Abstract
Objective
Although prematurity is a risk factor for developing deformational plagiocephaly (DP), to our knowledge, there are no studies that have analyzed the impact of a premature birth on the duration of head orthosis therapy and the extent of the reduction in asymmetry during treatment.
Materials and Methods
We examined 239 patients with DP who were undergoing head orthosis therapy. Depending on the gestational age, they were assigned to either a premature (gestational age of < 37 weeks) or a full-term (gestational age of ≥ 37 weeks) group. Head shape was analyzed using 3D-stereophotogrammetry at the start and end of treatment. We performed multiple linear regression analyses to evaluate the impact of prematurity on the duration of therapy and the extent of the reduction in asymmetry, taking age and the initial asymmetry of an infant’s head into account.
Results
Head orthosis therapy led to a significant reduction in asymmetry in both groups. Using multiple linear regression analyses, we demonstrated that age at the start of treatment, the initial asymmetry and prematurity, significantly influenced the duration of therapy. Patients who were born at an earlier gestational age experienced a shorter treatment length. However, prematurity did not affect the extent of the reduction in cranial asymmetry that was achieved.
Conclusions
Along with age at the start of treatment and the initial asymmetry, prematurity is significantly correlated with the duration of head orthosis therapy, but not with the extent of the reduction in asymmetry achieved.
Clinical Relevance
Knowledge of these findings is important for clinicians when planning treatment and discussing the effectiveness of head orthosis therapy with the parents of premature infants with DP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positional asymmetries of the head are the most common skull deformities in infancy [1,2,3]. Along with a unilateral flattening of the occiput, an ipsilateral anterior shift of the ear, an ipsilateral protrusion of the forehead, and facial scoliosis are possible further characteristics of a deformational plagiocephaly (DP) [4,5,6,7]. Due to the reasonable recommendation that children should sleep on their backs to avoid sudden infant death syndrome, the incidence of these positional head asymmetries has increased significantly [3]. Besides to this supine sleeping position, which is the main risk factor for developing DP, many other risk factors such as male sex [8,9,10,11], limited intrauterine space (e.g., multiple birth) [8, 9, 12], and restricted mobility due to muscular torticollis [13] may likewise contribute to the development of this condition. Prematurity also seems to be a very important risk factor [14,15,16,17,18]: the cranial bone of premature infants is more malleable and might therefore be more susceptible to deformation [19, 20]. Furthermore, premature infants have poorer neck muscle tone, meaning that their head is placed in a lateral position most of the time [16, 21].
In young patients (less than about 6 months of age) with only moderate asymmetry, it is generally recommended that active repositioning or physical therapy should be commenced to correct the asymmetry [22]. In older patients, or where there is severe skull asymmetry, or in cases where the above-mentioned therapies have failed to normalize the skull shape, molding head orthosis therapy is an additional option [22, 23]. This so-called helmet therapy guides further growth of an infant’s head in order to correct the deformational asymmetry. The effectiveness of the different therapeutic approaches mainly depends on age at the start of therapy and the initial asymmetry [2, 24]. In general, growth is the precondition for successful therapy [23], with the literature stating that there is a reduced growth potential with increasing age [25], but there may be a difference between full-term and premature infants.
Several studies have analyzed the impact of an infant’s age on how successful helmet therapy is [2, 24, 26, 27], but to our knowledge, none has investigated the influence of prematurity. For this reason, the aims of this study were to assess the impact of preterm birth on (1) the extent of the reduction in asymmetry, and (2) the duration of the head orthosis therapy in infants with DP.
Materials and methods
Subjects
A total of 239 Caucasian infants (33% female, 67% male) with DP were included in the study. With reference to Moss and Mortenson et al. [28, 29], only infants with a cranial vault asymmetry (CVA) of more than 3 mm were included. All the children underwent head orthosis therapy to correct their positional head asymmetry. Patients with craniosynostosis, microcephaly, severe comorbidities, or congenital anomalies were excluded.
The cohort was divided depending on gestational age:
-
Premature group (44 patients): infants with a gestational age of < 37 weeks
-
Full-term group (195 patients): infants with a gestational age of ≥ 37 weeks
Methods
The research was conceived as a prospective longitudinal study, was approved by the local ethics committee, and was carried out in compliance with the Declaration of Helsinki. All the parents or legal guardians gave their oral and written consent to participating in the investigation.
Course of therapy
All the patients had already undergone active repositioning therapy in combination with either physiotherapy or osteopathy without achieving a satisfactory head shape. After premature craniosynostosis had been ruled out clinically and by sonography, we performed a 3D-stereophotogrammetric image (T1) using the 3dMDhead System® (Atlanta, Georgia). An individual molding head orthosis (“helmet”) was then manufactured (Cranioform®, Alpnach, Switzerland), and therapy was commenced. The parents were instructed that their infant must wear the helmet for about 23 h per day. At regular consultations every 4–5 weeks, the helmet was adjusted according to changes in the head shape. At these appointments, further 3D-stereophotogrammetric images were performed to verify a proper course of treatment and to reevaluate sufficient growth for further head orthosis therapy. In consultations between doctors and parents, treatment ended after a satisfactory head shape was achieved. A final 3D-stereophotogrammetric image (T2) was then performed.
Three-dimensional analysis
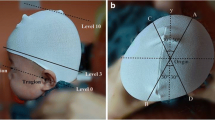
A single experienced examiner conducted all the analyses using professional 3D-software (Cranioform® Analytics 4.0). The 3D-datasets were aligned three dimensionally in a coordinate system using four reference points (nasion point “N,” subnasal point “Sn,” and the tragus points “Tr” on both sides), as depicted in Fig. 1. This method guarantees repeatable and reliable alignment of the datasets, thereby enabling longitudinal analyses to be performed.
For some assessments, another plane (measurement plane) was defined by shifting the XY-plane up to the level of the maximum anterior–posterior length of the infant’s head (Fig. 2). Three symmetry-related variables were used to assess the asymmetry of the skulls (Fig. 3a, b):
-
1.
30°-CVA (Cranial Vault Asymmetry) in mm:
Measurement of the two diagonals at a 30° angle to the Y-axis at the level of the measurement plane. The difference between these diagonals is the 30°-CVA.
-
2.
30°-CVAI (Cranial Vault Asymmetry Index) in %:
30°-CVA in relation to the length of the shorter 30°-diagonal.
-
3.
PCAI (Posterior Cranial Asymmetry Index) in %:
The cranial volume above the XY-plane is divided into four partial volumes by the XZ-plane and the YZ-plane. The difference between the two posterior volumes was divided by the smaller posterior volume and multiplied by 100.
Fifty randomly selected 3D-datasets were measured twice by the same examiner to determine the intra-rater reliability.
Statistics
All the collected data were analyzed using the software SPSS Statistics Version 25.0 for Windows (IBM, Ehningen, Germany).
Intra-rater reliability was verified by calculating the Pearson product-moment correlation for all the variables of the 50 3D-datasets that had been analyzed twice.
Normal distribution of the data was considered due to the size of our cohort, and we therefore used parametric analyses for all the statistics.
T-tests for independent samples were calculated to assess whether there were significant group differences between the premature and the full-term groups at T1, as well as for group-specific differences in changes of the symmetry-related parameters (ΔT1-T2). We used t-tests for dependent samples to compare the symmetry-related variables for each group at T1 and T2.
In a second step, we analyzed whether gestational age was a predictor of the extent of the reduction in asymmetry or the duration of therapy. As we know from previous investigations that both age at the start of therapy and initial asymmetry are independent predictors of the extent of the reduction in asymmetry [2, 24], we considered these two parameters in different multiple linear regression analyses. For both the initial asymmetry and the extent of the reduction in asymmetry, we investigated 2D- (30°-CVA at T1 / Δ 30°-CVA) and 3D-variables (PCAI at T1 / Δ PCAI). The characteristics of these multiple linear regression analyses are depicted in Table 1. The coefficient of determination (R2), the p value, and the regression coefficient (B) for all the multiple regression analyses are presented in the results.
The level of significance was set at 5% for all the analyses.
Results
General findings
The intra-rater reliability of all the symmetry-related variables was very high (all the correlation coefficients were > 0.900, with all the p values ≤ 0.05).
The cohort of 239 patients with DP (33% female / 67% male) was divided into two groups in relation to gestational age: 44 patients (= 18% of the total cohort; 30% female/70% male) with a gestational age of M = 33.1 weeks (SD = 3.2; range: 25.0–36.0) were placed in the premature group, and 195 infants (= 82% of the total cohort; 33% female / 67% male) with a gestational age of M = 39.0 weeks (SD = 1.3; range: 37.0–42.0) were assigned to the full-term group. This difference in gestational age was statistically significant, with p < 0.001.
Findings at the start of therapy
The average age of all the 239 patients with DP at the start of therapy was M = 27.9 weeks (SD = 6.6; range: 14.1–50.1). The infants in the premature group were significantly older (p < 0.001) at the start of treatment than those in the full-term group: in the premature group, therapy started at M = 32.3 weeks (SD = 7.1; range: 22.8–50.1), and at M = 26.9 weeks (SD = 6.1; range: 14.1–49.0) for the full-term group.
There were no significant differences between the groups with respect to the symmetry-related variables at the start of therapy (Table 2).
Findings during the course of therapy
There was a significant reduction in asymmetry for all the parameters in the premature and full-term groups when comparing the symmetry-related variables at T1 and T2 (all p < 0.001). The extend of the reduction was not statistically different between the two groups (Table 3). The average treatment time for the total cohort was M = 20.2 weeks (SD = 6.0; range: 8.3–42.0). There was no statistically significant difference with respect to this parameter (p = 0.945) between the premature (M = 20.3 weeks; SD = 6.3; range: 8.4–42.0) and the full-term group (M = 20.2 weeks; SD = 6.0; range: 8.3–40.1).
Multiple linear regression analyses
Extent of the reduction in asymmetry
The results of the multiple linear regression analyses with respect to the extent of the reduction in asymmetry (models 1a and 1b) are depicted in Tables 4 and 5.
There were significant relationships with the predictors age at T1 and the initial asymmetry (30°-CVA at T1, PCAI at T1) in both the multiple linear regression analyses with the extent of the reduction in asymmetry as the criterion (Δ 30°-CVA, respectively Δ PCAI). The younger the patients at the start of therapy, and the more severe the initial asymmetry, the greater the extent of the reduction in asymmetry during treatment. However, there were no significant relationships between gestational age and the extent of the asymmetry reduction during therapy in the regression analyses. We were unable to improve the explanation of the variance in the dependent variable (R2) in these multiple linear regression analyses by adding gestational age as an additional predictor.
Duration of therapy
The results of the multiple linear regression analyses concerning the duration of therapy (models 2a and 2b) are depicted in Tables 6 and 7.
There were significant relationships with all three predictors, age at T1, initial asymmetry (30°-CVA at T1, respective PCAI at T1) and gestational age, for these multiple linear regression analyses. The duration of therapy is lengthened with increasing age at the start of therapy, greater initial asymmetry, and a higher gestational age.
By adding the gestational age as an additional predictor, the explanation of the variance of the dependent variable (R2) was improved from 17.4% to 20.5% in model 2a and from 18.3% to 21.9% in model 2b.
Discussion
Head orthosis therapy is an effective treatment for patients with DP. In recent years, several studies have demonstrated that especially the patient’s age at the start of therapy and the initial asymmetry have a significant impact on how successful this treatment option is [2, 24]. However, the influence of premature birth on the effectiveness and duration of this therapy has not been studied, even though prematurity is one of the main risk factors for developing DP [14,15,16,17,18,19,20]. In the present study, we analyzed the impact of prematurity on the extent of the reduction in asymmetry and the duration of head orthosis therapy in affected infants with respect to age at the start of therapy and initial asymmetry.
A total of 239 infants, subdivided into a premature and a full-term group who successfully underwent head orthosis therapy, were investigated longitudinally. Of this cohort, 18% were born prematurely, which was double the incidence than in the general population in Germany [30]. This significantly higher number reflects prematurity as a risk factor for the development of DP. About two thirds of our cohort were male and only one third female, confirming the findings of previous studies, which highlight that there is double the incidence of this condition in the male sex [3, 8,9,10,11, 14]. The premature and the full-term groups were comparable in relation to their gender distribution.
All the cranial measurements were performed using 3D-stereophotogrammic images, enabling a high level of accuracy and reproducibility to be achieved [24]. As previously described, there was very high intra-rater reliability for all the examined variables [24, 25, 31]. Cranial asymmetry was assessed using three symmetry-related variables: 30°-CVA and 30°-CVAI as 2D-variables and PCAI as a 3D-variable. The advantage of this volumetric variable is that the 3D-asymmetry is no longer reduced to 2D-measurements [24].
At the start of treatment, there were no significant differences in all the symmetry-related variables in the two groups. During head orthosis therapy, there was also a similar extent of reduction in asymmetry for these variables in both groups. However, there was a significant difference concerning age at the start of therapy: the premature group was significantly older. This may be explained by the fact that premature infants frequently suffer from concomitant conditions such as respiratory distress syndrome [32] or renal diseases [33,34,35,36,37], meaning that the parents of these children may not initially prioritize the correction of positional asymmetry. As a result, only comparing the premature and the full-term groups using t-tests was statistically inadequate, as this method does not consider the different ages at the start of therapy, which is known to have a direct impact on the effectiveness of therapy. We therefore performed multiple linear regression analyses. The advantage of this method is the opportunity it provides to evaluate the impact of different predictors on a criterion at the same time.
For the regression analyses, we selected the two well-known predictors - age at the start of therapy and initial asymmetry [2, 24] - and enhanced them by the gestational age. The predictors’ influence on the two different criterions – (1) the extent of the reduction in asymmetry and (2) the duration of therapy – was then calculated. We used both 2D- (30°-CVA and Δ 30°-CVA) and 3D-variables (PCAI and Δ PCAI) for all the symmetry-related parameters (initial asymmetry and extent of the reduction in asymmetry), which produced four different multiple linear regression analyses (Table 1). Our results demonstrate that prematurity does not influence the extent of the reduction in asymmetry brought about by head orthosis therapy. In the two multiple regression analyses that had the extent of the reduction in asymmetry as the criterion (models 1a and 1b), there was a significant correlation with age at the start of treatment and initial asymmetry, but this was not the case for gestational age. In contrast, in the two multiple linear regression analyses that had the duration of therapy as the criterion, all predictors demonstrated a significant correlation (models 2a and 2b). The duration of therapy was shorter in infants who were born at an earlier gestational age. This confirms the assumption that premature infants have a higher growth potential within the first months of life, although direct causality is not possible to determine by linear regression analysis. There was no difference for the 2D- and 3D-variables for quantifying the asymmetry, but the explanation of the variance of all the predictors for this criterion was better when 3D-variables were used.
Conclusion
This study analyzed 239 patients with DP to determine the impact of a premature birth on the duration of head orthosis therapy and on the extent of the reduction in asymmetry during treatment. Age at the start of therapy, the initial asymmetry of an infant’s head and prematurity were all significantly correlated with the length of therapy, with the premature infants requiring treatment for a shorter period. The extent of the reduction in asymmetry was, in contrast, unaffected by prematurity. Knowledge of these findings is important for clinicians when planning treatment and discussing the effectiveness of head orthosis therapy with the parents of premature infants with DP.
References
Cunningham ML, Heike CL (2007) Evaluation of the infant with an abnormal skull shape. Curr Opin Pediatr 19(6):645–651. https://doi.org/10.1097/MOP.0b013e3282f1581a
Freudlsperger C, Steinmacher S, Saure D, Bodem JP, Kuhle R, Hoffmann J, Engel M (2016) Impact of severity and therapy onset on helmet therapy in positional plagiocephaly. J Craniomaxillofac Surg 44(2):110–115. https://doi.org/10.1016/j.jcms.2015.11.016
Mawji A, Vollman AR, Fung T, Hatfield J, McNeil DA, Sauve R (2014) Risk factors for positional plagiocephaly and appropriate time frames for prevention messaging. Paediatr Child Health 19(8):423–427. https://doi.org/10.1093/pch/19.8.423
Smartt JM Jr, Elliott RM, Reid RR, Bartlett SP (2011) Analysis of differences in the cranial base and facial skeleton of patients with lambdoid synostosis and deformational plagiocephaly. Plast Reconstr Surg 127(1):303–312. https://doi.org/10.1097/PRS.0b013e3181f95cd8
Lo LJ, Marsh JL, Pilgram TK, Vannier MW (1996) Plagiocephaly: differential diagnosis based on endocranial morphology. Plast Reconstr Surg 97(2):282–291
Netherway DJ, Abbott AH, Gulamhuseinwala N, McGlaughlin KL, Anderson PJ, Townsend GC, David DJ (2006) Three-dimensional computed tomography cephalometry of plagiocephaly: asymmetry and shape analysis. Cleft Palate Craniofac J 43(2):201–210. https://doi.org/10.1597/04-174.1
Argenta LC, David LR, Wilson JA, Bell WO (1996) An increase in infant cranial deformity with supine sleeping position. J Craniofac Surg 7(1):5–11
De Bock F, Braun V, Renz-Polster H (2017) Deformational plagiocephaly in normal infants: a systematic review of causes and hypotheses. Arch Dis Child 102(6):535–542. https://doi.org/10.1136/archdischild-2016-312018
Joganic JL, Lynch JM, Littlefield TR, Verrelli BC (2009) Risk factors associated with deformational plagiocephaly. Pediatrics 124(6):e1126–e1133. https://doi.org/10.1542/peds.2008-2969
Peitsch WK, Keefer CH, LaBrie RA, Mulliken JB (2002) Incidence of cranial asymmetry in healthy newborns. Pediatrics 110(6):e72. https://doi.org/10.1542/peds.110.6.e72
Bialocerkowski AE, Vladusic SL, Wei Ng C (2008) Prevalence, risk factors, and natural history of positional plagiocephaly: a systematic review. Dev Med Child Neurol 50(8):577–586. https://doi.org/10.1111/j.1469-8749.2008.03029.x
Losee JE, Mason AC, Dudas J, Hua LB, Mooney MP (2007) Nonsynostotic occipital plagiocephaly: factors impacting onset, treatment, and outcomes. Plast Reconstr Surg 119(6):1866–1873. https://doi.org/10.1097/01.prs.0000259190.56177.ca
Shweikeh F, Nuno M, Danielpour M, Krieger MD, Drazin D (2013) Positional plagiocephaly: an analysis of the literature on the effectiveness of current guidelines. Neurosurg Focus 35(4):E1. https://doi.org/10.3171/2013.8.FOCUS13261
Linz C, Kunz F, Bohm H, Schweitzer T (2017) Positional skull deformities. Dtsch Arztebl Int 114(31-32):535–542. https://doi.org/10.3238/arztebl.2017.0535
Ifflaender S, Rudiger M, Konstantelos D, Wahls K, Burkhardt W (2013) Prevalence of head deformities in preterm infants at term equivalent age. Early Hum Dev 89(12):1041–1047. https://doi.org/10.1016/j.earlhumdev.2013.08.011
Baum JD, Searls D (1971) Head shape and size of pre-term low-birthweight infants. Dev Med Child Neurol 13(5):576–581
Elliman AM, Bryan EM, Elliman AD, Starte D (1986) Narrow heads of preterm infants--do they matter? Dev Med Child Neurol 28(6):745–748
Littlefield TR, Kelly KM, Pomatto JK, Beals SP (1999) Multiple-birth infants at higher risk for development of deformational plagiocephaly. Pediatrics 103(3):565–569. https://doi.org/10.1542/peds.103.3.565
McPherson GK, Kriewall TJ (1980) The elastic modulus of fetal cranial bone: a first step towards an understanding of the biomechanics of fetal head molding. J Biomech 13(1):9–16
Kriewall TJ, McPherson GK, Tsai AC (1981) Bending properties and ash content of fetal cranial bone. J Biomech 14(2):73–79
Ifflaender S, Rudiger M, Konstantelos D, Lange U, Burkhardt W (2014) Individual course of cranial symmetry and proportion in preterm infants up to 6 months of corrected age. Early Hum Dev 90(9):511–515. https://doi.org/10.1016/j.earlhumdev.2014.03.008
Steinberg JP, Rawlani R, Humphries LS, Rawlani V, Vicari FA (2015) Effectiveness of conservative therapy and helmet therapy for positional cranial deformation. Plast Reconstr Surg 135(3):833–842. https://doi.org/10.1097/PRS.0000000000000955
Pogliani L, Mameli C, Fabiano V, Zuccotti GV (2011) Positional plagiocephaly: what the pediatrician needs to know. A review. Childs Nerv Syst 27(11):1867–1876. https://doi.org/10.1007/s00381-011-1493-y
Kunz F, Schweitzer T, Kunz J, Wassmuth N, Stellzig-Eisenhauer A, Bohm H, Meyer-Marcotty P, Linz C (2017) Head orthosis therapy in positional plagiocephaly: influence of age and severity of asymmetry on effect and duration of therapy. Plast Reconstr Surg 140(2):349–358. https://doi.org/10.1097/PRS.0000000000003517
Meyer-Marcotty P, Kunz F, Schweitzer T, Wachter B, Bohm H, Wassmuth N, Linz C (2018) Cranial growth in infants - A longitudinal three-dimensional analysis of the first months of life. J Craniomaxillofac Surg 46(6):987–993. https://doi.org/10.1016/j.jcms.2018.04.009
Seruya M, Oh AK, Taylor JH, Sauerhammer TM, Rogers GF (2013) Helmet treatment of deformational plagiocephaly: the relationship between age at initiation and rate of correction. Plast Reconstr Surg 131(1):55e–61e. https://doi.org/10.1097/PRS.0b013e3182729f11
Kluba S, Kraut W, Reinert S, Krimmel M (2011) What is the optimal time to start helmet therapy in positional plagiocephaly? Plast Reconstr Surg 128(2):492–498. https://doi.org/10.1097/PRS.0b013e31821b62d6
Moss SD (1997) Nonsurgical, nonorthotic treatment of occipital plagiocephaly: what is the natural history of the misshapen neonatal head? J Neurosurg 87(5):667–670. https://doi.org/10.3171/jns.1997.87.5.0667
Mortenson PA, Steinbok P (2006) Quantifying positional plagiocephaly: reliability and validity of anthropometric measurements. J Craniofac Surg 17(3):413–419
Schleussner E (2013) The prevention, diagnosis and treatment of premature labor. Dtsch Arztebl Int 110(13):227–235; quiz 236. https://doi.org/10.3238/arztebl.2013.0227
Kunz F, Schweitzer T, Grosse S, Wassmuth N, Stellzig-Eisenhauer A, Bohm H, Meyer-Marcotty P, Linz C (2019) Head orthosis therapy in positional plagiocephaly: longitudinal 3D-investigation of long-term outcomes, compared with untreated infants and with a control group. Eur J Orthod 41(1):29–37. https://doi.org/10.1093/ejo/cjy012
Banerjee S, Fernandez R, Fox GF, Goss KCW, Mactier H, Reynolds P, Sweet DG, Roehr CC (2019) Surfactant replacement therapy for respiratory distress syndrome in preterm infants: United Kingdom national consensus. Pediatr Res 86(1):12–14. https://doi.org/10.1038/s41390-019-0344-5
South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, Shaltout HA, O'Shea TM, Washburn LK (2019) Renal function and blood pressure are altered in adolescents born preterm. Pediatr Nephrol 34(1):137–144. https://doi.org/10.1007/s00467-018-4050-z
Kaiser JR, Tilford JM, Simpson PM, Salhab WA, Rosenfeld CR (2004) Hospital survival of very-low-birth-weight neonates from 1977 to 2000. J Perinatol 24(6):343–350. https://doi.org/10.1038/sj.jp.7211113
Keijzer-Veen MG, Kleinveld HA, Lequin MH, Dekker FW, Nauta J, de Rijke YB, van der Heijden BJ (2007) Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis 50(4):542–551. https://doi.org/10.1053/j.ajkd.2007.06.015
White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR (2009) Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54(2):248–261. https://doi.org/10.1053/j.ajkd.2008.12.042
de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB (2012) Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59(2):226–234. https://doi.org/10.1161/HYPERTENSIONAHA.111.181784
Funding
No funding was received for this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (The study was carried out in compliance with the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital of Würzburg).
Informed consent
All the parents or legal guardians gave their oral and written consent to participating in the investigation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunz, F., Schweitzer, T., Dörr, A. et al. Craniofacial growth in infants with deformational plagiocephaly: does prematurity affect the duration of head orthosis therapy and the extent of the reduction in asymmetry during treatment?. Clin Oral Invest 24, 2991–2999 (2020). https://doi.org/10.1007/s00784-019-03159-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03159-6