Abstract
Objectives
This study evaluated factors associated with vertical root fracture in endodontically treated teeth, using a cone-beam computed tomography (CBCT) image database.
Materials and methods
The sample for this case-control study consisted of 81 CBCT scans of teeth with vertical root fracture (VRF) and 81 CBCT scans of non-fractured teeth, matched by age, sex, and tooth position. The analyzed variables included dentin thickness, an intraradicular post, an adjacent implant, and a missing adjacent tooth. Student’s t test test was used to compare the quantitative variables. The chi-square test was used to compare the categorical variables. Logistic regression was performed to evaluate the association between the presence of VRF and the independent factors assessed.
Results
The mean dentin thickness of fractured teeth was 1.3 mm, whereas that of non-fractured ones was 1.5 mm (p < 0.001). There was no difference between the fractured and non-fractured groups, regarding implant frequency or missing adjacent tooth (p > 0.05). There were a significantly larger number of teeth with posts in the fractured versus non-fractured group (p = 0.007). However, dentin thickness ≤ 1.3 mm was the only factor associated with VRF in the multiple regression model (OR = 3.60, 95%CI = 1.76–7.37).
Conclusions
Dentin thickness may influence the development of VRF. Dentin thickness ≤ 1.3 mm is associated with a greater likelihood of fracture than ≥ 1.4 mm.
Clinical relevance
This study suggests there may be a minimum amount of safe dentin thickness that should be preserved after endodontic instrumentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vertical root fracture (VRF) is defined as a complete or incomplete fracture that extends longitudinally along the root [1]. The prevalence of VRF ranges from 3.69 to 31.5% [2,3,4]. It is more frequent in women aged older than 40 years, and the most affected teeth are mandibular molars and maxillary premolars [5, 6].
The etiology of VRF is multifactorial, and its occurrence is more common in endodontically treated teeth [7]. The microstructural changes in dentin over the years, excessive instrumentation, excessive irrigation with highly concentrated chemical solutions, and excessive compaction force during lateral condensation may increase susceptibility to VRF [8,9,10]. Other potential risk factors for VRF are endodontic retreatment [11] and overfilled roots [12].
Extensive dentin removal in endodontic instrumentation may result in tooth weakening [13]. Root canal wear during instrumentation can range from 14 to 45% of the dental structure [14], resulting in the removal of a mean dentin volume of approximately 2 to 3 mm3 [15, 16]. Knowing how much dentin can be removed without causing considerable weakening is a real challenge.
In addition to the weakening caused by endodontic treatment, other factors can put teeth at risk for VRF. A case series reported the presence of implants adjacent to endodontically treated teeth as a possible VRF-related factor [17]. According to this report, close placement of implants could cause an imbalance in the dissipation of occlusal forces [17, 18]. Based on this principle, one of our hypotheses was that a missing adjacent tooth would also cause a disparity in the dissipation of forces, making the remaining teeth of the dental arch more susceptible to root fractures.
VRF diagnosis poses a great challenge to the clinician, because VRFs have non-specific signs and symptoms, thus mimicking other dental conditions [6]. In addition, radiographic diagnosis of VRFs can be difficult due to limitations inherent to two-dimensional exams, such as the overlap of dental and bone structures [19]. Cone-beam computed tomography (CBCT) allows a three-dimensional evaluation of these structures and has been found to be more accurate than periapical radiography in detecting VRFs [20, 21]. However, CBCT could present a low sensibility in relation to VRF detection [22], influenced by the presence of artifacts [23, 24] and technical exposure parameters [25].
VRF is one of the main causes of extraction of endodontically treated teeth [3, 26]. Moreover, late diagnosis leads to damage to periodontal tissues, such as significant alveolar bone loss, which directly impacts a patient’s rehabilitation treatment [27]. Knowledge of the factors related to the development of a VRF may help prevent it, and also contribute to establishing procedures for early VRF diagnosis. Some of these factors have not yet been completely elucidated, such as the minimum amount of remaining dentin thickness and the presence of adjacent implants. Thus, this study aimed to evaluate the factors associated with VRF in endodontically treated teeth, using a CBCT image database.
Materials and methods
Study design
A case-control study was designed and conducted following the guidelines set by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) to describe case-control studies [28]. The present research protocol was approved by the ethics committee of the university (CAAE 80421917.0.0000.5083).
Sample selection
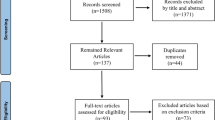
Eighty-one CBCT scans of endodontically treated teeth with VRF (cases) were selected and compared with CBCT scans of endodontically treated teeth without VRF (controls). The case and control groups were matched by a ratio of 1:1 for age (± 5 years), sex, and tooth. The CBCTs were provided from the image database of a private oral radiologic clinic, so they were not performed with research purposes. The VRF group was selected from radiological reports with suspicion of VRF and the record of a positive confirmation of VRF obtained by exploratory surgery. The controls were selected from CBCT scans, with no evidence of VRF, requested for the planning of third molar extraction or endodontic evaluation. The scans were acquired by i-CAT Next Generation (Imaging Sciences International, Hatfield, PA, USA), under the following exposure protocol: voxel 0.125–0.2, FOV 16 × 4 cm, mA 5, and kVp 120.
A pilot study was performed to calculate the sample size, using a convenience sample of 20 CBCT images of teeth with VRF and 20 matched control teeth (without VRF). Considering root dentin thickness as the main tested risk factor for VRF (exposure variable)—≤ 1.3 mm (exposed) and ≥ 1.4 mm (unexposed)—the proportion of exposed teeth was 54.2% (n = 11) for the cases and 35.0% (n = 7) for the controls. Other parameters included a 5% level of significance (type I error rate) and 80% power (20% type II error rate). Based on this information, the minimum odds ratio (OR) to detect was 2.2, and the estimated sample size was 81 cases and 81 controls for this matched case-control study (one-sided test).
The variables analyzed included dentin thickness, an intraradicular post, an adjacent implant, and missing adjacent teeth. Adjacent implants and adjacent teeth were considered as such when they were next to the analyzed tooth.
Radiological reports of endodontically treated teeth, which included descriptive images of VRFs and information on the clinical confirmation of VRF, were previously selected. Then, the entire volume was evaluated following multiplanar reformatting to confirm the presence of a clear fracture line. A fracture line was so considered when a radiolucent/hypodense linear image was observed simultaneously and continuously in the axial, coronal, and sagittal planes.
To permit the use of digital applications and to aid in dental measurement procedures, all the CBCT scans obtained from the oral radiologic clinic database that aimed the investigation of dental fractures, or the planning of third molar extraction or endodontic evaluation, were obtained in disclusion. This procedure is helpful if the CBCT scan is captured for dental measurements, and in cases that having accurate occlusal details is currently indispensable.
After defining the case group, the patient profile (sex, age, and tooth) of these scans was recorded. The control group was selected according to similar characteristics specified in the case group. For this purpose, an initial selection was made based on the radiological reports, gender, age, and tooth. After this preselection, a simple, random draw was performed to choose 1 control from the eligible scans. All CBCT scans were acquired with a FOV of 16 × 4 cm, following the As Low As Reasonably Achievable (ALARA) principle. The CBCT scans, performed between 2014 and 2018, from patients with single-rooted and/or multirooted endodontically treated teeth were included in the study. CBCT scans with artifacts that hindered their evaluation were excluded, as well as teeth with internal root resorption and extensive periapical lesions.
Image analysis
DICOM files were exported to CS 3D Imaging Software version 3.1.9 (Carestream Health, Rochester, NY, USA) and ITK-SNAP version 3.8.0 (www.itksnap.org) and analyzed by a single radiologist with 10 years of experience, in a dark environment, on a Dell XPS X8700 computer with a 28-in. color monitor, an Intel 3.4-GHz processor, 12-GB memory, and 2-TB HD (Dell, Austin, TX, USA).
Dentin thickness was evaluated using the CS 3D Imaging Software, with the linear measurement tool. Oblique slicing was used to adjust the planes on the long axis of the tooth. The evaluations were performed on oblique coronal and oblique sagittal slices. The roots were divided proportionally into three parts, and measured at three points (cervical, middle, and apical thirds—1 mm from the apex), in each dental aspect (buccal, lingual, mesial, and distal) (Fig. 1). The final measurement was the simple arithmetic mean of the measurements of each aspect. The measurements of multirooted teeth were performed on the fractured root. In regard to the teeth that had two root canals in a root and significant presence of dentin between them, the dentin thickness between the canals was included in the evaluation. The same points were evaluated in the mesial and distal aspects.
Statistical analysis
Statistical analysis was performed using the SPSS 24 software (Statistical Package for Social Sciences, IBM Corp, NY, USA). Fifteen percent of the sample was reevaluated and the intraclass correlation coefficient (ICC) was calculated to evaluate the intraexaminer agreement of the measurements for dentin thickness. The Kolmogorov-Smirnov test was used to evaluate normality of the sample. Student’s t test was used to compare the dentin thickness between the fractured and non-fractured groups. The categorical variables were compared using the chi-square test. Statistical significance was set at p < 0.05. Logistic regression was performed to evaluate the association between the presence of VRF and the independent factors assessed.
Results
The intraexaminer agreement (intraclass correlation coefficient) was excellent for dentin thickness (0.8). Of the 81 teeth selected with root fractures, 58% (n = 47) were single-rooted and 42% (n = 34) were multirooted. For case-control study purposes, the non-fractured group had the same proportions. The sample consisted mostly of women (63%), with a mean age of 51 years, and the most frequently assessed teeth were mandibular molars (21%) and maxillary premolars (21%).
The mean dentin thickness for teeth with VRF was 1.54 (± 0.28), 1.45 (± 0.24), and 0.96 (± 0.15) mm for the cervical, middle, and apical thirds, respectively. These measurements in the control group were 1.85 (± 0.30), 1.62 (± 0.23), and 1.03 (± 0.14) mm for the cervical, middle, and apical thirds, respectively. Figure 2 shows that dentin thickness was significantly lower for teeth with VRF in all regions of the root—cervical (p < 0.001), middle (p < 0.001), and apical (p = 0.002).
The measurements of the dentin thickness are described in Table 1. A thinner dentin wall was found for teeth with VRFs (p < 0.001). The dentin thickness of the fractured tooth with an intraradicular post was 1.25 mm (± 0.21), whereas the mean value of the fractured tooth without an intraradicular post was 1.38 mm (± 0.15; p = 0.004). The presence of an intraradicular post was significantly higher in the fractured group (p = 0.007). The presence of implants adjacent to fractured teeth was 8.6%, and missing adjacent teeth represented 19.8%. Similar percentages were found for the control group (Table 1).
Logistic regression analysis (Table 2) was performed to identify factors associated with the occurrence of VRF. The independent variables were dentin thickness—dichotomized as ≤ 1.3 mm and ≥ 1.4 mm, based on the mean value of dentin thickness of the fractured group, the presence of intraradicular post, adjacent implant, and missing adjacent tooth.
The bivariate logistic regression models showed that lower dentin thickness (OR = 4.24; 95% CI = 2.19–8.18) and the presence of an intraradicular post (OR = 2.4; 95% CI = 1.26–4.53) were associated with VRF (Table 2). However, as the presence of an intraradicular post was highly associated with lower dentin thickness, due to root canal preparation, this variable lost statistical significance in the multiple regression model. Hence, the only factor that was considered associated with VRF was the thickness of the root dentin (p < 0.001).
Discussion
The multifactorial etiology of VRFs and their non-specific symptoms make diagnosis a challenge in clinical practice [6]. Identification of the factors causing a VRF is crucial for its diagnosis and prevention. The present study evaluated some factors associated with the development of VRFs in endodontically treated teeth, using CBCT images. The results showed that dentin thickness ≤ 1.3 mm was a risk factor, regardless of the presence of an intraradicular post, adjacent implants, or missing adjacent tooth.
In the present investigation, we executed a case-control study to evaluate the association of some factors with VRF. According to the case-study design, the case and the control groups were matched for age, sex, and tooth type to reduce the influence of these variables as confounding factors. We performed tomographic measurements of dentin thickness in the CBCT scans based on the premise that this exam provides an accurate means to measure dentin thickness, even in the presence of metallic artifacts [29]. According to a previous study, there is a very high correlation between CBCT and micro-CT in the measurement of dentin thickness, with the last being considered the reference standard [30], showing that CBCT can be used to accurately assess radicular dentin wall thickness [29].
The CBCT scans used in this investigation were obtained with a voxel size varying from 0.125–0.2 mm, which according to Ozer et al. [31] and Bragatto et al. [32], allows an accurate analysis for VRF diagnosis. Additionally, the voxel sizes used in the present study (0.125–0.2 mm) show no differences between them in respect to the detection of VRF in teeth with metallic posts [23].
In the intent to prevent measurement bias, all the analyses were performed by a single experienced radiologist, showing an excellent intraexaminer agreement for all the measurements. A single examiner provides more consistency in observations, improves distinction among the groups, and suppresses the interexaminer variation [33].
The minimum amount of remaining dentin not promoting considerable weakening after endodontic treatment has not yet been determined. In this study, the mean thickness of the fractured teeth was 1.3 mm, whereas that of the non-fractured teeth was 1.5 mm. Mireku et al. [8] found similar values in their evaluation of the effects of dentin thickness on extracted single-rooted teeth, treated endodontically with intraradicular post, where the fractured and non-fractured teeth had a mean thickness of 1.24 mm (± 0.35) and 1.52 mm (± 0.27), respectively. Unlike our investigation, which determined dentin thickness at three points, theirs evaluated it along the fracture line, and their approach, sample size, and study design were different from ours. Accordingly, our results suggest that there is a possible minimum amount of safe dentin thickness after root canal treatment.
Our findings imply that teeth with less remaining dental tissue are more susceptible to VRF due to the weakening of the dental structure. Marchi et al. [34] evaluated the effect of remnant thickness of bovine dental roots on fracture resistance, with different intraradicular systems, and found that teeth with less root structure are less resistant to fracture, regardless of the type of post used. In addition, our results demonstrated that when an intraradicular post was involved, the dentin thickness was significantly smaller. Accordingly, the clinician should be aware of the greater chances of fracture in post-preparation procedures when the dentin thickness of roots is ≤ 1.3 mm.
In the logistic regression, the odds ratio found for dentin thickness indicates that teeth endodontically treated with a dentin remnant ≤ 1.3 mm are 3.6 times more likely to present a root fracture, in comparison with teeth with a greater thickness. Although the presence of a post may be independently associated with the development of VRF (OR = 2.3; 95% CI = 1.26–4.53), this variable was not a risk factor in the multiple regression model (OR = 1.49; 95% CI = 0.72–3.05). In a retrospective cohort study, Maddalone et al. [35] reported that the presence of a post was a risk factor for VRF (OR = 15; 95%CI = 6.84–33.59). However, the authors of the mentioned study did not evaluate dentin thickness, whereas we tested the effect of both the dentin thickness and the presence of the post, in a bivariate logistic regression model. Our results indicated that dentin thickness was the main factor associated with VRF, regardless of the presence of an intraradicular post.
It was also postulated that the combination of excessive occlusal overload on endodontically treated teeth adjacent to dental implants may potentially contribute to the development of VRF [17]. This assumption was based on the finding that dental implants do not offer the protective action of proprioception, which may result in occlusal overload, and also on the fact that endodontically treated teeth have reduced proprioception and fracture resistance. In this respect, Rosen et al. [17] related a possible association between the presence of implants and the development of VRFs in endodontically treated teeth. The results of the present study did not show a significant difference between the case and the control groups regarding the presence of implants. From a clinical perspective, the interpretation of results was impaired, because it was not known if the implant was placed before or after the occurrence of the VRF, or if the dental implant was really related to occlusal overload in the region of the analyzed teeth. Despite the plausibility of this theory, the only evidence currently available regarding the relation between the presence of dental implants and VRF in endodontically treated teeth comes from only a few reported cases [17].
Likewise, in the present study, we found no significant relation between missing adjacent teeth and VRFs. Our hypothesis was that a missing adjacent tooth would also cause a disparity in the dissipation of forces and occlusal overload in the endodontic tooth region. However, our study design did not allow us to determine whether the patient indeed wore a removable partial prosthesis in the missing tooth region, an important factor that would directly influence the dissipation of occlusal forces.
The limitations of this study are related to the use of an image database that did not contain all the clinical information on the patients analyzed, as well as the lack of information on the type of restoration and post, and on their use with an adhesive or non-adhesive system.
New studies using micro-CT should be carried out to establish and reinforce the concept of an optimal minimal thickness of dentin that should be maintained in endodontic treatments. Further research to understand related, yet unelucidated factors, such as the presence of adjacent implants, is also necessary.
The results of the present study showed the influence of dentin thickness on the development of VRF and emphasize the importance of preserving utmost tooth structure in endodontic procedures, and in preparations for intraradicular post placement. The findings related to dentin thickness can alert professionals to the possibility of root fractures in teeth with smaller thicknesses, and guide them in more conservative protocols.
Conclusion
Based on our results, we concluded that dentin thickness influences the development of VRF. Dentin thickness ≤ 1.3 mm is associated with a greater likelihood of fracture, compared with thicknesses ≥ 1.4 mm, regardless of the presence of intraradicular posts. Missing adjacent teeth and the presence of implants are factors that do not seem significant for the development of VRFs.
References
Rivera E, Walton RE (2008) Cracking the cracked tooth code: detection and treatment of various longitudinal tooth fractures. Endodontics: colleagues for excellence. American Association of Endodontists (Summer):1-8. http://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/ecfesum08.pdf. Accessed 12 November 2019
Morfis A (1990) Vertical root fractures. Oral Sur Oral Med Oral Pathol 69:631–635. https://doi.org/10.1016/0030-4220(90)90248-q
Touré B, Faye B, Kane AW, Lo CM, Niang B, Boucher Y (2011) Analysis of reasons for extraction of endodontically treated teeth: a prospective study. J Endod 37:1512–1515. https://doi.org/10.1016/j.joen.2011.07.002
Yoshino K, Ito K, Kuroda M, Sugihara N (2015) Prevalence of vertical root fracture as the reason for tooth extraction in dental clinics. Clin Oral Investig 19:1405–1409. https://doi.org/10.1007/s00784-014-1357-4
PradeepKumar AR, Shemesh H, Jothilatha S et al (2016) Diagnosis of vertical root fractures in restored endodontically treated teeth: a time-dependent retrospective cohort study. J Endod 42:1175–1180. https://doi.org/10.1016/j.joen.2016.04.012
Liao WC, Tsai YL, Wang CY, Chang MC, Huang WL, Lin HJ, Liu HC, Chan CP, Chang SH, Jeng JH (2017) Clinical and radiographic characteristics of vertical root fractures in endodontically and nonendodontically treated teeth. J Endod 43:687–693. https://doi.org/10.1016/j.joen.2016.12.009
Seo DG, Yi YA, Shin SJ, Park JW (2012) Analysis of factors associated with cracked teeth. J Endod 38:288–292. https://doi.org/10.1016/j.joen.2011.11.017
Mireku AS, Romberg E, Fouad AF, Arola D (2010) Vertical fracture of root filled teeth restored with posts: the effects of patient age and dentine thickness. Int Endod J 43:218–225. https://doi.org/10.1111/j.1365-2591.2009.01661.x
Barreto MS, Moraes RA, da Rosa RA et al (2012) Vertical root fractures and dentin defects: effects of root canal preparation, filling, and mechanical cycling. J Endod 38:1135–1139. https://doi.org/10.1016/j.joen.2012.05.002
Uzunoglu E, Aktemur S, Uyanik MO, Durmaz V, Nagas E (2012) Effect of ethylenediaminetetraacetic acid on root fracture with respect to concentration at different time exposures. J Endod 38:1110–1113. https://doi.org/10.1016/j.joen.2012.04.026
García-Guerrero C, Parra-Junco C, Quijano-Guauque S, Molano N, Pineda GA, Marín-Zuluaga DJ (2018) Vertical root fractures in endodontically-treated teeth: a retrospective analysis of possible risk factors. J Investig Clin Dent 9:e12273. https://doi.org/10.1111/jicd.12273
PradeepKumar AR, Shemesh H, van Loveren C et al (2019) Impact of apical extent of root canal filling on vertical root fracture: a case-control study. Int Endod J 52:1283–1289. https://doi.org/10.1111/iej.13134
Tavanafar S, Karimpour A, Karimpour H, Saleh AM, Saeed MH (2015) Effect of different instrumentation techniques on vertical root fracture resistance of endodontically treated teeth. J Dent (Shiraz) 16:50–55 http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4476116/. Accecced 10 Nov 2019
Sousa K, Andrade-Junior CV, Silva JM, Duarte MAH, De-Deus G, Silva EJNL (2015) Comparison of the effects of TripleGates and Gates-Glidden burs on cervical dentin thickness and root canal area by using cone beam computed tomography. J Appl Oral Sci 23:164–168. https://doi.org/10.1590/1678-775720130542
Moura-Netto C, Palo RM, Camargo CHR, Pameijer CH, Bardauil MRRS (2013) Micro-CT assessment of two different endodontic preparation systems. Braz Oral Res 27:26–30. https://doi.org/10.1590/s1806-83242012005000029
Elnaghy AM, Elsaka SE (2014) Evaluation of root canal transportation, centering ratio, and remaining dentin thickness associated with ProTaper Next instruments with and without glide path. J Endod 40:2053–2056. https://doi.org/10.1016/j.joen.2014.09.001
Rosen E, Beitlitum I, Tamse A, Taschieri S, Tsesis I (2016) Implant-associated vertical root fracture in adjacent endodontically treated teeth: a case series and systematic review. J Endod 42:948–952. https://doi.org/10.1016/j.joen.2016.03.021
Rosen E, Goldberger T, Tamse A, Nemcovsky CE, Breslauer S, Beitlitum I, Tsesis I (2017) Implant-associated cracked teeth: case series. Evid Based Endod 2:6. https://doi.org/10.1186/s41121-017-0012-3
Patel S, Dawood A, Whaites E, Pitt Ford T (2009) New dimensions in endodontic imaging: part 1. Conventional and alternative radiographic systems. Int Endod J 42:447–462. https://doi.org/10.1111/j.1365-2591.2008.01530.x
Wang P, Yan XB, Lui DG, Zhang WL, Zhang Y, Ma XC (2011) Detection of dental root fractures by using cone-beam computed tomography. Dentomaxillofac Radiol 40:290–298. https://doi.org/10.1259/dmfr/84907460
da Silveira PF, Vizzotto MB, Liedke GS, da Silveira HLD, Montagner F, da Silveira HED (2013) Detection of vertical root fractures by conventional radiographic examination and cone beam computed tomography–an in vitro analysis. Dent Traumatol 29:41–46. https://doi.org/10.1111/j.1600-9657.2012.01126.x
Chavda R, Mannocci F, Andiappan M, Patel S (2014) Comparing the in vivo diagnostic accuracy of digital periapical radiography with cone-beam computed tomography for the detection of vertical root fracture. J Endod 40:1524–1529. https://doi.org/10.1016/j.joen.2014.05.011
Junqueira RB, Verner FS, Campos CN, Devito KL, do Carmo AMR (2013) Detection of vertical root fractures in the presence of intracanal metallic post: a comparison between periapical radiography and cone-beam computed tomography. J Endod 39:1620–1624. https://doi.org/10.1016/j.joen.2013.08.031
e Silva DDM, Campos CN, Carvalho ACP, Devito KL (2018) Diagnosis of mesiodistal vertical root fractures in teeth with metal posts: influence of applying filters in cone-beam computed tomography images at different resolutions. J Endod 44:470–474. https://doi.org/10.1016/j.joen.2017.08.030
Yamamoto-Silva FP, Siqueira CFO, Silva MAGS et al (2018) Influence of voxel size on cone-beam computed tomography-based detection of vertical root fractures in the presence of intracanal metallic posts. Imaging Sci Dent 48:177–184. https://doi.org/10.5624/isd.2018.48.3.177
Tzimpoulas NE, Alisafis MG, Tzanetakis GN, Kontakiotis EG (2012) A prospective study of the extraction and retention incidence of endodontically treated teeth with uncertain prognosis after endodontic referral. J Endod 38:1326–1329. https://doi.org/10.1016/j.joen.2012.06.032
Tsesis I, Rosen E, Tamse A, Taschieri S, Kfir A (2010) Diagnosis of vertical root fractures in endodontically treated teeth based on clinical and radiographic indices: a systematic review. J Endod 36:1455–1458. https://doi.org/10.1016/j.joen.2010.05.003
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
Xu J, He J, Yang Q, Huang D, Zhou X, Peters OA, Gao Y (2017) Accuracy of cone-beam computed tomography in measuring dentin thickness and its potential of predicting the remaining dentin thickness after removing fractured instruments. J Endod 43:1522–1527. https://doi.org/10.1016/j.joen.2017.03.041
Ordinola-Zapata R, Bramante CM, Versiani MA, Moldauer BI, Topham G, Gutmann JL, Nuñez A, Duarte MAH, Abella F (2017) Comparative accuracy of the clearing technique, CBCT and micro-CT methods in studying the mesial root canal configuration of mandibular first molars. Int Endod J 50:90–96. https://doi.org/10.1111/iej.12593
Özer SY (2011) Detection of vertical root fractures by using cone beam computed tomography with variable voxel sizes in an in vitro model. J Endod 37:75–79. https://doi.org/10.1016/j.joen.2010.04.021
Bragatto FP, Iwaki Filho L, Kasuya AV, Chicarelli M, Queiroz AF, Takeshita WM, Iwaki LC (2016) Accuracy in the diagnosis of vertical root fractures, external root resorptions, and root perforations using cone-beam computed tomography with different voxel sizes of acquisition. J Conserv Dent 19:573–577. https://doi.org/10.4103/0972-0707.194029
Mutch L, Johnson M, Morley R (1989) Follow up studies: design, organization, and analysis. Arch Dis Child 64:1394–1402. https://doi.org/10.1136/adc.64.10_spec_no.1394
Marchi G, Mitsui F, Cavalcanti A (2008) Effect of remaining dentine structure and thermal-mechanical aging on the fracture resistance of bovine roots with different post and core systems. Int Endod J 41:969–976. https://doi.org/10.1111/j.1365-2591.2008.01459.x
Maddalone M, Gagliani M, Citterio CL, Karanxha L, Pellegatta A, Del Fabbro M (2018) Prevalence of vertical root fractures in teeth planned for apical surgery. A retrospective cohort study. Int Endod J 51:969–974. https://doi.org/10.1111/iej.12910
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee of the university (CAAE 80421917.0.0000.5083) and conducted in accordance with the 1964 Helsinki declaration.
Informed consent
For this type of study (retrospective study), formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, L.R., de Lima, K.L., Santos, A.A. et al. Dentin thickness as a risk factor for vertical root fracture in endodontically treated teeth: a case-control study. Clin Oral Invest 25, 1099–1105 (2021). https://doi.org/10.1007/s00784-020-03406-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03406-1