Abstract
Objective
To investigate the effects of probiotics, Lactobacillus paracasei SD1, on the quantities of Streptococcus mutans in saliva and plaque samples of preschool children.
Design
This randomized trial recruited 487 preschool children from eight childcare centers. Participants were assigned to receive a 6-month course of placebo milk daily (group I), probiotic milk either daily (group II) or three days a week (triweekly, group III). The absolute quantities of S. mutans and total lactobacilli in the saliva and plaque samples at baseline (T0), after intervention (T6), and 6 months after discontinuation (T12) were assessed by qPCR.
Results
Of 487 children, 354 completed all follow-up periods. However, only 268 children (3.2 ± 0.8 years old; groups I = 86, II = 89, and III = 93) provided adequate saliva for qPCR. Whereas the quantities of S. mutans were significantly decreased in groups II and III compared to group I in the saliva and plaque samples at T6 and T12, those of total lactobacilli were significantly increased (p < 0.0167). There was no difference in the quantities of S. mutans or total lactobacilli between groups II and III at any period. Significant changes in the quantities of S. mutans and total lactobacilli lasted until T12 compared to T0 (p < 0.0167).
Conclusions
Probiotic administration daily or triweekly reduces S. mutans quantities, whereas it increases total lactobacilli quantities that persists at least 6 months after discontinuation in the saliva and plaque samples of preschool children.
Clinical relevance
Daily or triweekly consumption of L. paracasei SD1 supplemented in milk may help prevent dental caries in preschool children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics were originally used in medicine and have been a topic of extensive research in the fields of cariology, periodontology, mucositis, and halitosis in the past two decades [1]. Dental caries, a common preventable and multifactorial oral disease affecting all age groups worldwide [2], is caused by a shift of microbial compositions from physiologic to pathogenic bacteria. Streptococcus mutans plays an important role as an early colonizer in the dental biofilm, in which it produces acids and resists an acidic condition, resulting in demineralization of the tooth structure [3]. Dental caries is considered a non-communicable disease similar to hypertension, diabetes mellitus, and cardiovascular diseases; modifications of human lifestyles would, therefore, help prevent these diseases [1]. Early childhood caries adversely affects the general health of children and influences socioeconomic burdens of countries [4]. Consequently, caries prevention as early as the first 1,000 days of life by introducing probiotics in routine diets would be beneficial to modulate the ecology of oral microorganisms in the biofilm, leading to a reduction of cariogenic bacteria [1].
For caries prevention, Lactobacilli are commonly used as probiotic strains [5]. Previous studies have shown that L. paracasei SD1, isolated from caries-free Thai preschool children [6], exerts various effects in the oral cavity, including inhibition of mutans streptococci, production of less acids than other lactobacilli, bacterial adherence to oral epithelial cells [7], synthesis of bacteriocin [8], production of antioxidants [9], and immunomodulation by enhancing salivary human neutrophil peptide 1-3 levels [10]. Five previous clinical trials, using L. paracasei SD1 as probiotic supplementation in Thai adults, adolescents, children, and patients with clefts, have demonstrated bacterial changes in saliva and a favorable microbial symbiosis [10,11,12,13,14]. Whereas mutans streptococci were significantly decreased, the probiotic strain was significantly increased and persisted after discontinuation in conjunction with caries reduction. However, these five trials were conducted in a small cohort within southern Thailand and all participants obtained probiotics daily during the trial period. Accordingly, conducting a multicenter randomized controlled trial in a larger population of young children for an early exposure is necessary to verify the beneficial effects of L. paracasei SD1. Although it is generally recommended that probiotics be consumed daily for continuing benefits [1], daily consumption may not be practical for everyone. Moreover, concerns are raised whether children who miss some doses of probiotics would still receive benefits. This study designed with different dose intervals and conducted in different regions should lead to a more practical implementation of public health policy.
It was hypothesized that (1) the effect of early exposure to probiotics on the reduction of cariogenic bacteria in the saliva and the plaque is sustained after discontinuation, and (2) the frequency of probiotic administration either daily or 3 days in a week (triweekly) has similar impacts on the control of cariogenic microorganisms. The aim of this study was, thus, to determine the quantities of S. mutans, total lactobacilli, and total bacteria in saliva and plaque samples of healthy preschool children, who consumed milk supplemented with probiotics once daily or triweekly or without probiotics as a placebo, at baseline (T0), immediately after 6 months of probiotic intervention (T6), and 6 months after probiotic discontinuation (T12).
Material and methods
Participants
Eligible participants included healthy preschool children (aged 1-5 years) who had normal height and weight for their ages within 1.5 standard deviation of the Thai children growth chart and with a minimum weight of 10 kg. Exclusion criteria were 1) medically compromised children, who were at risk of episodic infections or prone to frequent uses of antibiotics, 2) allergy to cow milk or lactose intolerance, 3) receiving antibiotics within two weeks prior to the study in order to allow reestablishment of normal oral microflora [15], 4) having more than four cavitated teeth [12], and 5) refusal to participate by parents or legal guardians. Written informed consent was obtained before enrolling each child into the trial. This multicenter, stratified-randomized, double-blind, and placebo-controlled trial with three randomization arms (1:1:1), lasting for one year, was registered with the Thai Clinical Trial Registry (TCTR20170511002; http://www.clinicaltrials.in.th), being part of the WHO’s Registry Network, Clinical Trials identifier (registration on May 10, 2017, first enrollment on May 22, 2017).
Probiotic and placebo milk powder
The probiotic milk powder was prepared by a spray drying technique, containing viable 1.8x107 CFU/ml of L. paracasei SD1 in 20% skimmed milk and < 4% humidity according to Teanpaisan et al., 2015 [11]. The placebo was the exact same milk powder devoid of L. paracasei SD1. Both probiotic and placebo milk were identical in appearance, scent, weight, and packaging. A single dose of 3 g of the milk powder was packaged for daily intake and sealed in plastic bags labeled specifically for each participant. The packages were stored at 4 °C until delivery to the childcare center monthly. The viability of L. paracasei SD1 in milk powder has been shown to last 6 months when being stored at 4 °C [16]. The powder was dissolved in 50 ml of child’s milk at room temperature by caregivers or parents, who monitored the child intake on weekdays or weekends/holidays, respectively, and recorded the days that each child had consumed milk in the child’s logbook. In addition, the caregivers recorded occurrence of any specific clinical symptoms, including fever, respiratory infection, allergy, diarrhea, constipation, school absence due to illnesses, and health care utilization in the logbook. The participants were allowed to withdraw from the study if they were suspected to be allergic to the milk or experienced any abnormal symptoms, including fever, nausea, vomiting, abdominal pain, or diarrhea. After each month, both the empty and full plastic bags of each child were collected to confirm the percentage of compliance, which was calculated by the number of days that participants had consumed milk divided by the total number of days for 6 months (Table 1).
Randomization, allocation, concealment, and blinding
After enrollment, participants were randomly assigned to one of the three study arms (placebo, daily probiotics, and triweekly probiotics), by stratified randomization using block of six in each childcare center. Children who consumed the placebo milk powder were classified in the placebo (group I), while those who consumed the probiotic milk powder once daily for 6 months were grouped in the daily probiotics (group II). The triweekly probiotics (group III) included children who consumed the probiotic milk powder in any 3 days of a week and the placebo milk powder for the remaining 4 days. Participants, caregivers, parents, clinical examiners, investigators, laboratory staff, and research assistants who entered data to a database were blinded to the assigned groups until the codes were opened after statistical analysis.
Saliva collection
Saliva samples were collected from each child during the same time, i.e., mid-morning, at T0, T6, and T12. The unstimulated whole saliva was collected as much as possible by spitting into a small cup, while children who could not spit or were uncooperative, sterile 1.0-ml droppers were used to collect their saliva. The saliva samples were transferred to 1.5-ml sterile microcentrifuge tubes and stored on ice for a few hours while being transported to the research laboratories. The saliva samples were immediately stored at − 80 °C until being used for DNA extraction.
Oral examination and plaque sample collection
Oral examination by trained and calibrated pediatric dentists, with inter- and intra-examiner reliability greater than 0.8, was performed using light sources, mouth mirrors, and ball-ended periodontal probes (probe WHO-621). The visible dental biofilm index was recorded at T0, T6, and T12 using the overall index modified by Santos and Soviero (2006) [17, 18] as follows: 1 = absence of visible biofilm; 2 = thin biofilm, easily removed; 3 = thick biofilm, firmly adhered to either anterior or posterior teeth; 4 = thick biofilm, firmly adhered to both anterior and posterior teeth. Afterwards, the pooled plaque samples were collected using sterile toothpicks to scrape plaque from the mesial, distal, buccal, and lingual surfaces of all erupted teeth, placed into pre-weighed 1.5-ml sterile microcentrifuge tubes, and stored dry for a few hours while being transported to the laboratories. Each plaque sample was weighed, mixed with 200 μl of Tris-EDTA buffer, and stored at − 80 °C until being used for DNA extraction.
Dental caries status was recorded at T0, T6, and T12 according to the modified Nyvad criteria for caries assessment [19,20,21]; code 0, sound surface; code 1, active non-cavitated lesion; code 2, active cavitated lesion; code 3, inactive non-cavitated lesions; code 4, inactive cavitated lesions; code 5, filling; code 6, filling associated with active caries; code 7 missing due to caries. The overall status for each tooth was determined by the greatest severity among all tooth surfaces of each tooth and presented as follows; caries free, non-cavitated, and cavitated. The number of unerupted or partially erupted teeth was also recorded.
DNA extraction
DNA isolation from the saliva and plaque samples in the three intervention groups at T0, T6, and T12 was performed in only the samples whose saliva volume was at least 500 μl. A 500-μl and a 200-μl volume of saliva and reconstituted plaque samples, respectively, were thawed, vortexed, and centrifuged at 10,000 rpm for 5 min. The resultant pellet was resuspended in 200 μl of 20 mg/ml freshly prepared lysozyme (Amresco Inc., Solon, OH, USA) and incubated at 37 °C for 30 min to lyze the bacterial cell wall. Bacterial DNA was then extracted, purified, and eluted in 40 μl of Tris-EDTA buffer, using the PureDirex Genomic DNA Isolation kit (Bio-Helix Co., LTD., Keelung City, Taiwan) according to the manufacturer’s instructions.
Absolute quantification of bacterial DNA
To establish a standard curve for each microorganism, S. mutans ATCC25175 for S. mutans, Escherichia coli ATCC25922 for total bacteria and S. sobrinus ATCC33478 as a negative control, were cultured on 5% blood agar (DifcoTM, Sparks, MD, USA), while L. fermentum ATCC14931 for total lactobacilli was cultured on de Man Rogosa and Sharpe (MRS) agar (DifcoTM) anaerobically at 37 °C for 24–48 h. A single colony of E. coli ATCC25922, S. mutans ATCC25175, or S. sobrinus ATCC33478 was inoculated in Brain Heart Infusion broth (DifcoTM), and a single colony of L. fermentum ATCC14931 was inoculated in MRS broth (DifcoTM), overnight at 37 °C anaerobically. The bacterial pellets were collected after centrifugation at 5,000 rpm for 10 min, washed twice with phosphate-buffered saline (PBS), pH 7.0, and adjusted to an optical density of 2.0 at 600 nm using a UV/VIS spectrophotometer (Biochrom Ltd., Cambridge, UK). The bacterial cell suspensions were twofold diluted until 1:4096 and divided into two aliquots. Whereas the first aliquot was streaked on agar plates for bacterial count as CFU/ml using the aforementioned cultivation method, the second was used for DNA isolation as aforementioned to determine the cycle threshold (Ct) of qPCR using the CFX96 TouchTM Real-Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A linear standard curve was plotted for each bacterial species from log10 CFU/ml against the corresponding Ct and showed a high correlation coefficient (R2 > 0.99).
A 20-μl aliquot of qPCR master mix, comprising 5 μl of purified DNA template from saliva or plaque sample, 2 μl of a specific primer pair, 3 μl of nuclease-free water, and 10 μl of 2x SensiFAST SYBR® No-ROX mix (Bioline Reagent Ltd., Foster City, CA, USA), was amplified using the CFX96 TouchTM Real-Time PCR detection system (Bio-Rad Laboratories, Inc). The primer sequences for detecting each bacterial species are presented in Table 2 [22,23,24]. The qPCR conditions for both unknown samples and bacterial genomic DNA were as follows; 1 cycle at 95 °C for 10 min and 1 cycle at 50 °C for 2 min, followed by 40 cycles at 95 °C for 20 s; at 60 °C, 58 °C, or 60 °C for S. mutans, total lactobacilli, or total bacteria, respectively, for 20 s; and at 72 °C for 25 s. The melting curve analysis for targeted bacterial species demonstrated a sharp peak at the expected melting temperature, indicating that qPCR products were homogeneous without primer-dimers, and the chosen primers specifically amplified the target DNA. Additionally, DNA isolated from S. sobrinus ATCC33478 was used as a negative control for qPCR analysis of S. mutans and total lactobacilli, and the findings showed no amplified products (data not presented). The absolute quantities of S. mutans, total lactobacilli, and total bacteria in the saliva and plaque samples were extrapolated by comparing the Ct value of each unknown sample with the standard curve for S. mutans ATCC25175, L. fermentum ATCC14931, and Escherichia coli ATCC25922, respectively. The quantities of bacteria in the saliva samples were reported as log10 CFU/ml, whereas those in the plaque samples were normalized by the plaque weight and expressed as log10 CFU/mg. All qPCR reactions for saliva and plaque samples were performed in duplicate, and their averages were used in statistical analysis.
Statistical analysis
Descriptive analysis was used to describe mean age, sex, the number of non- or partially erupted teeth, caries status, including caries-free, non-cavitated and cavitated teeth, and visible plaque index at baseline. ANOVA was used to compare continuous variables and the Chi-square or Fisher exact test was used to compare categorical variables among the three groups. Statistical significance was accepted at p < 0.05. To compare bacterial quantities, non-parametric statistical tests were used due to the non-normal distribution of the data. For intergroup comparisons, the Kruskal-Wallis H test was used to determine the differences in bacterial quantities at each period, followed by multiple comparisons using the Mann-Whitney U test with Bonferroni adjustment. For intragroup comparisons, the Friedman test was used to determine the differences in bacterial quantities among the three periods (T0, T6, and T12), followed by multiple comparisons using the Wilcoxon signed rank test with Bonferroni adjustment. Statistical significance for both intergroup and intragroup comparisons was accepted at p < 0.0167. The Pearson’s correlation was used to evaluate the correlations of bacterial quantities between the saliva and plaque samples. All analyses were performed using SPSS® software version 17.0 for Windows (IBM, Armonk, NY, USA).
Results
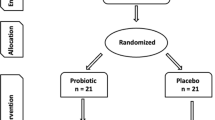
Of 1050 children assessed for eligibility, 487 children from eight childcare centers of two provinces, Satun (n = 250) and Chiang Mai (n = 237) in the southern and northern regions of Thailand, respectively, were registered in this trial (Fig. 1). Of 487 registered participants, 354 (72.69%) completed all follow-up periods (T6 and T12; Fig. 1). The reasons for dropout included failure to comply with the research protocol, withdrawal due to allergy, and relocation, as summarized in Fig. 1. However, only 268 of 354 children (75.70%), comprising 86 in group I, 89 in group II, and 93 in group III, provided adequate saliva volume (at least 500 μl) at T0, T6, and T12 for qPCR analysis (Fig. 1). The baseline variables for participants, including mean age, sex, the number of unerupted or partially erupted teeth, that of carious teeth, the visible plaque index, or the average percentage of compliance, were not found to be different among the three groups (Table 1).
Reduction of S. mutans by the probiotic strain
At T6 and T12, there were significant decreases in the median quantities of S. mutans, expressed as log10 CFU/ml and log10 CFU/mg for saliva and plaque samples, respectively, in the children receiving daily probiotics (group II) and the children receiving triweekly probiotics (group III) compared to the children receiving the placebo (group I) (p < 0.0033; Fig. 2a, b). As anticipated, the median quantities of total lactobacilli were significantly increased in both the saliva and plaque samples in groups II and III compared to those in group I (p < 0.0167; Fig. 2c, d, respectively). In contrast to significant changes in the median quantities of S. mutans or total lactobacilli by probiotic administration, those of total bacteria in the saliva or plaque samples were not found to be different among the three groups at any periods (Fig. 2e or f, respectively), indicating true reduction in the quantities of S. mutans by probiotic consumption. Moreover, the median quantities of S. mutans or of total lactobacilli in the saliva or plaque samples were not found to be different between the two probiotic groups at T0, T6, or T12 (Fig. 2a–d). Note that the median quantity of S. mutans in the saliva samples at T0 was significantly lower in group I than in groups II and III (p < 0.00033; Fig. 2a), whereas that of total lactobacilli in the saliva samples at T0 was significantly greater in group I than in groups II and III (p < 0.00033; Fig. 2c).
Box plot graphs presenting median quantities along with interquartile ranges (25th to 75th percentiles) of aStreptococcus mutans in saliva, bStreptococcus mutans in plaque, c total lactobacilli in saliva, d total lactobacilli in plaque, e total bacteria in saliva and f total bacteria in plaque. The measurement units for saliva and plaque samples are log10 CFU/ml and log10 CFU/mg, respectively. Empty, gray, and hatched boxes represent the placebo, daily probiotic, and triweekly probiotic groups, respectively. The horizontal line in each box represents the median of the data, and X within each box represents the mean. Empty circles are outliers. T0, T6, and T12 are the time points at baseline, after 6 months of probiotic intervention, and 6 months after probiotic discontinuation, respectively. *p < 0.0167, **p < 0.0033, ***p < 0.00033
Sustained decreases in S. mutans and increases in total lactobacilli quantities after probiotic discontinuation
At T6 and T12, similar trends of changes in the median quantities of S. mutans and total lactobacilli in both the saliva and plaque samples were found both in the children receiving daily (group II) and triweekly probiotics (group III; Fig. 3a, b, respectively). Particularly for the median quantities of total lactobacilli in both the saliva and plaque samples from groups II and III, there were significant increases at T6 and T12 compared to T0 (p < 0.0033). On the contrary, there were significant decreases in the median quantities of S. mutans found only in the saliva samples from groups II and III at T6 and T12 compared to T0 (p < 0.0033; Fig. 3a), whereas a significant decrease was found in the plaque samples of group II at T6 (p < 0.0033; Fig. 3b). Taken together, these findings suggest sustained effects on microbial shifts after probiotic discontinuation for at least 6 months and saliva samples as a better surrogate for microbial quantification than plaque samples. Without probiotic consumption in the placebo group, there were significant increases in the median quantities of S. mutans, and significant decreases in those of total lactobacilli were found in both the saliva and plaque samples at T6 and T12 compared to T0 (p < 0.0167; Fig. 3a, b, respectively). With respect to the median quantities of total bacteria, significant increases were found at T6 and T12 compared to T0 in the saliva samples of all three groups (p < 0.0167; Fig. 3a), but not in the plaque samples (Fig. 3b), confirming saliva samples as a better surrogate for detection of changes in microbial quantities. Details of the medians together with the interquartile ranges of S. mutans, total lactobacilli, and total bacteria quantities in saliva and plaque samples at T0, T6, and T12 are summarized in Table 3.
Linear graphs presenting bacterial quantities as median log10 counts (y-axis) of Streptococcus mutans (square), total lactobacilli (circle), and total bacteria (triangle) in the a saliva and b plaque samples. The measurement units for saliva and plaque samples are log10 CFU/ml and log10 CFU/mg, respectively. The dotted, solid, and dashed lines represent the placebo, daily probiotic, and triweekly probiotic groups, respectively. T0, T6, and T12 are the time points at baseline, after 6 months of probiotic intervention and 6 months after probiotic discontinuation, respectively. The significant differences between T6 or T12 and T0 within each group are illustrated. Note that the interquartile ranges are omitted from the linear graphs, but are presented in Table 3. *p < 0.0167, **p < 0.0033, ***p < 0.00033
Weak correlations for the quantities of S. mutans and total lactobacilli between saliva and plaque samples
When all data from the three periods and the three intervention groups were pooled and compared between the saliva (log10 CFU/ml) and plaque (log10 CFU/mg) samples, significantly, but weakly, positive correlations were found in the quantities of S. mutans (r = 0.206, p < 0.001; Fig. 4a) and in those of total lactobacilli (r = 0.036, p < 0.001; Fig. 4b).
Significantly, but weakly, positive correlations of aStreptococcus mutans (S. mutans) quantities and b total lactobacilli quantities from pooled data from the three intervention groups (placebo, daily probiotics, and triweekly probiotics) at all three periods (at baseline, after 6 months of probiotic intervention and 6 months after probiotic discontinuation) between the plaque (y-axis) and saliva (x-axis) samples. Regression lines shows positive correlations of S. mutans (a) and total lactobacilli (b) quantities between the plaque and the saliva samples
Discussion
This large-scale multicenter trial, in which several hundreds of preschool children were registered, demonstrates the beneficial effect of probiotic supplementation with L. paracasei SD1 on a significant reduction in cariogenic bacteria, S. mutans, which is in agreement with our previous studies using standard cultivation to quantify salivary mutans streptococci [11,12,13,14]. The reduction of S. mutans by probiotic consumption is also supported by a number of studies analyzed in three systematic reviews, showing that various probiotic strains could significantly reduce cariogenic mutans streptococci [25,26,27]. Furthermore, some studies in a review by Zaura and Twetman, 2019 [28], showed that probiotics could even reduce caries incidence, but the authors suggested that further long-term clinical studies are needed. In this study, we also found that our probiotic strain could decrease caries progression while increase caries regression at T6 and T12, suggesting that reduction of S. mutans by probiotic consumption would ultimately lead to impede demineralization while facilitate remineralization processes. The details of these findings will be published in a separate article. The possible mechanism of action for the probiotic L. paracasei SD1 may be due to the production of bacteriocin, named “paracasin SD1,” which possesses potent antimicrobial activities against cariogenic bacteria (S. mutans, Streptococcus. sobrinus) and periodontopathogens (Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis) as well as Candida albicans [7, 8]. Moreover, the inhibitory effect of paracasin SD1 was shown to work in a broad pH range from 3.0 to 8.0 [8]. Our finding is also in line with another proposed inhibitory action of L. paracasei by co-aggregation with S. mutans to prevent S. mutans adherence to the tooth surfaces [29]. The bacterial co-aggregation in saliva results in elimination of S. mutans from the oral cavity by swallowing, which corresponds to more significant decreases in the quantities of S. mutans observed in the saliva than in the plaque samples (Fig. 3).
This field trial showed that the efficacy of probiotic consumption on S. mutans reduction in several childcare centers meets the criteria of a phase 2 trial, defined by a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food [30]. In such guidelines, it is recommended that “human trials be repeated by more than one center for confirmation of results.” Furthermore, significant increases in the quantities of total lactobacilli at T6 and T12 compared with T0 imply that L. paracasei SD1 administered either daily or triweekly accounts for an increase in the amounts of total lactobacilli in the oral cavity, which can be detected in saliva and constituted in the dental plaque even 6 months after probiotic discontinuation. Accordingly, the beneficial effect on S. mutans reduction could be observed at least 6 months after probiotic intervention, possibly as a result of persistent microbial modulation by the probiotic L. paracasei SD1, which is consistent with the findings from our previous studies [12, 24]. Such increases in the quantities of total lactobacilli are expected, given that most probiotics are lactobacillus species, and the increases are in line with a previous systematic review and meta-analysis [27]. Due to the possible cariogenicity of lactobacilli, their use for caries prevention may be of concern. However, according to a number of systematic reviews and meta-analyses [25,26,27] as well as our previous studies [11,12,13,14], the use of probiotics is generally considered safe, and neither adverse effects to oral health nor increased caries risks have been reported so far in clinical trials. It is suggested that not all lactobacillus species can induce caries [31], which is in agreement with our finding that only certain lactobacillus species, Lactobacillus salivarius, was a strong acid producer [32]. In addition, L. salivarius was found to be associated with high caries in children [6].
The ability of L. paracasei SD1 to adhere to the tooth surface or to colonize in dental plaque has not yet been tested; however, the ability of L. paracasei SD1 to adhere to oral epithelial cells has been previously demonstrated [7]. It is likely that the oral epithelial cells could be a reservoir for L. paracasei SD1 to be continuously present in the oral cavity even 6 months after probiotic discontinuation. Moreover, by using an arbitrary primer-PCR method to analyze the bacterial DNA fingerprints, the identical pattern of L. paracasei SD1 DNA fingerprints could be found in saliva of volunteers who consumed the probiotic milk [12]. Taken together, early exposure to probiotics, especially during the first 1000 days of life, would have a long-term benefit for prevention of dental caries in young children.
The dosing interval (daily or triweekly) was designed to evaluate the minimal dosage required for the presence and persistence of probiotic benefits over microbial compositions. Our results suggest that the benefits of triweekly consumption are not different from those of daily consumption in terms of the significant reduction of S. mutans quantities or the significant increase in total lactobacilli quantities. Both triweekly and daily probiotic groups showed a similar trend for the reduction of S. mutans in both the saliva and plaque samples at T6 and T12 compared with T0, although the daily probiotic group showed greater decreases in the quantities of S. mutans than did the triweekly group (Fig. 3). Therefore, the inhibitory action of L. paracasei SD1 against S. mutans was found to be greater and more consistent in the daily probiotic than the triweekly probiotic group, suggesting that when promising and stable inhibition against cariogenic bacteria from probiotics is expected, the daily dosage is preferable, yet the triweekly dosage is still acceptable in some circumstances.
Even by stratified randomization, the placebo group in our study began with a significantly smaller median quantity of S. mutans (Fig. 2a), and a significantly greater median quantity of total lactobacilli (Fig. 2b), than those of the two probiotic groups at T0 in the saliva samples. These microbial differences among the three intervention groups may have occurred by chance. However, when children in the placebo group were 6 months and one year older, the median quantities of S. mutans and of total lactobacilli were, instead, significantly greater and smaller, respectively, than in the other two probiotics groups in both the saliva and plaque samples (Fig. 2a, b). This suggests that without the beneficial effects from consumption of L. paracasei SD1, the quantities of S. mutans in the placebo group tended to continually increase with age in both the saliva and plaque samples, whereas the quantities of total lactobacilli in this group tended to decrease. Besides the aforementioned changes in the quantities of S. mutans and total lactobacilli, total bacteria in the saliva samples of the placebo group tend to increase with age (Fig. 3a). All of these microbial changes in the placebo group are consistent with the results of Damle et al., 2016 [33], which demonstrated that children tend to have high levels of oral bacteria, especially mutans streptococci, when they are older.
When data on the quantities of S. mutans (Fig. 4a) and those of total lactobacilli (Fig. 4b) in the three intervention groups at the three periods were pooled, weakly, albeit significantly, positive correlations, were found between the saliva and plaque samples. To the best of our knowledge, there has not yet been a correlation study comparing the microbial quantities between the two oral samples upon probiotic administration. The weak correlations can be explained by the fact that microorganisms live in the plaque biofilm as a somewhat static and stable community compared with much more dynamic changes and fluctuations in both types and quantities of microorganisms in the saliva [34]. Moreover, all tooth surfaces are constantly bathed in the saliva so that it may better reflect changes in the microbial compositions than would the plaque samples [33]. This is confirmed by more drastic alterations in the amounts of both S. mutans and total lactobacilli found in the saliva samples than in the plaque samples upon probiotic administration (Fig. 3). However, saliva collection is not easy in young children due to their difficulty in spitting and uncooperative behaviors. This is why only about three quarters of all saliva samples collected from eligible children (Fig. 1) had an adequate volume, i.e., at least 500 μl, required for DNA extraction [35].
The reason why L. fermentum was used to establish a standard curve for total lactobacilli is due to its predominance (>80%) among other lactobacilli strains found in the oral cavity of Thai preschool children [6], so the quantities of L. fermentum could well represent those of total lactobacilli in the saliva and plaque samples. Furthermore, we chose to amplify DNA of total lactobacilli after consumption of milk supplemented with the probiotic L. paracasei SD1 was due to the limitation to design a primer pair specific only for the L. paracasei SD1 because of the almost identical sequences between the paracasei and the casei species [24]. Moreover, our previous study had shown a positive correlation between the quantities of total lactobacilli and those of L. paracasei/casei, as being quantified by qPCR [24]. A significant increase in the amounts of total lactobacilli after consumption of the probiotic L. paracasei SD1 was also demonstrated by a cultivation method [11]. Consumption of the L. paracasei SD1 would, therefore, mainly contribute to an increase in the DNA amounts of total lactobacilli observed in the saliva and plaque samples of both probiotic groups (Fig. 3a, b, respectively), indicating that total lactobacilli can be used as a surrogate for monitoring changes in the quantities of the probiotic L. paracasei SD1. Regarding future studies, it is of our interest to determine the beneficial effects on both S. mutans and caries reduction by a combination of L. paracasei SD1 with the other probiotic strain in a prospective study, which lasts longer than 6 months after probiotic discontinuation. In addition, the effects of consumption of the probiotic L. paracasei SD1 on salivary flow and buffering capacity are needed to be further examined, although we have previously shown that there was no difference in salivary pH in the probiotic group compared with that in the placebo group [11, 12].
Conclusions
In summary, a 6-month course of daily and triweekly consumption of the probiotic, L. paracasei SD1, significantly reduces the quantities of the cariogenic bacteria, S. mutans, in both saliva and plaque, and the decreasing effect persists for at least 6 months after discontinuation. Although the daily consumption yields a better benefit in terms of S. mutans reduction than the triweekly consumption, the triweekly dosage is still acceptable and is a more practical method of implementation to reduce the cost of probiotic milk in some developing countries. Nevertheless, in children with high caries risk, the daily consumption of probiotics is highly recommended to better control the quantity of S. mutans at the initial phase.
References
Twetman S, Jørgensen MR, Keller MK (2017) Fifteen years of probiotic therapy in the dental context: what has been achieved? J Calif Dent Assoc 45(10):539–545
Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W (2015) Global burden of untreated caries: a systematic review and metaregression. J Dent Res 94(5):650–658. https://doi.org/10.1177/0022034515573272
Hoare A, Marsh PD, Diaz PI (2017) Ecological therapeutic opportunities for oral diseases. Microbiol Spectr 5(4). https://doi.org/10.1128/microbiolspec.BAD-0006-2016
Nora AD, Rodrigues CS, Rocha RO, Soares FZM, Braga MM, Lenzi TL (2018) Is caries associated with negative impact on oral health-related quality of life of pre-school children? A systematic review and meta-analysis. Pediatr Dent 40(7):403–411
Coqueiro AY, Bonvini A, Raizel R, Tirapegui J, Rogero MM (2018) Probiotic supplementation in dental caries: is it possible to replace conventional treatment? Nutrire 43(1):6–9. https://doi.org/10.1186/s41110-018-0064-3
Piwat S, Teanpaisan R, Thitasomakul S, Thearmontree A, Dahlen G (2010) Lactobacillus species and genotypes associated with dental caries in Thai preschool children. Mol Oral Microbiol 25(2):157–164. https://doi.org/10.1111/j.2041-1014.2009.00556.x
Teanpaisan R, Piwat S, Dahlen G (2011) Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol 53(4):452–459. https://doi.org/10.1111/j.1472-765X.2011.03132.x
Wannun P, Piwat S, Teanpaisan R (2014) Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe 27:17–21. https://doi.org/10.1016/j.anaerobe.2014.03.001
Chooruk A, Piwat S, Teanpaisan R (2017) Antioxidant activity of various oral Lactobacillus strains. J Appl Microbiol 1:271–279. https://doi.org/10.1111/jam.13482
Wattanarat O, Makeudom A, Sastraruji T, Piwat S, Tianviwat S, Teanpaisan R, Krisanaprakornkit S (2015) Enhancement of salivary human neutrophil peptide 1-3 levels by probiotic supplementation. BMC Oral Health 15:19. https://doi.org/10.1186/s12903-015-0003-0
Teanpaisan R, Piwat S (2014) Lactobacillus paracasei SD1, a novel probiotic, reduces mutans streptococci in human volunteers: a randomized placebo-controlled trial. Clin Oral Investig 18(3):857–862. https://doi.org/10.1007/s00784-013-1057-5
Teanpaisan R, Piwat S, Tianviwat S, Sophatha B, Kampoo T (2015) Effect of long-term consumption of lactobacillus paracasei SD1 on reducing mutans streptococci and caries risk: a randomized placebo-controlled trial. Dent J (Basel) 3(2):43–54. https://doi.org/10.3390/dj3020043
Ritthagol W, Saetang C, Teanpaisan R (2014) Effect of probiotics containing Lactobacillus paracasei SD1 on salivary mutans streptococci and lactobacilli in orthodontic cleft patients: a double-blinded, randomized, placebo-controlled study. Cleft Palate Craniofac J 51(3):257–263. https://doi.org/10.1597/12-243
Pahumunto N, Piwat S, Chankanka O, Akkarachaneeyakorn N, Rangsitsathian K, Teanpaisan R (2018) Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontol Scand 76(5):331–337. https://doi.org/10.1080/00016357.2018.1453083
Wilson W, Taubert KA, Gewitz M et al (2007) Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 138(6):739–745 747-760
Teanpaisan R, Chooruk A, Wannun A, Wichienchot S, Piwat S (2012) Survival rates of human-derived probiotic Lactobacillus paracasei SD1 in milk powder using spray drying. Songklanakarin J Sci Technol 34:241–245
Santos APPD, Soviero VM (2006) Comparison between two visible biofilm indices in the primary dentition. J Clin Pediatr Dent 30(4):292–295. https://doi.org/10.17796/jcpd.30.4.9n1852u276583r3v
Santos AP, Sellos MC, Ramos ME, Soviero VM (2007) Oral hygiene frequency and presence of visible biofilm in the primary dentition. Braz Oral Res 21(1):64–69. https://doi.org/10.1590/S1806-83242007000100011
Nyvad B, Machiulskiene V, Baelum V (1999) Reliability of a new caries diagnostic system differentiating between active and inactive caries lesions. Caries Res 33(4):252–260. https://doi.org/10.1159/000016526
Sellos MC, Soviero VM (2011) Reliability of the Nyvad criteria for caries assessment in primary teeth. Eur J Oral Sci 119(3):225–231. https://doi.org/10.1111/j.1600-0722.2011.00827.x
Nyvad B, Baelum V (2018) Nyvad criteria for caries lesion activity and severity assessment: a validated approach for clinical management and research. Caries Res 52(5):397–405. https://doi.org/10.1159/000480522
Oho T, Yamashita Y, Shimazaki Y, Kushiyama M, Koga T (2000) Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol Immunol 15(4):258–262
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148(Pt 1):257–266. https://doi.org/10.1099/00221287-148-1-257
Pahumunto N, Sophatha B, Piwat S, Teanpaisan R (2019) Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: A double-blind, randomized, controlled study. J Dent Sci. https://doi.org/10.1016/j.jds.2019.01.008
Twetman S (2012) Are we ready for caries prevention through bacteriotherapy? Braz Oral Res 26(Suppl 1):64–70. https://doi.org/10.1590/S1806-83242012000700010
Twetman S, Stecksén-Blicks C (2008) Probiotics and oral health effects in children. Int J Paediatr Dent 18(1):3–10. https://doi.org/10.1111/j.1365-263X.2007.00885.x
Gruner D, Paris S, Schwendicke F (2016) Probiotics for managing caries and periodontitis: Systematic review and meta-analysis. J Dent 48(5):16–25. https://doi.org/10.1016/j.jdent.2016.03.002
Zaura E, Twetman S (2019) Critical Appraisal of Oral Pre- and Probiotics for Caries Prevention and Care. Caries Res 53(5):514–526. https://doi.org/10.1159/000499037
Lang C, Bottner M, Holz C, Veen M, Ryser M, Reindl A, Pompejus M, Tanzer JM (2010) Specific Lactobacillus/Mutans Streptococcus co-aggregation. J Dent Res 89(2):175–179. https://doi.org/10.1177/0022034509356246
Joint Food and Agriculture Organization of the United States/World Health Organization (FAO/WHO) Working Group (2002) Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London Ontario, Canada. http://www.fao.org/3/a-a0512e.pdf. Accessed 27 May 2019
Caufield PW, Li Y, Dasanayake A, Saxena D (2007) Diversity of lactobacilli in the oral cavities of young women with dental caries. Caries Res 41(1):2–8. https://doi.org/10.1159/000096099
Piwat S, Teanpaisan R, Dahlén G, Thitasomakul S, Douglas CW (2012) Acid production and growth by oral Lactobacillus species in vitro. J Investig Clin Dent 3(1):56–61. https://doi.org/10.1111/j.2041-1626.2011.00098.x
Damle SG, Loomba A, Dhindsa A, Loomba A, Beniwal V (2016) Correlation between dental caries experience and mutans streptococci counts by microbial and molecular (polymerase chain reaction) assay using saliva as microbial risk indicator. Dent Res J (Isfahan) 13(6):552–559. https://doi.org/10.4103/1735-3327.197035
Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML, Tonetti MS, Wade WG, Zaura E (2016) The oral microbiome – an update for oral healthcare professionals. Br Dent J 221(10):657–666. https://doi.org/10.1038/sj.bdj.2016.865
Koni AC, Scott RA, Wang G, Bailey ME, Peplies J, Bammann K, Pitsiladis YP (2011) DNA yield and quality of saliva samples and suitability for large-scale epidemiological studies in children. Int J Obes 35(Suppl 1):S113–S118. https://doi.org/10.1038/ijo.2011.43
Acknowledgments
The authors wish to thank Dr. M. Kevin O Carroll, Professor Emeritus of the University of Mississippi School of Dentistry, USA, and Faculty Consultant at Chiang Mai University Faculty of Dentistry, Thailand, for his assistance in the preparation of the manuscript.
Funding
This trial was financially supported by the Health Systems Research Institute, Ministry of Public Health, Thailand (#HSRI60-023) to R.T.; by the National Research Council of Thailand (#DEN600045a) to S.P.; by a Postdoctoral Fellowship from Prince of Songkla University, Thailand to N.P.; by the Intramural Endowment Fund, Faculty of Dentistry, Chiang Mai University, Thailand to A.N.; and by the Food Innovation and Packaging Center, Chiang Mai University, and the Thailand Research Fund (#BRG6080001) to S.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving human participants in this trial were in accordance with the ethical standards of the Ethics Committee, Faculty of Dentistry, Prince of Songkla University (#EC5912-50-L-HR) and the Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University (#10/2018), and with the Declaration of Helsinki (1964) and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manmontri, C., Nirunsittirat, A., Piwat, S. et al. Reduction of Streptococcus mutans by probiotic milk: a multicenter randomized controlled trial. Clin Oral Invest 24, 2363–2374 (2020). https://doi.org/10.1007/s00784-019-03095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03095-5