Abstract
Objective
Non-invasive esthetic treatment options for stained arrested caries lesions have not been explored. This study aimed to develop laboratory models to create stained-remineralized caries-like lesions (s-RCLs) and to test the efficacy of bleaching on their esthetic treatment.
Materials and methods
One hundred twelve enamel/dentin specimens were prepared from human molars, embedded, and had their color measured spectrophotometrically at baseline and after demineralization. They were randomly divided into four groups (n = 14) based on the staining/remineralization protocols for a total of 5 days: G1, no staining/no remineralization; G2, no staining/remineralization in artificial saliva (AS); G3, non-metallic staining/remineralization with sodium fluoride/AS; and G4, metallic staining/remineralization with silver diamine fluoride/AS. The lesion mineral loss (ΔZ) and depth (L) were measured using transverse microradiography along with color change (ΔE). Specimens were bleached and color was re-evaluated. Data were analyzed using ANOVA models followed by Fisher’s PLSD tests (α = 0.05).
Results
s-RCLs in G4 were significantly (p < 0.001) darker than G3, G2, and G1 regardless of substrate type and condition. s-RCLs in G2, G3, and G4 showed significantly lower ΔZ and L than G1 (all p < 0.001), confirming occurrence of remineralization. G4 exhibited significantly lower ΔZ and L compared to G2 (p < 0.001). Bleaching was more effective in non-metallic than in metallic stained lesions regardless of substrate type (p < 0.001).
Conclusion
The proposed models created distinct s-RCLs. Non-metallic s-RCLs were lighter and more responsive to bleaching compared to metallic s-RCLs.
Clinical relevance
The developed experimental models allow the further investigation of the efficacy and safety of different clinical strategies for the esthetic management of s-RCLs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arrested caries lesions (ACLs) present highly mineralized surfaces [1], along with frequent and undesirable dark discolorations due to pigment incorporation in the remineralization process. The pigmentation may be exacerbated if remineralization is driven by a metallic salt, such as silver diamine fluoride [2]. Esthetic management options for a stained ACL (s-ACL) include surgical interventions [3], such as dental microabrasion and placement of restorations. However, microabrasion can cause significant loss of the tooth structure (up to 200 μm) [4], while inadequate restorations may result in poor marginal adaptation, plaque retention, sensitivity, secondary caries, and possibly periodontal disease [5].
We have previously proposed dental bleaching as a non-invasive option to esthetically treat s-ACLs [6]. To further understand the clinical impact of this approach, it is important to systematically test its efficacy. The study of standardized stained lesions with very sensitive evaluation methods is an important experimental aspect that cannot be easily satisfied in clinical conditions. Therefore, the development of in vitro simulation models seems relevant. Common discoloration causes of s-ACLs may involve food pigments, byproducts released during the proteolytic processes, and the presence of trapped organic debris and metallic ions within the tooth structure [7]. Evidence shows that metallic stains are more difficult to treat [8].
In the present study, we proposed and tested two in vitro models to create stained-remineralized caries-like lesions (s-RCLs), based on the incorporation of metallic and non-metallic pigments during the remineralization of previously developed caries-like lesions [9, 10]. These models will allow the study of efficacy and safety aspects related to the dental bleaching treatment of s-RCLs. Our null hypotheses were (1) there were no differences in the color change, mineral loss, and lesion depth of metallic and non-metallic stained-remineralized caries-like lesion (s-RCL) models; (2) the use of bleaching agent on the created s-RCL models had no significant effect on the esthetic outcome.
Materials and methods
Experimental design
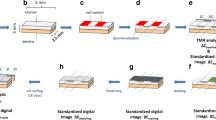
The main experimental factor was staining/remineralization at four levels (negative control, positive control, non-metallic, and metallic); secondary experimental factors derived from the main factor were substrate condition at two levels (sound and demineralized, to control for differences between superficial and sub-superficial staining, respectively), and substrate type at two levels (enamel and dentin, independently analyzed). The study outcomes were color change (ΔE) measured at three time points (after demineralization, staining/remineralization cycling, and bleaching) and mineral content (ΔZ) and lesion depth (L), measured after staining/remineralization cycling (Fig. 1). Color measurements were performed by spectrophotometry, while mineral loss and lesion depth were determined by transverse microradiography (TMR).
Specimen preparation
Enamel and dentin slabs (4 × 4 × 2 mm) were sectioned from the buccal and lingual area of human molars. Enamel specimens were obtained from the middle third of the crowns, while dentin specimens were taken from the cervical third of the roots, using a low-speed diamond saw (Isomet, Buehler, Lake Bluff, IL, USA). Specimens were embedded in acrylic resin blocks (10 × 10 × 8 mm; Varidur, Buehler); each block consisted of one slab of enamel and dentin. They were flattened using a sequence of 500, 1200, 2400, and 4000 grit silicon carbide paper (MDFuga, Struers Inc., Cleveland, OH, USA), polished with 1-μm diamond suspension (Struers Inc.) and sonicated in a detergent solution (Micro-90, International Products Corporation, Burlington, USA).
Following the polishing procedure, the specimens were placed under running deionized water for 3 min. Specimens were stored in moist conditions at 4 °C in a refrigerator (Kenmore; Whirlpool, Benton Harbor, MI, USA).
Caries-like lesion creation
Adhesive unplasticized polyvinyl chloride tapes (TapeCase Ltd., Wheeling, IL, USA) were used to cover half of the surface (2 × 4 mm) of each specimen (enamel and dentin), leaving the other half exposed. The lesion was initiated by placing specimens in a carboxymethylcellulose (CMC) demineralizing solution as described by Lippert et al. (2011) [11]. Briefly, specimens were demineralized for 7 days in a solution containing 0.1 M lactic acid, 4.1 mM Ca (as CaCl2·2H2O), 8.0 mM PO4 (as KH2PO4), and 1.0% w/v CMC (Sigma-Aldrich Co., St. Louis, MO, USA), with pH adjusted to 5.0, at 37 °C.
Staining, remineralization, and cycling
After demineralization, specimens were subdivided into four groups based on the different remineralizing protocols. Group 1 (negative control) was stored in 100% relative humidity throughout the experiment, with no immersion in artificial saliva or any other treatment. Group 2 (positive control) was immersed in artificial saliva (2.20 g/l gastric mucin, 1.45 mM CaCl2·2H2O, 5.40 mM KH2PO4, 28.4 mM NaCl, 14.9 mM KCl, pH 7.0) [12], which was changed daily. Group 3 (non-metallic stain) was incubated in a combined coffee (The Folger Coffee Company Inc., Orrville, OH, USA) and tea (Nestea, Nestle Inc., Glendale, CA, USA) solution prepared based on the manufacturer’s instructions and used immediately after preparation. Specimens were kept in stirring staining solution at ~ 37 °C for 8 h, rinsed, allowed to air dry, and treated with 2% sodium fluoride (NaF) gel (Sultan Healthcare Inc., York, PA, USA) for 4 min, before being immersed in artificial saliva overnight. Group 4 (metallic stain) was stored in a staining solution with iron, based on the protocol described by Stookey et al. [13]. Specimens were placed on a rotating rod (~ 37 °C incubator), which alternately exposed them to air and to a solution (800 ml/cycle, total of 4 l) consisting of 26 ml Micrococcus luteus BA13 (American Type Culture Collection, Manassas, VA, USA), 2.7 g coffee (Instant Folgers Crystals; Folgers, Orrville, OH, USA), 2.7 g tea (Nestea, Nestlé), 2 g gastric porcine mucin (American Laboratories, Omaha, NE, USA), and 0.04 g ferric chloride (Fisher Scientific, Fair Lawn, NJ, USA) dissolved into 800 ml of sterilized trypticase soy broth. After each cycle (8 h/day), specimens were rinsed, allowed to air dry, treated with 38% SDF Advantage Arrest (Elevate Oral Care LLC, West Palm Beach, FL, USA) for 2 min, and immersed in artificial saliva overnight.
Color assessment
L*a*b* (Commision Internationale de l’Eclairage) values were taken for each specimen. Measurements were performed using a spectrophotometer, Minolta Chroma meter CR- 241 (Minolta Camera Co., Osaka, Japan) with a focus of 0.3 mm. Color measurements were taken at baseline, and immediately after demineralization, cycling and bleaching. All measurements were repeated three times. The means of the L*a*b* values were measured and the color difference (ΔE = {(ΔL*)2 + (Δa*)2 + (Δb*)2}1/2) was calculated [14] after demineralization (ΔEDemin: demineralization-baseline), cycling (ΔECycling: staining-demineralization), and bleaching (ΔEBleaching: bleaching-staining).
Lesion mineral content and lesion depth assessment
Specimens were mounted on acrylic rods and sectioned with a hard tissue microtome (Silverstone-Taylor Hard Tissue Microtome, Series 1000 Deluxe, USA). One section from enamel (100 μm ± 20 μm) and one from dentin (180 μm ± 20 μm) were obtained from each specimen, including both the sound and demineralized areas. The sections were kept moist and mounted with an aluminum stepwedge on high-resolution glass plates Type I A (Microchrome Technology Inc., San Jose, CA, USA), then X-rayed at 20 kV and 30 mA at a distance of 42 cm for 65 min.
The film was developed in a Kodak d-19 developer for 3 min, placed in a stop bath (Kodak 146-4247) for 45 s, and then fixed (Kodak 146-4106) for 3 min. All plates were then rinsed in deionized water for 15 min and air dried. Microradiographs were examined with a Zeiss EOM microscope in conjunction with the TMR software v.3.0.0.11 (Inspektor Research Systems BV, Amsterdam, The Netherlands). A window (approx. 400 × 400 μm) representative of the entire lesion area and not containing any cracks, debris, or other alterations was selected for analysis. Integrated mineral loss (ΔZ), is the product of lesion depth and the mineral loss over that depth. The lesion depth, L, is per definition the distance from the outer tissue surface to the point where the mineral content is 95% of that of the sound hard tissue. This would mean 83% mineral for enamel, assuming the sound enamel mineral content being 87% volume/volume percent [15, 16]. Likewise, for dentin, this would mean the area obtained by plotting the volume percent mineral profile towards dentin depth in each dentin section, with the sound dentin set as 48 vol% mineral [17].
Dental bleaching efficacy
After transverse microradiography (TMR) analysis, specimens were covered with acid-resistant nail varnish (Sally Hansen Advanced Hard as Nails Nail Polish, USA) except for the area to be exposed to the bleaching agent. They were treated with 40% hydrogen peroxide (pH 6.0 to 8.5) (Opalescence® Boost, Ultradent Products, Inc., South Jordan, UT, USA). A 0.5–1.0-mm thick layer of the bleaching gel was applied to the specimen surface. After 20 min, the bleaching gel was gently wiped using a cotton pellet. This bleaching treatment was repeated two more times (total of 60 min of treatment time, as recommended by the manufacturer’s instructions). After the third bleaching application, each specimen was rinsed with running deionized water for 1 min, blot-dried, and stored moist at 4 °C for color measurements, which were performed immediately after.
Statistical analysis
The color change calculated after demineralization, staining/remineralization cycling, and bleaching for each dental substrate was analyzed using three-way ANOVA, with factors for stain type, substrate condition, and time points as well as all two-way and three-way interactions among the factors. Specimen and condition combination were allowed to have different variances, and random effects were added to the model.
The mineral loss and lesion depth change after treatment was analyzed using two-way ANOVA for each substrate, with factors for stain type and substrate condition as well as interaction between stain type and condition.
All pair-wise comparisons from ANOVA analysis were made using Fisher’s Protected Least Significant Differences to control the overall significance level at 5%. Prior to the study, calculations showed that with a planned sample size of 14 per group, the study was designed to have 80% power to detect a 2.2 ΔE difference between groups, assuming a 5% significance level and standard deviation of 2.0.
Results
Color change
For both enamel and dentin, means of color change were significantly different among stain types, substrate conditions, and time points (all p < 0.0001). All two-way and three-way interactions among stain types, substrate conditions, and time points were significant (p < 0.0001).
In lesioned enamel, G4 presented significantly (p ≤ 0.001) darker stains after cycling compared to G3, G2, and G1, with no significant difference between control groups. G3 was more responsive (p < 0.001) to bleaching compared to G4, G1, and G2; no significant differences were found in control groups (Table 1). In sound enamel, G4 presented significantly (p ≤ 0.001) darker stains after cycling compared to G3, G2, and G1, with no significant differences found among G3, G2, and G1. No significant differences were found among groups after bleaching (Table 2).
In lesioned and sound dentin, G4 showed significantly (p ≤ 0.001) darker stains after cycling compared to G3, G2, and G1, with no significant difference between control groups. G3 was more responsive (p < 0.001) to bleaching compared to the other groups with no significant differences among them (Tables 3 and 4).
The numerical values and comparisons within and among treatments for stain types, substrate conditions, and time points for lesioned and sound enamel are found in Tables 1 and 2 respectively; while lesioned and sound dentin are in Tables 3 and 4, respectively.
Mineral loss and lesion depth
Significant differences were found for stain type, substrate condition, and their interaction (all p < 0.001), for both outcomes studied (mineral loss and lesion depth), in both enamel and dentin. Regarding lesioned enamel, G4 had the least significant (p < 0.001) change in mineral loss and lesion depth (indicating SDF protective effect) compared to controls. G3 was not significantly different from G4 and G2, yet, significantly different (p < 0.001) from G1 (Table 5).
In lesioned dentin, G4 had the least significant (p < 0.001) change in mineral loss and lesion depth compared to G3, G2, and G1. Regarding mineral loss, G3, G2, and G1 were significantly (p < 0.001) different between each other. Regarding lesion depth, G3 and G2 were not significantly different yet both were significantly (p < 0.001) different from G1 (Table 6).
The numerical values and comparisons within and among treatments for stain types and substrate conditions for enamel and dentin are in Tables 5 and 6, respectively.
Discussion
The different cycling protocols used in this study created remineralized lesions with the stains (non-metallic, metallic) confined within the tooth structure, potentially resembling stained arrested caries lesions. NaF and SDF were chosen based on both their remineralization potentials and varying staining abilities. Standardizing the use of only one of the remineralizing agents would not allow us to obtain the desired level of stain differentiation, as SDF would result in a silver/metallic stain for both groups due to the deposition of metallic stains (silver phosphate), whereas NaF would not result in the pronounced metallic stain desired for the metallic staining group. The color change observed after staining (ΔE range 5.3–49.3) was greater than 3.3 units. ΔE values equal or greater than 3.3 are considered to be clinically perceptible, indicating that our staining process resulted in a distinct visual discoloration in all specimens [18].
Despite differences among study protocols, our TMR results were similar to other studies, as the three experimental groups (G2, G3, and G4, treated with artificial saliva, NaF, and SDF respectively) showed significant remineralization compared to the negative control group G1 [9,10,11, 19, 20]. In the present study, we tested remineralization efficacy by comparing treatment groups. We observed that SDF had lower ΔZ and L values compared to NaF, indicating its better remineralization efficacy [21, 22]. This might be explained by the higher fluoride concentration of SDF compared to NaF (44,800 ppm F versus 9040 ppm F), which is in agreement with previous studies, in which higher levels of fluoride provided better remineralization [2, 21, 23].
Furthermore, SDF and NaF present different protective mechanisms. NaF facilitates remineralization by calcium, fluoride, and phosphate deposition on the tooth surface to form calcium fluoride and fluoridated apatite crystals, which are acid-resistant and present lower solubility than enamel [24]. On the other hand, SDF forms a range of insoluble or sparingly soluble compounds including calcium fluoride, silver phosphate, and silver protein complexes [25, 26]. These precipitate on the dental surface, forming an insoluble protective layer that decreases demineralization [25, 26]. The overall combined effect of these factors might have played a substantial role in groups treated with SDF compared to NaF groups. This observation is in line with some reports suggesting that the synergistic effect of combining fluoride with other minerals, including iron, calcium, or tin, decreases demineralization [27, 28].
To better represent a clinical condition of a patient seeking professional bleaching, an in-office bleaching agent was used, as it is known to have higher concentrations of hydrogen peroxide in order to penetrate deeper into the histological structure, increase the oxidative power, and result in a fast bleaching result [29]. Although metallic stains had substantial increase in ΔE (black discoloration) compared to non-metallic stains (orange/brown discoloration), they were less responsive to bleaching treatment. Non-metallic stains, on the other hand, had the highest significant improvement in color change compared to all groups.
The better response of non-metallic (G3) stains to the bleaching treatment compared to the metallic (G4) ones might be explained by their inherent chemical compositions [6]. This can be explained by the bleaching process, as peroxide agents (highly unstable) dissociate upon tooth contact into water, oxygen, and free radicals. This oxidation process is increased in the presence of complex organic stains [29], represented in G3 (orange/brown discoloration) by coffee and tea pigments. On the contrary, the dark discoloration in G4 arose from the additive effect of silver component in SDF and the combination of iron, coffee, and tea from the staining solution, which cannot be broken down and bleached like organic chromophores [8].
Different substrate conditions (sound versus demineralized) were used to better represent differences in staining susceptibility. We observed that previously demineralized dental surfaces significantly incorporated more stains within the lesion during remineralization and were more difficult to bleach compared to sound substrates. This might be justified by the changes within the substrate structure throughout the demineralization process, since demineralized enamel with enlarged pore size [30] allowed for deeper penetration and of non-metallic and metallic stains during cycling, compared to sound enamel. This difference between sound and demineralized substrates indicates the successful incorporation of stains within the tooth structure. This finding corroborates those of a previous study, which observed that demineralized enamel surfaces were more susceptible to absorb external stains than sound enamel [14].
In general, dentin behaved similarly to enamel even though they were not statistically compared in this study. The overall staining level in dentin was higher compared to enamel, especially in G4. Although both substrates were exposed to SDF, the difference in stain susceptibility might be explained by the substantial differences in their structures [31]. The darker stains in dentin might be due to the fact that SDF penetrated deeper into dentin structure, depositing more stain compared to enamel [8]. This was probably enhanced by the fact that dentin demineralization occurred at much higher degree compared to enamel. Despite this difference, dentin remineralization seemed to be similar to that of enamel, although one would expect the presence of organic matrix to make it more difficult to achieve than in enamel [32], as spontaneous mineral precipitation on the demineralized dentin organic matrix is unlikely to happen [33].
We proposed developing stained-remineralized caries-like lesion models to explore the bleaching treatment efficacy in vitro. This model can be useful as a screening tool, when searching and developing novel strategies for the esthetic management of stained arrested caries lesions. However, despite the advantages of in vitro protocols with regard to their ability to be highly standardized and to allow for the control of different variables (lesion type, depth, stain, etc.), a significant limitation is their inability to adequately simulate the complex biological processes involved in creating s-RCL. Therefore, clinical extrapolation of the results should be done with caution.
In summary, the s-RCL models developed in this study allow investigations of mechanistic aspects related to the efficacy and safety of different bleaching treatments as conservative options for the esthetic management of these lesions in a relatively quick, affordable, and systematic fashion. It is essential to understand the different variables (stain types and remineralizing agents) that contribute to the development of s-RCL models. The different staining models created different discolorations, with the non-metallic model showing better bleaching efficacy than the metallic model. Hence, our null hypotheses were rejected. These observations not only give us a better mechanistic understanding of the process, but also suggest specific clinical recommendations for the esthetic management of stained-remineralized lesions.
Conclusion
The laboratory models developed in this investigation are able to create stained-remineralized caries-like lesions in both enamel and dentin. Non-metallic stains are lighter in color and more responsive to bleaching treatment compared to metallic stains.
References
Schupbach P, Lutz F, Guggenheim B (1992) Human root caries: histopathology of arrested lesions. Caries Res 26:153–164
Rosenblatt A, Stamford TC, Niederman R (2009) Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res 88:116–125. https://doi.org/10.1177/0022034508329406
Holmgren C, Gaucher C, Decerle N, Domejean S (2014) Minimal intervention dentistry II: part 3. Management of non-cavitated (initial) occlusal caries lesions--non-invasive approaches through remineralisation and therapeutic sealants. Br Dent J 216:237–243. https://doi.org/10.1038/sj.bdj.2014.147
Sundfeld RH, Sundfeld-Neto D, Machado LS, Franco LM, Fagundes TC, Briso AL (2014) Microabrasion in tooth enamel discoloration defects: three cases with long-term follow-ups. J Appl Oral Sci 22:347–354
Ababnaeh KT, Al-Omari M, Alawneh TN (2011) The effect of dental restoration type and material on periodontal health. Oral Health Prev Dent 9:395–403
Al-Angari SS, Hara AT (2016) A conservative approach to esthetically treat stained arrested caries lesions. Quintessence Int 47:499–504. https://doi.org/10.3290/j.qi.a36010.
Watts A, Addy M (2001) Tooth discolouration and staining: a review of the literature. Br Dent J 190:309–316. https://doi.org/10.1038/sj.bdj.4800959a
Horst JA, Ellenikiotis H, Milgrom PL (2016) UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications and consent. J Calif Dent Assoc 44:16–28
Lee RC, Kang H, Darling CL, Fried D (2014) Automated assessment of the remineralization of artificial enamel lesions with polarization-sensitive optical coherence tomography. Biomed Opt Express 5:2950–2962. https://doi.org/10.1364/boe.5.002950
Kielbassa AM, Shohadai SP, Schulte-Monting J (2001) Effect of saliva substitutes on mineral content of demineralized and sound dental enamel. Support Care Cancer 9:40–47
Lippert F, Lynch RJ, Eckert GJ, Kelly SA, Hara AT, Zero DT (2011) In situ fluoride response of caries lesions with different mineral distributions at baseline. Caries Res 45:47–55. https://doi.org/10.1159/000323846
Al Dehailan L, Martinez-Mier EA, Lippert F (2015) The effect of fluoride varnishes on caries lesions: an in vitro investigation. Clin Oral Investig 20:1655–1662. https://doi.org/10.1007/s00784-015-1648-4
Stookey GK, Burkhard TA, Schemehorn BR (1982) In vitro removal of stain with dentifrices. J Dent Res 61:1236–1239
Ahrari F, Akbari M, Mohammadpour S, Forghani M (2015) The efficacy of laser-assisted in-office bleaching and home bleaching on sound and demineralized enamel. Laser Ther 24:257–264. https://doi.org/10.5978/islsm.15-OR-15
Angmar B, Carlstrom D, Glas JE (1963) Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J Ultrastruct Res 8:12–23
de Josselin de Jong E, ten Bosch JJ, Noordmans J (1987) Optimised microcomputer-guided quantitative microradiography on dental mineralised tissue slices. Phys Med Biol 32:887–899
van der Veen MH, Tsuda H, Arends J, ten Bosch JJ (1996) Evaluation of sodium fluorescein for quantitative diagnosis of root caries. J Dent Res 75:588–593. https://doi.org/10.1177/00220345960750011201
Johnston WM, Kao EC (1989) Assessment of appearance match by visual observation and clinical colorimetry. J Dent Res 68:819–822
Preston KP, Smith PW, Higham SM (2008) The influence of varying fluoride concentrations on in vitro remineralisation of artificial dentinal lesions with differing lesion morphologies. Arch Oral Biol 53:20–26. https://doi.org/10.1016/j.archoralbio.2007.08.001
Hellen A, Mandelis A, Finer Y, Amaechi BT (2011) Quantitative evaluation of the kinetics of human enamel simulated caries using photothermal radiometry and modulated luminescence. J Biomed Opt 16:071406. https://doi.org/10.1117/1.3564909
Mei ML, Ito L, Cao Y, Li QL, Lo EC, Chu CH (2013) Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent 41:809–817. https://doi.org/10.1016/j.jdent.2013.06.009
Shah SGBV, Chawla S, Venkataraghavan K, Choudhary P, Ganesh M, Trivedi K (2014) Efficacy of silver diamine fluoride as a topical fluoride agent compared to fluoride varnish and acidulated phosphate fluoride gel: an in vivo study. J Pediatr Dent 2:5–12
Zhi QH, Lo EC, Kwok AC (2013) An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J 58:50–56. https://doi.org/10.1111/adj.12033
Tenuta LM, Cury JA (2010) Fluoride: its role in dentistry. Braz Oral Res 24(Suppl 1):9–17
Yu DG, Kimura Y, Fujita A, Hossain M, Kinoshita JI, Suzuki N, Matsumoto K (2001) Study on acid resistance of human dental enamel and dentin irradiated by semiconductor laser with Ag (NH3)2F solution. J Clin Laser Med Surg 19:141–146. https://doi.org/10.1089/10445470152927973
Liu BY, Lo EC, Li CM (2012) Effect of silver and fluoride ions on enamel demineralization: a quantitative study using micro-computed tomography. Aust Dent J 57:65–70. https://doi.org/10.1111/j.1834-7819.2011.01641.x
Zhang Y, Huang RZ (2016) Study on enhancement effect and mechanism of the acid resistance of enamel by Fe2+ and F. Shanghai Kou Qiang Yi Xue 25:42–46
Byeon SM, Lee MH, Bae TS (2016) The effect of different fluoride application methods on the remineralization of initial carious lesions. Restor Dent Endod 41:121–129. https://doi.org/10.5395/rde.2016.41.2.121
Bortolatto JF, Pretel H, Floros MC, Luizzi AC, Dantas AA, Fernandez E, Moncada G, de Oliveira OB Jr (2014) Low concentration H(2)O(2)/TiO_N in office bleaching: a randomized clinical trial. J Dent Res 93:66s–71s. https://doi.org/10.1177/0022034514537466
Kidd EA, Fejerskov O (2004) What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res 83 Spec No C:C35–C38
Cadavid AS, Lince CM, Jaramillo MC (2010) Dental caries in the primary dentition of a Colombian population according to the ICDAS criteria. Braz Oral Res 24:211–216
Chen Z, Cao S, Wang H, Li Y, Kishen A, Deng X, Yang X, Wang Y, Cong C, Wang H, Zhang X (2015) Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries. PLoS One 10:e0116553. https://doi.org/10.1371/journal.pone.0116553
Klont B, ten Cate JM (1991) Susceptibility of the collagenous matrix from bovine incisor roots to proteolysis after in vitro lesion formation. Caries Res 25:46–50
Acknowledgments
This manuscript is part of a dissertation submitted by Sarah Al-Angari to Indiana University School of Dentistry, in partial fulfillment of the requirements for the PhD degree in Dental Sciences. The authors would like to thank Dr. Armando Soto-Rojas and Dr. Bruce Matis for their insightful comments and discussions.
Funding
The work was supported by the Oral Health Research Institute, Indiana University School of Dentistry, Indianapolis, IN, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Local Institutional Review Board approval was obtained for the use of unidentified extracted human teeth (NS0911-07). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Al-Angari, S.S., Lippert, F., Platt, J.A. et al. Bleaching of simulated stained-remineralized caries lesions in vitro. Clin Oral Invest 23, 1785–1792 (2019). https://doi.org/10.1007/s00784-018-2590-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2590-z