket showed a significant difference. DSPP dentin sialophosphoprotein, DMP1 dentin matrix protein-1, OPN osteopontin, ALP alkaline phosphatase
Comparison of groups B and T with either subgroups B1 or B2
For DSPP and ALP markers, the subgroups expressed significantly greater levels than groups B and T (P < 0.05). For DMP1, the subgroups showed a significantly higher expression than group T (P < 0.05). OPN marker was expressed significantly greater in the subgroup B1 than group B (P < 0.05), and in the subgroup B2 than group T (P < 0.05) (Fig. 4a and b).
Comparison of groups B and T with either subgroups T1, T2, or BT
For DSPP and ALP markers, groups B and T showed that the higher levels were expressed significantly than the subgroups (P < 0.05). For DMP1 and OPN markers, group B had significantly greater levels when compared with the subgroups (P < 0.05) (Fig. 4c–e).
Among the subgroups, B1 and B2 showed the higher expression levels of odonto/osteogenic genes. So, we compared the subgroups B1 and B2 with the groups B and T. For DSPP and ALP, both subgroups showed the higher significantly levels than groups B and T (P < 0.05). For DMP1, the expression was significantly increased in the subgroups B2 than groups B and T (P < 0.05), and in the subgroup B2 than B1 (P < 0.05). OPN was expressed significantly higher in the subgroups B2 than groups B and T (P < 0.05), and in the subgroup B1 than group T (P < 0.05) (Fig. 4f).
Discussion
Despite the level of attention and interest by researchers and clinicians to regenerative endodontics, several studies on immature non-infected human teeth showed that the current tissue engineering protocols do not result in true regeneration of pulp dentin complex [15, 16]. Cell proliferation and differentiation are dependent on the timely and spatial presence of specific growth factors. In the present study, for the first time, we aimed to evaluate the effect of co-stimulation with BMP-2 and TGF-β1 on differentiation of hDPSCs with different timing of delivery, compared to treatment with each growth factor alone. We also examined the overall effect of time of the growth factor administration on the expression of differentiation markers. Results of the present study will be for future experiments/studies in the field of regenerative endodontics to develop effective strategies for odontogenic differentiation of hDPSCs.
We found no significant effect of TGF-ß1, BMP-2, or their combination on the proliferation of DPSCs, except for BMP-2 at day 14. Outcome of previous studies were contraindicatory regarding the effect of TGF-ß1 on cell proliferation. Use of TGF-ß1 inhibited DNA synthesis in DPSCs in one study [17], while it increased the DNA content of human pulp cells in other studies [12, 18]. The anti-proliferative mechanism of TGF-β signaling pathway might be contributing to the regulation of some proteins that drive the G1 phase of the cell cycle [19]. Regarding to the proliferative effect of BMP-2, it has been reported that exogenous BMP-2 has an important role in the odonto/osteoblast differentiation of DPSCs but does not affect cell proliferation [4].
In an attempt to determine the best delivery timing of co-delivery of TGF-β1 and BMP-2 on odonto/osteogenesis of DPSCs, we tested 3 ways of adding these 2 factors to DPSC cultures. The highest mineralization and odonto/osteogenic gene expressions were observed when TGF-β1 was added for the first 7 days of incubation with a continuous application of BMP-2 for 14 days compared with the other groups. However, a continuous application of either growth factors showed better results than a continuous application of TGF-β1 with the addition of BMP-2 either in the early or late stage or a continuous application of both growth factors in combination for 14 days. This finding indicates that modulating the application time of growth factors in the combined application is important factor. The lower levels of mineralization in subgroups T1 and T2 in which TGF-β1 was continuously applied in a TGF-β1/BMP-2 combination might be caused by the fact that both growth factors can induce the overexpression of the DNA-binding protein inhibitor Id1 [20]. However, we did not quantify the results of mineralization, which is an inherent limitation for alizarin red staining experiments.
Previous studies showed that the transcription levels of DSPP and DMP1 increased continuously during odontoblastic differentiation of hDPSCs and reached the greatest level after 14 days of culture [21, 22]. Results of the present study showed that increasing the time of experiments to 14 days is necessary to rigorously evaluate the expression levels of odontogenic markers. On the other hand, the effect of time on expression levels of osteogenic markers (i.e., OPN and ALP) was mixed, positive for OPN and negative for ALP. In several odonto/osteoblast differentiation studies, the peak ALP expression was observed on day 7 [23, 24]. Overall, ALP is known as an indicator for early odonto/osteogenic differentiation [25].
The present study showed that “time of growth factor delivery” had a positive effect on the expression levels of odontogenic markers (i.e., DSPP and DMP1). Studies on stage-specific BMP deletions in mice revealed their importance after initial tooth formation. Previous studies reported that BMP-2 might be involved in the early tooth morphogenesis as well as the late odontoblast differentiation or mineral secretion. BMP-2 conditional knockout mice displayed abnormal tooth phenotypes with delayed odontoblast differentiation, abnormal dentin tubules, and decreased tooth-related gene expression [15]. In a knocked-out mice study, a total loss of BMP signaling led to an arrested tooth development at bud stage [26]. These studies show that BMP-2 provides an early temporal, non-redundant signal for directed and organized tooth mineralization. Our experiments showed that BMP-2 is an important factor for the initiation and continuation of odontogenic differentiation which should be present at early and late stages. In our experiments, B groups with continuous exposure to BMP-2 showed significantly higher expressions of DSPP and DMP1 compared to T groups with continuous exposure to TGF-β1.
Studies on mice with stage-specific TGF-β1 deletions revealed an essential role for TGF-β1 signaling in dentin mineralization [27]. When TGF-β was inhibited, the volume of dentin formed was not influenced, but its organization was impaired [28]. Tooth initiation, morphogenesis, and cytodifferentiation were not affected in a TGFβ-1 null mutation mouse model. However, profound changes were found at later stages of differentiation due to lack of TGF-β1 [29]. Based on these reports, TGF-β1 is mainly involved in terminal differentiation of odontoblasts. The present study showed that continuous exposure to TGF-β1 did not improve the expression of odontogenic markers, proving that early stimulation with TGF-β1 has no benefit for odontogenic differentiation.
The interaction between BMP and TGFβ signaling is another important issue since BMP and TGFβ can act antagonistic, or synergistic. BMPs and TGFβs act through the canonical or non-canonical signaling pathways. However, the output of this crosstalk is complex and their interaction has not been clearly studied in dentin regeneration. The overall expression of markers in B groups was greater than T groups in all time points. The greater outcome of B1 and B2 subgroups could be due to a synergistic effect via the interaction of BMP and TGF-β signaling. While both early and late exposure to TGF-β1 enhanced the expression of DSPP in groups B1 and B2, only late exposure to TGF-β1 resulted in significant increase in expression of DMP-1 in group B2. Similar findings were observed in the expression of OPN at day 14. These results showed a synergistic interaction between BMP-2 and TGF-β1 based on time of delivery. Previous studies showed that co-stimulation of human mesenchymal stem cells with TGF-β1 and BMP-9 results in significant increase in the expression of OPN [30].
In the current study, we selected DSPP, DMP1, ALP, and OPN as the related markers to evaluate the odontoblast differentiation. Our RT-PCR data showed that 14-day treatment with BMP-2 in combination with 7-day delivery of TGF-ß1 upregulated the expression of odontogenic specific markers, which means that hDPSCs are more likely to differentiate to a dentin-forming cell, possibly an odontoblast-like cell, by using this regimen. Meanwhile 14 days of treatment with TGF-ß1 downregulated the expression of DSPP and DMP-1 which means that this regimen could reduce the probability of hDPSCs differentiating to a dentin-generating cell. In other words, exposures starting with BMP-2 supplemented with late TGF-ß1 are better strategies for differentiation studies on hDPSCs. Follow-up in vitro and in vivo studies could better demonstrate the efficacy of these strategies.
Growth factors have short half-life, and thus, repeated administrations are required to maintain the therapeutic concentration. Implementing this strategy in an in vivo setting could be challenging. Designing a delivery system that could have a controlled release capacity can address this challenge.
Conclusion
The present study highlights the importance of timing in delivering growth factors for guided dentin regeneration. Continuous stimulation with BMP-2 was the key in odontogenic differentiation of hDPSCs, which was further enhanced by co-stimulation with TGF-β1. Continuous stimulation with TGFβ-1 did not improve the differentiation process of hDPSCs.
References
Chrepa V, Joon R, Austah O, Diogenes A, Hargreaves KM, Ezeldeen M, Ruparel NB (2020) Clinical outcomes of immature teeth treated with regenerative endodontic procedures—a San Antonio study. J Endod 46:1074–1084. https://doi.org/10.1016/j.joen.2020.04.008
Thesleff I, Nieminen P (1996) Tooth morphogenesis and cell differentiation. Curr Opin Cell Biol 8:844–850. https://doi.org/10.1016/s0955-0674(96)80086-x
Jernvall J, Thesleff I (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19–29. https://doi.org/10.1016/s0925-4773(99)00322-6
Saito T, Ogawa M, Hata Y, Bessho K (2004) Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod 30:205–208. https://doi.org/10.1097/00004770-200404000-00005
Qin W, Yang F, Deng R, Li D, Song Z, Tian Y, Wang R, Ling J, Lin Z (2012) Smad 1/5 is involved in bone morphogenetic protein-2-induced odontoblastic differentiation in human dental pulp cells. J Endod 38:66–71. https://doi.org/10.1016/j.joen.2011.09.025
Li J, Huang X, Xu X, Mayo J, Bringas P, Jiang R, Wang S, Chai Y (2011) SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development 138:1977–1989. https://doi.org/10.1242/dev.061341
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56:709–721. https://doi.org/10.1016/j.archoralbio.2010.12.008
Vaahtokari A, Vainio S, Thesleff I (1991) Associations between transforming growth factor beta 1 RNA expression and epithelial-mesenchymal interactions during tooth morphogenesis. Development 113:985–994
Bellamy C, Shrestha S, Torneck C, Kishen A (2016) Effects of a bioactive scaffold containing a sustained transforming growth factor-β1-releasing nanoparticle system on the migration and differentiation of stem cells from the apical papilla. J Endod 42:1385–1392. https://doi.org/10.1016/j.joen.2016.06.017
Wang W, Dang M, Zhang Z, Hu J, Eyster TW, Ni L, Ma PX (2016) Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta biomater 36:63–72. https://doi.org/10.1016/j.actbio.2016.03.015
Aksel H, Huang GT (2017) Combined effects of vascular endothelial growth factor and bone morphogenetic protein 2 on odonto/osteogenic differentiation of human dental pulp stem cells in vitro. J Endod 43:930–935. https://doi.org/10.1016/j.joen.2017.01.036
He H, Yu J, Liu Y, Lu S, Liu H, Shi J, Jin Y (2008) Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int 32:827–834. https://doi.org/10.1016/j.cellbi.2008.03.013
Chang Y-C, Chang M-C, Chen Y-J, Liou J-U, Chang H-H, Huang W-L, Liao W-C, Chan C-P, Jeng P-Y, Jeng J-H (2017) Basic fibroblast growth factor regulates gene and protein expression related to proliferation, differentiation, and matrix production of human dental pulp cells. J Endod 43:936–942. https://doi.org/10.1016/j.joen.2017.01.024
Homayounfar N, Verma P, Nosrat A, El Ayachi I, Yu Z, Romberg E, Huang GT-J, Fouad AF (2016) Isolation, characterization, and differentiation of dental pulp stem cells in ferrets. J Endod 42:418–424. https://doi.org/10.1016/j.joen.2015.12.002
Wu L, Wang F, Donly KJ, Wan C, Luo D, Harris SE, MacDougall M, Chen S (2015) Establishment of immortalized mouse Bmp2 knock-out dental papilla mesenchymal cells necessary for study of odontoblastic differentiation and odontogenesis. J Cell Physiol 230:2588–2595. https://doi.org/10.1002/jcp.25061
Nosrat A, Kolahdouzan A, Khatibi AH, Verma P, Jamshidi D, Nevins AJ, Torabinejad M (2019) Clinical, radiographic, and histologic outcome of regenerative endodontic treatment in human teeth using a novel collagen-hydroxyapatite scaffold. J Endod 45:136–143. https://doi.org/10.1016/j.joen.2018.10.012
Chan CP, Lan WH, Chang MC, Chen YJ, Lan WC, Chang HH, Jeng JH (2005) Effects of TGF-beta s on the growth, collagen synthesis and collagen lattice contraction of human dental pulp fibroblasts in vitro. Arch Oral Biol 50:469–479. https://doi.org/10.1016/j.archoralbio.2004.10.005
Chang HH, Chang MC, Wu IH, Huang GF, Huang WL, Wang YL, Lee SY, Yeh CY, Guo MK, Chan CP, Hsien HC, Jeng JH (2015) Role of ALK5/Smad2/3 and MEK1/ERK signaling in transforming growth factor beta 1-modulated growth, collagen turnover, and differentiation of stem cells from apical papilla of human tooth. J Endod 41:1272–1280. https://doi.org/10.1016/j.joen.2015.03.022
Massagué J, Blain SW, Lo RS (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295–309. https://doi.org/10.1016/s0092-8674(00)00121-5
Song X, Liu S, Qu X, Hu Y, Zhang X, Wang T, Wei F (2011) BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim Biophys Sin 43:796–804. https://doi.org/10.1093/abbs/gmr074
Hao J, Yang H, Cao Y, Zhang C, Fan Z (2020) IGFBP5 enhances the dentinogenesis potential of dental pulp stem cells via JNK and ErK signalling pathways. J Oral Rehabil 47:1557–1565. https://doi.org/10.1111/joor.13047
Lv T, Wu Y, Mu C, Liu G, Yan M, Xu X, Wu H, Du J, Yu J, Mu J (2016) Insulin-like growth factor 1 promotes the proliferation and committed differentiation of human dental pulp stem cells through MAPK pathways. Arch Oral Biol 72:116–123. https://doi.org/10.1016/j.archoralbio.2016.08.011
Lin Z, Wang JS, Lin L, Zhang J, Liu Y, Shuai M, Li Q (2014) Effects of BMP2 and VEGF165 on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Exp Ther Med 7:625–629. https://doi.org/10.3892/etm.2013.1464
Wu J, Huang GT, He W, Wang P, Tong Z, Jia Q, Dong L, Niu Z, Ni L (2012) Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J Endod 38:614–622. https://doi.org/10.1016/j.joen.2012.01.014
Claassen H, Kampen WU, Kirsch T (1996) Localization of collagens and alkaline phosphatase activity during mineralization and ossification of human first rib cartilage. Histochem Cell Biol 105:213–219. https://doi.org/10.1007/BF01462294
Yang G, Yuan G, Ye W, Cho KW, Chen Y (2014) An atypical canonical bone morphogenetic protein (BMP) signaling pathway regulates Msh homeobox 1 (Msx1) expression during odontogenesis. J Biol Chem 289:31492–31502. https://doi.org/10.1074/jbc.M114.600064
Thyagarajan T, Sreenath T, Cho A, Wright JT, Kulkarni AB (2001) Reduced expression of dentin sialophosphoprotein is associated with dysplastic dentin in mice overexpressing transforming growth factor-beta 1 in teeth. J Biol Chem 276:11016–11020. https://doi.org/10.1074/jbc.M010502200
Oka S, Oka K, Xu X, Sasaki T, Bringas P Jr, Chai Y (2007) Cell autonomous requirement for TGF-beta signaling during odontoblast differentiation and dentin matrix formation. Mech Dev 124:409–415. https://doi.org/10.1016/j.mod.2007.02.003
D’Souza RN, Cavender A, Dickinson D, Roberts A, Letterio J (1998) TGF-β1 is essential for the homeostasis of the dentin-pulp complex. Eur J Oral Sci 106:185–191. https://doi.org/10.1111/j.1600-0722.1998.tb02174
Li RD, Deng ZL, Hu N, Liang X, Liu B, Luo J, Chen L, Yin L, Luo X, Shui W, He TC, Huang W (2012) Biphasic effects of TGFβ1 on BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep 45:509–514. https://doi.org/10.5483/bmbrep.2012.45.9.053
Acknowledgments
The authors would like to thank Prof. Alireza Akbarzadeh Baghban for statistical analysis.
Funding
Research reported in this publication was supported by Elite Researcher Grant Committee under award number 996576 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Saeed Asgary and Ali Nosrat; methodology: Saeed Asgary and Hassan Torabzadeh; formal analysis and investigation: Sayna Shamszadeh, Ali Nosrat, and Simzar Hosseinzadeh; writing — original draft preparation: Sayna Shamszadeh; writing — review and editing: Hassan Torabzadeh and Ali Nosrat; supervision: Saeed Asgary. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Intact/sound third molars were obtained from patients at the Oral and Maxillofacial Surgery Department at Shahid Beheshti Dental School. The approval was obtained by the Ethics Committee at National Institutes for Medical Research Development (NIMAD) (IR.NIMAD.REC.1399.262).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shamszadeh, S., Asgary, S., Torabzadeh, H. et al. Cytokine co-stimulation effect on odontogenic differentiation of stem cells. Clin Oral Invest 26, 4789–4796 (2022). https://doi.org/10.1007/s00784-022-04443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04443-8