Abstract
Objectives
The objective of this study was to investigate selected physical properties of nine contemporary and recently marketed glass ionomer cement (GIC) and four resin-modified glass ionomer cement (RMGI) dental restorative materials.
Materials and methods
Specimens (n = 12) were fabricated for fracture toughness and flexure strength using standardized, stainless steel molds. Testing was completed on a universal testing machine until failure. Knoop hardness was obtained using failed fracture toughness specimens on a microhardness tester, while both flexural modulus and flexural toughness was obtained by analysis of the flexure strength results data. Testing was completed at 1 h, 24 h, 1 week, and then at 1, 3, 6, and 12 months. Mean data was analyzed with Kruskal-Wallis and Mann-Whitney (p = 0.05).
Results
Physical properties results were material dependent. Physical properties of the GIC and RMGI products were inferior at 1 h compared to that at 24 h. Some improvement in selected physical properties were noted over time, but development processes were basically concluded by 24 h. A few materials demonstrated improved physical properties over the course of the evaluation.
Conclusions
Under the conditions of this study:
-
1.
GIC and RMGI physical property performance over time was material dependent;
-
2.
Polyalkenoate maturation processes are essentially complete by 24 h;
-
3.
Although differences in GIC physical properties were noted, the small magnitude of the divergences may render such to be unlikely of clinical significance;
-
4.
Modest increases in some GIC physical properties were noted especially flexural modulus and hardness, which lends support to reports of a maturing hydrogel matrix;
-
5.
Overall, GIC product physical properties were more stable than RMGI;
-
6.
A similar modulus reduction at 6 months for both RMGI and GIC produced may suggest a polyalkenoate matrix change; and
-
7.
Globally, RMGI products demonstrated higher values of flexure strength, flexural toughness, and fracture toughness than GIC materials.
Clinical relevance
As compared to RMGI materials, conventional glass ionomer restorative materials demonstrate more stability in physical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glass ionomer cements (GIC) were invented and developed by Wilson and Kent in the early 1970s to overcome shortcomings associated with silicate restorative cements [1, 2]. GIC restorative materials generally consist of a mixture of various polyalkenoate (polyacrylic) acids and tartaric acid that react with a fluoroaluminosilcate glass. The acid-base setting reaction between the acid and the glass surface liberates metal cations that allows crosslinking between the polyalkenoate chains [3, 4], with additional maturation beyond 24 h consisting of continued polymer chain cross linking and hydrogel matrix maturation formation with physical property improvement [5,6,7,8]. GICs are self-adhesive materials that bond to tooth hard tissues through chemical bonding, consisting of ionic bonds between polyalkenoate acid carboxyl groups and enamel and dentin hydroxyapatite in enamel and dentin [9, 10]. GIC restorative materials have been improved with formulation changes to enhance physical properties and clinical handling characteristics [4, 11, 12]. These modifications have included the use of alternative polyacids [4, 13, 14], water-activated dehydrated polyacid powders [4, 13, 15], cermets [16], metal additions [17,18,19], smaller glass particle size [20], antibacterial agents [21, 22], novel glass compositions [13, 23], and most recently aluminum-free glasses [24].
Resin-modified GICs (RMGICs) were first developed as bases and liners but were soon further modified to serve as direct restorative materials, hopefully to overcome early moisture sensitivity and lower mechanical properties associated with the GIC restoratives of that era [10, 25, 26]. Similar to GIC materials, RMGICs contain not only an acid-base reaction but also a resin free radical polymerization [10, 27, 28]. The resin content is added either with a direct monomer addition such as 2-hydroxyethyl methacrylate (HEMA) or uniquely grafted on the polyalkenoate acid chain. These monomers polymerize either by visible light curing (VLC) photo activation or an internal chemical reaction [10, 26]. RMGI materials also demonstrate bonding to tooth structure, as demonstrated by X-ray photoelectron spectroscopy and infrared spectroscopy as well as hybrid layer micromechanical interlocking similar to, but not to the same extent as resin adhesive systems [29,30,31,32,33]. Although the exact composition of each GIC and RMGI material is generally proprietary, the polymerized resin composition in the early RMGI products was earlier estimated to be approximately 4.5–6% [34]. Unfortunately, the resin and polyalkenoate setting reactions compete as the resin retards the polyalkenoate reaction by stereo-chemical distortion of the polyalkenoate acid chains making reaction sites less available. Furthermore, RMGIs contain less water for the polyalkenoate reaction to proceed as resin is added at the expense of water [9, 35,36,37].
Glass ionomer setting reactions have been investigated by several different methods to include infrared spectroscopy [38], Raman spectroscopy [39], NMR spectroscopy [40], pH measurements [41], rheology [42, 43], dielectric spectroscopy [44,45,46,47], inductively coupled plasma optical emission spectroscopy (ICP-OES) [48], and thermal analysis techniques [49]. Although some laboratory novel GIC formulations have shown the capacity for continuation of the acid-base reaction [27], it has been established by different analysis methods that the GIC polyalkenoate acid-base reaction is essentially complete by 24 h [19, 27, 38, 40, 50]. However, the continued changes in both the organic and inorganic GIC matrix past 24 h remains a source of interest as some GIC products continue to display an increase in physical properties with time [51,52,53,54]. The initial setting reaction and subsequent matrix formation is a multifaceted phenomenon, while Nicholson [4] relates the possibility of an intermediary phase that may influence in the timing of these processes. Moreover, Dickey et al. [55] recently reported evidence of a complex intermediary phase that delayed polyalkenoate crosslinking, but this was only observed to be particular to the glasses used. After acid-base reaction completion, both Stamboulis et al. [40] and Zainuddin et al. [56] reported the formation of a network and a hydrated silica gel phase, consisting of either a pure silicate or a mixed silicate phosphate matrix that contributed to physical property improvement with continued matrix maturation [6].
Both GIC and RMGI materials have displayed excellent clinical performance with atraumatic restoration treatment (ART) [57,58,59,60,61,62], as well as definitive restorations in both primary and permanent teeth [63,64,65,66,67,68,69,70,71]. Several GIC and RMGI restorative products have been recently marketed but lack independent verification of reported physical properties. The purpose of this study was to evaluate selected physical properties, namely flexural strength, flexural modulus, Knoop hardness, fracture toughness, flexural toughness and resiliency of newer GIC, and RMGI restorative materials compared with materials that have enjoyed marketing tenure. The null hypotheses were that there will be no difference in the physical properties between the tested restorative materials.
Materials and methods
The restorative materials used in this study were comprised of four RMGI products and nine GIC products and are listed in Tables 1 and 2, respectively. Twelve specimens were fabricated for each test. Fracture toughness and flexural strength specimens were fabricated using standardized, stainless steel molds (Sabri Dental Enterprises, Downers Grove, IL, USA) whose exact dimensions are described in each particular test. Materials were placed into respective molds on a mylar strip-covered glass slab with a second mylar strip placed on top of the filled mold. A glass microscope slide was then placed over the top mylar strip and digital pressure was applied to form a uniformly flat surface. GIC materials were allowed to set for the manufacturer recommended setting time in an oven at 35 °C. RMGI materials were polymerized using a light-emitting-diode (LED) visible-light-curing (VLC) unit (Bluephase G2, Ivoclar-Vivadent, Amherst, NY, USA) for 20 s in an overlapping fashion on both sides. The performance of the VLC unit was periodically assessed (~ 1000 mw/cm2) using a laboratory-grade laser power meter (10A-V1, Ophir-Spiricon, North Logan, UT, USA). Specimens were removed from the respective molds and refined as needed using surgical scalpel blades removing any flash material from the edges. Specimens were then stored under dark conditions in a 0.2 M physiologic phosphate-buffered saline environment at 37 °C and 98 ± 1% humidity until testing. Testing occurred at 1 h, 24 h, 1 week, and then at 1, 6, and 12 months after fabrication.
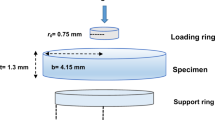
Flexure strength specimens were formed in a 2 × 2 × 25 mm stainless steel mold (Sabri Dental Enterprises) as formerly described and were tested on a three-point bend apparatus mounted on a universal testing machine (Alliance RT/5, MTS Corporation, Eden Prairie, MN, USA). Specimens were stressed using a cross head speed of 0.5 mm/min until failure with the maximum force recorded. Flexure strength results was determined using the formula F = 3FI/2bh2, where F = maximum load recorded (N), I = distance between supports (mm), b = width of specimen (mm), and h = height of specimen (mm). Flexural modulus was determined using the slope of the linear portion of the flexure strength stress-strain curve while flexural toughness was determined from the same data by integrating the area under the entire flexural testing stress/strain curve. Fracture toughness specimens were fabricated as described earlier using a stainless steel mold 2 × 5 × 25 mm in accordance with the single edge notch beam method as described in ASTM Standard E399. Each specimen’s dimensions were measured in three equally spaced positions along the specimen with the mean recorded. Testing occurred in a three-point bend apparatus with a crosshead speed of 0.5 mm/min in a universal testing machine (Alliance RT/5) until failure. The true notch length was measured post-testing with a digital measuring microscope (Hirox 7700, Hirox USA, Hackensack, NJ, USA). The fracture toughness calculation was accomplished using the following equation: KIC = (3PLa1/2/2tw2) × f(a/w) where P = failure load (N), L = distance between the support rollers (mm), a = measured notch length (mm), t = specimen thickness (mm), w = specimen width (mm), and f(a/w) = 1.93–3.07(a/w) + [14.53(a/w)2 – 25.11(a/w)3] + 25.80(a/w)4. Surface hardness was determined using post-testing fracture toughness specimens (n = 12). Eleven Knoop hardness (KH) indentations were made taken in an alternating fashion over a 5.25-mm distance with 0.5 mm spacing between indentations using a microhardness tester (OmniMet MHT, Buehler Manufacturing, Inc., Lake Bluff, IL, USA) using a 100-g load and a dwell time of 10 s. Indentions were measured under 50× magnification with the representative specimen hardness determined by the mean of the 11 calculated measurements. A total of 12 specimens were tested for each material, with the mean of the samples representing sample mean. All property mean testing results were submitted first to the Shapiro-Wilk and Bartlett’s test, which identified irregularities in both the distribution and variance within the mean data. Therefore, the data was analyzed using Kruskal-Wallis with Dunn’s post hoc testing performed with a 95% level of confidence (p = 0.05). Statistical analysis was performed using SPSS 21 (IBM/SPSS, Chicago, IL, USA).
Results
The GIC physical property testing mean results are listed in Tables 3, 4, 5, 6 and 7. The mean flexure strength results are presented in Table 3. All GIC products gained flexure strength between 1 and 24 h but increases with both Equia and Ketac Molar Quick were not significant. Fuji Triage, Ketac Molar Quick, Riva Self Cure Fast, Riva Self Cure HV, and Riva Silver attained highest flexure strength values at 1 week, while Chemfil Rock, Riva Protect, and Ketac Silver required 1 month to attain maximum flexure strength. These flexure strength values, despite some fluctuations, were essentially maintained for the remainder of the evaluation.
All GIC materials demonstrated modulus increase between 1 and 24 h (Table 4). Ketac Molar Quick, Ketac Silver, Riva Self Cure Fast, Riva Self Cure HV, and Riva Silver reached maximum modulus values at 1 week that was maintained at 12 months. Only Chemfil Rock and Riva Protect displayed a continual modulus improvement in which Chemfil Rock attained maximum modulus at 6 months, while Riva Protect demonstrated a slow modulus increase up to 12 months.
All GIC materials except for Equia, Ketac Molar Quick, and Riva Self Cure HV demonstrated a significant flexural toughness increase between 1 and 24 h (Table 5). Thereafter, material performance was individual achieving significant maximum toughness either at 1 week (Fuji Triage; Riva Self Cure Fast) or at 1 month (Chemfil Rock; Ketac Molar Quick; Ketac Silver). Interestingly, Equia displayed no toughness change beyond that observed at 1 h while the toughness of Riva Protect was stable after 24 h. All GIC materials demonstrated significant increase in hardness between 1 and 24 h (Table 6). However, Chemfil Rock, Equia, Riva Self Cure Fast, Riva Self Cure HV, and Riva Silver did not demonstrate any difference in hardness between the hardness established at 24 h and that observed at 12 months. Only Fuji Triage, Ketac Molar Quick, Ketac Silver, and Riva Protect manifested hardness increases beyond 24 h that was maintained at 12 months. Fracture toughness mean results are presented in Table 7. The fracture toughness of Chemfil Rock, Equia, Ketac Molar Quick, Riva SC Fast, and Riva Self Cure HV did not demonstrate any significant change over 12 months from that seen at 1 h. Fuji Triage, Ketac Silver, Riva Protect, and Riva Silver demonstrated increases in fracture toughness, with both Fuji Triage and Ketac Silver increasing in fracture toughness up to 12 months. Riva Protect achieved maximum fracture toughness at 1 week while Riva Silver fracture toughness reached maximum values at 6 months.
The RMGI restorative mean physical property results are displayed in Tables 8, 9, 10, 11, and 12. All RMGI materials demonstrated significant flexure strength increase between 1 and 24 h (Table 8). However, both Fuji II LC (after 24 h) and Ketac Nano (after 1 week) displayed progressive flexure strength decline over the 12-month evaluation. Both Riva LC and Riva LC HV acquired maximum flexure strength at 1 month that remained stable thereafter. Table 9 reveals that all RMGI materials exhibited significant modulus increase between 1 and 24 h, while Riva Self Cure HV demonstrated significant modulus improvement at 1 week. For flexural toughness (Table 10), both Fuji II LC and Ketac Nano mirrored the flexural modulus results with early maximum values that thereafter declined while Riva LC HV also demonstrated toughness diminution but only after 1 month. Only Riva LC demonstrated toughness increase that was significantly greatest at 6 months and stable afterward. When considering hardness (Table 11), both Fuji II LC and Ketac Nano displayed highest hardness values at 1 h. Subsequently, Fuji II LC subsequently displayed decay of hardness values while Ketac Nano remained stable. Riva LC increased hardness values up to 1 month while Riva LC HV reached its maximum stable hardness at 6 months. RMGI fracture toughness mean values can be seen in Table 12. The fracture toughness of both Fuji II LC and Ketac Nano were greatest at 1 h after which Fuji II LC exhibited declining fracture toughness while Ketac Nano remained stable after 24 h. The fracture toughness of Riva LC HV did not significantly change between 1 h and 12 months, while Riva LC demonstrated a significant increase between 1 and 24 h that was stable thereafter.
Discussion
This study encompassed the physical properties of nine GIC and four RMGI restorative products over a 12-month period. This study not only included products that have had lengthy market tenure but also included newer materials, some of which have not been previously reported in the scientific literature. Only precapsulated materials were chosen for this study in order to eliminate variables introduced by manual hand-mixing of glass ionomer materials [72, 73]. In addition to evaluating recently marketed materials, this study is somewhat novel in that 0.2 M phosphate-buffered saline (PBS) was used as a storage media. PBS represents a physiologic as well as a slightly basic environment that has been suggested to promote hydrolysis of some dental resins [74] as well as being the recommended storage media suggested by ISO 9917 [75]. Also, physical property evaluation started at 1 h after specimen preparation, as the authors maintain that this would represent perhaps a more realistic testing time at when restorative materials might be first subjected to oral forces.
Flexure testing encompasses both compressive and tensile force components and is considered a more discriminating and clinically relevant in vitro test [76, 77]. The GIC materials exhibited inferior flexure strength values at 1 h compared to that observed at 24 h at which time Chemfil Rock, Riva Silver, and Riva Self Cure fast demonstrated greater flexure strength than Ketac Molar Quick, Fuji Triage, and Riva Protect, with the other products being intermediary. Between 24 h and 1 week, physical property development significantly improved flexure strength for Fuji Triage, Ketac Molar Quick, Riva Self Cure Fast, Riva Self Cure HV, and Riva Silver. However, as after 24 h, Equia and Chemfil Rock flexure strength remained while up to 1 week the flexure strength of Fuji Triage, Ketac Molar Quick, Ketac Silver, Riva Self Cure Fast, and Riva Silver continued to increase, only Riva Protect, demonstrating further significant improvement to 1 month which subsequently stabilized. It could be reasonable to conjecture that the continued physical property development observed with these materials can be ascribed to matrix maturation. The flexure strength results in this study were similar to that reported by Hu et al. [78], but had higher values for Equia, Chemfil Rock, and Riva Self Cure than that from Zoergibel and Ilie [79], whose methodology differed from the present evaluation. Flexure strength values in this study for Riva Self Cure and Equia were similar to that reported by Bonifácio et al. [80] but lower for Ketac Molar Quick. Moreover, KetacMolar Quick findings were similar as that reported by Yamazaki et al. [81] while that of Ketac Molar and Ketac Silver were almost identical to that reported by Xie et al. [82]. A 1-h glass ionomer material physical properties study by Lucksanasombool et al. [83] reported flexural values higher than that found in this study, but those authors’ method varied from that accomplished in the present study.
RMGI products demonstrated a significant flexure strength increase between 1 and 24 h. Ketac Nano’s 12 month flexure strength, albeit some variation, was similar to that observed at 24 h. Fuji II LC’s flexure strength displayed a reduction after 1 month that stabilized while only Riva LC and Riva LC HV demonstrated increased flexure strength behavior that did not deteriorate. The flexure strength trend noted in this evaluation is similar to that reported by Azillah et al. [53] who likewise reported a decline in Fuji II LC flexure strength after 100 days. However, it must be noted that the data are not directly comparable, as Azillah and colleagues used four-point-bend testing methodology [53]. Furthermore, this study’s results are greater than reported by Xie et al. [84] and Weng et al. [85], who used higher loading rates. Moreover, this study’s RMGI flexure strength values are similar to that reported by Yamazaki et al. [81] and nearly identical to that reported for Fuji II LC by Xie et al. [82] as well as Culbertson [86]. The graphical flexure strength results for both GIC and RMGI products are available in Supplemental Figs. 1 and 2, respectively.
The GIC products mean modulus results graphically can be viewed in Supplemental Fig. 3. Under the conditions of this study, flexural modulus was determined from the linear portion of the flexure strength stress-strain curve. GIC products demonstrated significant modulus increase up to 1 and 3 months (Chemfil Rock 6 months) that overall remained stable. Although the group variance was small, results would probably not be of clinical significance. This modest modulus increase lends further support of a matrix maturation process [6]. The modulus results for Ketac Molar Quick, Riva Self Cure Fast, Riva Self Cure HV, and Riva Silver agrees with an observation made by Wren et al. [52]. Contrary to the flexural strength trends, RMGI materials demonstrated significant modulus increases (Supplemental Fig. 4). The only exception was Riva LC, of which 24-h modulus values remained stable thereafter. RMGI modulus development deserves additional thought. It is well established that the resin content in RMGI impedes the polyalkenoate reaction [35,36,37]. The resin presence distorts the polyalkenoate polymer chain that reduces available reaction sites as well as slowing the reaction due to less water content [35,36,37]. In an infrared spectroscopy study involving Fuji II LC, Young [38] reported that the resin free radical polymerization reaction was essentially complete 5 min after VLC exposure. Thereafter, the polyalkenoate acid-base reaction occurred largely supported by water absorption. Therefore, water uptake may compensate for the resin-influenced polyalkenoate reaction rate that may possibly compare to that observed with conventional GIC materials [38]. Also, using infrared spectroscopy, Wan et al. [50] found that the acid-base reaction in Fuji II LC continued for 96 h and thus RMGI water absorption appears to allow the acid-base reaction to occur longer compared with GIC materials, and may partially compensate for the inhibiting properties of the monomer system.
Flexural toughness is a seldom-reported feature in the dental scientific literature. Flexural toughness is determined by integrating the area under the total stress-strain curve, and toughness can be considered to represent a material’s ability to resist total catastrophic rupture. Hence, toughness may also reflect the material’s matrix organizational level. Toughness could be a function of a material’s flexural strength and modulus, as both affect both the length and slope of the stress-strain curve and consequently the area underneath. Curiously, besides some initial increase, the GIC materials did not largely experience great change in toughness over the course of the evaluation (Supplemental Fig. 6). Even with this level of change, under this study’s conditions, GIC flexure strength influenced toughness more than modulus for some of the materials (Supplemental Table 1). At 12 months, the flexural toughness values strongly correlated with the flexure strength of Chemfil Rock (r2 = 0.9), Equia (r2 = 0.83), and Riva SC HV (r2 = 0.85), while Fuji Triage and Riva Self Cure Fast displayed moderate correlation (r2 = 0.76 and 0.66, respectively). The RMGI flexural toughness values for Riva LC essentially mirrored flexural strength performance, largely demonstrating a significant toughness increase throughout the evaluation. However, Fuji II LC, Ketac Nano, and Riva LC HV exhibited toughness increases that were followed by progressive decline. Similar to GIC materials, both Fuji II LC and Ketac Nano flexure strength was found to exhibit a strong correlation (r2 = 0.84) with toughness (Supplemental Table 2). Review of the literature reveals little research reporting GIC and RMGI flexural toughness results, as this study may be the first to report 12-month results for these materials.
Surface hardness represents a material’s localized surface resistance to deformation, which has been suggested to be related to the underlying material matrix [48]. To minimize any RMGI potential surface microhardness irregularities due to the Polywave® LED light curing unit used [87], 11 Knoop hardness indentations spaced 0.5 mm apart in alternating fashion apart were made on each specimen. The mean of the 11 hardness values was then recorded as that specimen’s representative KHN value. This was accomplished for a total of 12 specimens. GIC materials demonstrated significant hardness increases compared to that observed at 1 h. Although some materials essentially did not display hardness increase beyond 24 h (Equia, Riva Protect, Riva Self Cure Fast, Riva Self Cure HV, and Riva Silver), other materials continued in surface hardness increase (Supplemental Fig. 7). Hardness results were lower for Chemfil Rock than that reported by Al-Angari et al. [88] but comparable for Equia and Ketac Molar Quick. Riva SC Fast hardness development mirrored that reported by Shiozawa et al. [51] while Ketac Silver hardness values were very similar to that reported by De Moor and Verbeeck [89]. The continued hardness development for Chemfil Rock, Fuji Triage, Ketac Molar Quick, and Ketac Silver provides further evidence of a maturation process of the hydrogel matrix.
Among the RMGI materials, Ketac Nano did not evidence any hardness changes beyond 1 h. Interestingly, Fuji II LC’s maximum hardness was observed at 1 h and thereafter displayed continued hardness deterioration below that observed initially. Riva LC developed maximum and stable hardness at 24 h, with only Riva LC HV manifested a slow but significant increase in Knoop hardness over the 12-month evaluation. As previously discussed, some RMGI products have been reported to exhibit a delayed acid-base reaction that may slowly progress up to 96 h after preparation [50, 82]. Although this reported delay could provide rationale for hardness improvement between 24 h and 1 week, but under the conditions of this study, no RMGI products demonstrated a hardness increase for this time period. Realistically, any improvement could be due to the continued hydrogel matrix maturation, but explanation for the late Riva LC HV hardness improvement is presently unclear. The only compositional difference between Riva LC HV and Riva LC is that Riva LC HV contains a higher powder/liquid ratio and may contain a slightly smaller amount of HEMA.
Fracture toughness has been described as an in vitro test that may correlate with clinical performance [77]. Fracture toughness is a material’s ability to resist crack propagation from a pre-existing flaw [90,91,92,93] with some correlation reported with Class IV restoration clinical performance [91] as well as resin composite marginal deterioration [92]. The graphical results of the fracture toughness testing for the GIC and RMGI products are shown in Supplemental Figs. 9 and 10, respectively. For the GIC materials, fracture toughness performance was material specific. Only Fuji Triage, Ketac Silver, Riva Protect, and Riva Silver displayed any significant increase in fracture toughness over the evaluation. However, low variance may render any significant results to be unlikely of clinical significance, and essentially GIC fracture toughness values changed very little during this evaluation. These results are somewhat perplexing, as the fracture toughness results did not follow the same trend evidenced by the other GIC physical property results. It may be noteworthy that the two cermets evaluated demonstrated higher fracture toughness values than the other GIC products. Perhaps the cermet microstructure metallic interfaces may afford additional crack deflection ability, but such is conjecture and remains to be seen. The limited GIC fracture toughness findings do seemingly reinforce a recent report by Baig et al. [92] that questions fracture toughness testing’s discriminatory value in GIC physical property evaluation. Nevertheless, fracture toughness results for Ketac Molar Quick were similar to that values reported by Yamazaki et al. [81]. Contrastingly, this study’s fracture toughness results were lower than that reported for Chemfil Rock, Equia, and Ketac Molar Quick by Al-Angari and colleagues [88], lower for Ketac Silver and Ketac Molar Quick reported by Ilie et al. [93], as well as the 1-h results reported for Fuji IX reported by Lucksanasombool et al. [83]. All these studies used different methodologies than the present study that could account for these differences. For RMGI materials, both Fuji II LC and Ketac Nano displayed significant decreases in fracture toughness after 1 h that somewhat stabilized for both materials for the remainder of the evaluation. The reason for this deminuation is presently unknown, but could possibly be due to irregularities between the resin and conventional polyalkenoate domains and/or matrix plasticization due to water absorption that has been reported for RMGI materials [94]. Riva LC HV demonstrated no fracture toughness changes from that observed at 1 h but Riva LC was the only material to demonstrate significantly greater fracture toughness development. Nonetheless, variance was small and any noted significant changes are unlikely to be of clinical relevance. RMGI fracture toughness reports are sparsely reported in the dental literature, but this study’s findings for Fuji II LC and Ketac Molar were similar to that reported by Yamazaki et al. [81], whereas lower for Fuji II LC, Ketac Molar, and Ketac Silver as reported by Ilie and colleagues [93].
The results of this study suggest that GIC restorative materials typically exhibited more physical property stability than some RMGI products. While global mean RMGI results may depict stability, in real contrast, some individual RMGI products at each observation time generally demonstrated significant declining mean values of flexural modulus and toughness, fracture toughness, and hardness. The deterioration of these RMGI physical properties have been attributed to water absorption due to the hydrophilic nature of the included resins [94,95,96]. Accordingly, Kanachanavasita et al. [94] reported that RMGI products absorbed water twice the amount compared to GIC materials. Moreover, these authors found that absorption exponentially increased during 30 days storage in artificial saliva, presumably due to RMGI matrix changes [94]. Small and colleagues [95] observed that RMGI materials displayed greater water absorption as compared to resin composites while Versluis et al. [96] noted that particular RMGI products displayed matrix expansion due to water absorption. Hence, water absorption with ensuing matrix plasticization may be considered as a contributor to RMGI physical property loss over time.
GIC and RMGI material global mean comparisons provide perspective between the two classes of products as well as identify general trends. Understandably, due to the marked disparity between the group sizes as well as the different nature between the two materials, statistical analysis was not pursued. The global flexure strength comparison is shown in Fig. 1. The RMGI restorative materials demonstrated higher flexure strength values than the GIC counterparts. Based on this singular comparison, a RMGI product might be the choice material for restorations exposed to functional forces. Realistically, as discussed earlier GIC performance reported by ART technique studies can attest their functional area suitability [57,58,59,60,61,62,63]. Moreover, GIC products largely demonstrated some increasing flexure strength trends, whereas RMGI products were noticed to display overall reduced flexure strength trends with time. In contrast to flexure strength, GIC products displayed higher modulus values than the RMGI products (Fig. 2). Yamazaki and colleagues [81] discussed that the RMGI resin matrix network’s viscoelastic nature could account for lower RMGI modulus values. Furthermore, the GIC products overall demonstrated increasing modulus trends which again may provide evidence supporting matrix network maturation [6, 7]. Interestingly, both GIC and RMGI materials demonstrated a parallel modulus decrease at 6 months, which might be conjectured to possible polyalkenoate network change at that time period.
When flexural toughness global results are reviewed (Fig. 3), GIC products demonstrate lower but stable flexural toughness. RMGI toughness was observed to decline with time but displayed increasing trends at 6 months. GIC products display greater hardness values as compared to the RMGI materials (Fig. 4) with the GIC products revealing a slight increasing trend up to 3 months. Lower RMGI hardness values have been attributed to the resin content’s ability to allow substantial creep under load with resultant recovery and stress relief [81]. Under the conditions of this study, the RMGI products’ hardness was stable after 1 week and did not demonstrate a hardness decline after 6 months as observed by Shiozawa et al. [51]. Figure 5 depicts that RMGI products globally demonstrate greater fracture toughness than the GIC restorative materials. The GIC brittle nature may account for this difference, whereas the RMGI viscoelastic nature may require more energy for crack propagation. In conclusion, global comparison overall depicts that RMGI products demonstrate higher flexure strength, flexural toughness, and fracture toughness than the GIC counterparts, and may allow conclusions that RMGI products could afford superior performance in functional areas. However, information is presented in this work that longitudinally GIC materials may exhibit more stability with selected physical properties and should also be considered with recent reports of GIC material longevity in adult functional environments [71, 97].
Conclusions
This study evaluated selected physical properties of nine GIC and four RMGI restorative materials over 12 months. Under the conditions of this study:
-
1.
GIC and RMGI physical property performance over time was material dependent;
-
2.
Polyalkenoate maturation processes are essentially complete by 24 h;
-
3.
Although differences in GIC physical properties were noted, the small magnitude of the divergences may render such to be unlikely of clinical significance;
-
4.
Modest increases in some GIC physical properties were noted especially flexural modulus and hardness, which lends support to reports of a maturing hydrogel matrix;
-
5.
Overall, GIC product physical properties were more stable than RMGI;
-
6.
A similar modulus reduction at 6 months for both RMGI and GIC produced may suggest a polyalkenoate matrix change; and
-
7.
Globally, RMGI products demonstrated higher values of flexure strength, flexural toughness, and fracture toughness than GIC materials.
References
Wilson AD, Kent BE (1971) The glass-ionomer cement, a new translucent dental filling material. J Appl Chem 21:313
Wilson AD, Batchelor RF (1967) Dental silicate cements I. The chemistry of erosion. J Dent Res 46:1078–1085
Wilson AD (1989) Developments in glass-ionomer cements. Int J Prosthodont 2:438–446
Nicholson JW (1998) Chemistry of glass-ionomer cements: a review. Biomaterials 19:485–494
Hatton PV, Brook IM (1992) Characterization of the ultrastructure of glass-ionomer (poly-alkenoate) cement. Br Dent J 173:275–277
Cattani-Lovente MA, Godin C, Meyer JM (1994) Mechanical behavior of glass ionomer cements affected by the long-term storage in water. Dent Mater 10:37–44
Matsuya S, Maeda T, Ohta M (1996) IR and NMR analyses of hardening and maturation of glass-ionomer cement. J Dent Res 75:1920–1927
Crisp S, Lewis BG, Wilson AD (1976) Characterization of glass-ionomer cements. 1. Long term hardness and compressive strength. J Dent 4:162–166
Sidhu SK, Nicholson JW (2016) A review of glass ionomer cements for clinical dentistry. J Funct Biomater 28. https://doi.org/10.3390/jfb7030016
Mitra SB (1991) Adhesion to dentin and physical properties of a light-cured glass-ionomer liner/base. J Dent Res 70:72–74
Baig MS, Fleming GJP (2015) Conventional glass-ionomer materials: a review of the developments in glass powder, polyacid liquid and the strategies of reinforcement. J Dent 42:897–912. https://doi.org/10.1016/j.jdent.2015.04.004
Khoroushi M, Keshani F (2013) A review of glass-ionomers: from conventional glass-ionomer to bioactive glass-ionomer. Dent Res J (Isfahan) 10:411–420
Baig MS, Dowling AH, Fleming GJ (2013) Hertzian indentation testing of glass-ionomer restoratives: a reliable and clinically relevant testing approach. J Dent 41:968–973. https://doi.org/10.1016/j.jdent.2013.04.004
Moshaverinia A, Roohpour N, Cheea WWL, Schricker SR (2012) A review of polyelectrolyte modifications in conventional glass-ionomer dental cements. J Mater Chem. https://doi.org/10.1039/c2jm14880c, www.rsc.org/materials
Prosser HJ, Powis DR, Brant PJ, Wilson AD (1984) Characterization of glass-ionomer cements. The physical properties of current materials. J Dent 12:231–240
McLean JW, Gasser O (1985) Glass cermet cements. Quintessence Int 16:333–343
Simmons JJ (1983) The miracle mixture: glass-ionomer and alloy powder. Tex Dent J 100:6–12
Williams JA, Billington RW, Pearson GJ (1992) The comparative strengths of commercial glass-ionomer cements with and without metal additions. Br Dent J 172:279–282
Wren AW, Kidari A, Cummins NM, Towler MR (2010) A spectroscopic investigation into the setting and mechanical properties of titanium containing glass polyalkenoate cements. J Mater Sci Mater Med 21:2355–2364. https://doi.org/10.1007/s10856-010-4089-2
Guggenberger R, May R, Stefan KP (1998) New trends in glass-ionomer chemistry. Biomaterials 19:479–483
Tüzüner T, Kuşgӧz A, Er K, Taşdemіr T, Buruk K, Kemer B (2011) Antibacterial activity and physical properties of conventional glass-ionomer cements containing chlorhexidine diacetate/cetrimide mixtures. J Esthet Restor Dent 23:46–56. https://doi.org/10.1111/j.1708-8240.2010.00385.x
Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C (2011) Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater 27:487–496. https://doi.org/10.1016/j.dental.2011.02.006
Boyd D, Towler MR, Watts S, Hill RG, Wren AW, Clarkin OM (2008) The role of Sr2+ on the structure and reactivity of SrO-CaO-ZnO-SiO2 ionomer glasses. J Mater Sci Mater Med 19:953–957
Kiri L, Boyd D (2015) Predicting composition-property relationships for glass ionomer cements: a multifactor central composite approach to material optimization. J Mech Behav Biomed Mater 46:285–291. https://doi.org/10.1016/j.jmbbm.2015.02.007
Sidhu SK, Watson TF (1995) Resin-modified glass ionomer materials. A status report for the American Journal of Dentistry. Am J Dent 8:59–67
Friedl KH, Powers JM, Hiller KA (1995) Influence of different factors on bond strength of hybrid ionomers. Oper Dent 20:74–80
Kakaboura A, Eliades G, Palaghias G (1996) An FTIR study on the setting mechanism of resin-modified glass ionomer restoratives. Dent Mater 12:173–178
Tay FR, Pashley EL, Huang C, Hashimoto M, Sano H, Smales RJ, Pashley DH (2001) The glass-ionomer phase in resin-based restorative materials. J Dent Res 80:1808–1812
Mitra SB, Lee CY, Bui HT, Tantbirojn D, Rusin RP (2009) Long-term adhesion and mechanism of bonding of a paste-liquid resin-modified glass-ionomer. Dent Mater 25:459–466. https://doi.org/10.1016/j.dental.2008.09.008
Fukuda R, Yoshida Y, Nakayama Y, Okazaki M, Inoue S, Sano H, Suzuki K, Shintani H, Meerbeek BV (2003) Bonding efficacy of polyalkenoic acids to hydroxyapatite, enamel, and dentin. Biomaterials 24:1861–1867
Yiu CK, Tay FR, King NM, Pashley DH, Sidhu SK, Neo JC et al (2004) Interaction of glass ionomer cements with moist dentin. J Dent Res 83:283–289
Yip HK, Tay FR, Ngo HC, Smales RJ, Pashley DH (2001) Bonding of contemporary glass ionomer cements to dentin. Dent Mater 17:456–470
Tay FR, Smales RJ, Ngo H, Wei SH, Pashley DH (2000) Effect of different conditioning protocols on adhesion of a GIC to dentin. J Adhes Dent 3:153–167
Hammesfahr PD Developments in resionomer systems. In: Hunt P (ed) Glass ionomers: The next generation. Proceedings of the 2nd International Symposium on Glass Ionomers, June 1994, Philadelphia, PA. International Symposia in Dentistry, PC, Philadelphia, pp 47–55
Berzins DW, Abey S, Costache MC, Wilkie CA, Roberts HW (2010) Resin-modified glass-ionomer setting reaction competition. J Dent Res 89:82–86. https://doi.org/10.1177/0022034509355919
Roberts HW, Berzins DC (2015) Early reaction kinetics of contemporary glass-ionomer restorative materials. J Adhes Dent 17:67–75. https://doi.org/10.3290/j.jad.a33526.
Yelamanchili Y, Darvell BW (2008) Network competition in a resin-modified glass-ionomer cement. Dent Mater 24:1065–1069. https://doi.org/10.1016/j.dental.2007.12.005
Young AM (2002) FTIR investigation of polymerization [sic] and polyacid neutralization kinetics in resin-modified glass-ionomer dental cements. Biomaterials 23:3289–3295
Young A, Sherpa G, Pearson B, Schottlander, Waters DN (2000) Use of Raman spectroscopy in the characterisation of the acid–base reaction in glass-ionomer cements. Biomaterials 21:1971–1979
Stamboulis A, Matsuya S, Hill RG, Law RV, Udoh K, Nakagawa M, Matsuya Y (2006) MAS-NMR spectroscopy studies in the setting reaction of glass ionomer cements. J Dent 34:574–581
Wasson EA, Nicholson JW (1993) Change in pH during setting of polyelectrolyte dental cements. J Dent 21:122–126
Griffin SG, Hill RG (1999) Influence of glass composition on the properties of glass polyalkenoate cements. Part I: influence of aluminium to silicon ratio. Biomaterials 20:1579–1586
Algera TJ, Kleverlaan CJ, Prahl-Andersen B, Feilzer AJ (2006) The influence of environmental conditions on the material properties of setting glass-ionomer cements. Dent Mater 22:852–856
Tay M, Braden M (1981) Dielectric properties of glass ionomer cements. J Dent Res 60:1311–1314
Watts C (1998) Analysis of reactions in glass-polyalkenoate/resin systems by dielectric impedance spectroscopy. Biomaterials 19:551–577
Babu TA, Ramesh KV, Sastry DL (2012) Studies on electrical and thermal properties of dental glass ionomer cement. J Biomed Sci Eng 5:638–638. https://doi.org/10.4236/jbise.2012.511078
ŠSantić A, Čalogović AM, Pavić L, Gladić J, Vučić Z, Lovrić D, Prskalo K, Janković B, Tarle Z, Moguš-Milanković A (2015) New insights into the setting processes of glass ionomer cements from analysis of dielectric properties. J Am Ceram Soc 98:3869–3876. https://doi.org/10.1111/jace.13830
Watts DC (1986) The development of surface hardness in visible light cured posterior composites. J Dent 14:169–174
Roberts HW, Berzins D (2014) Thermal analysis of contemporary glass-ionomer restorative materials. J Therm Anal Calorim 115:2099–2106. https://doi.org/10.1007/s10973-013-3428-1
Wan ACA, Yap AUJ, Hastings GW (1999) Acid-base complex reactions in resin-modified and conventional glass ionomer cements. J Biomed Mater Res 48:700–704. https://doi.org/10.1007/s00784-013-1074-4
Shiozawa M, Takahashi H, Iwasaki N (2014) Fluoride release and mechanical properties after 1-year water storage of recent restorative glass ionomer cements. Clin Oral Invest 18:1053–1060. https://doi.org/10.1007/s00784-013-1074-4
Wren AW, Coughlan A, Laffir FR, Towler MR (2013) Comparison of a SiO2-CaO-ZnO-SrO glass polyalkenoate cement to commercial dental materials: glass structure and physical properties. J Mater Sci Mater Med 24:271–280. https://doi.org/10.1007/s10856-012-4813-1
Azillah MA, Anstice HM, Pearson GJ (1998) Long-term flexural strength of three direct aesthetic restorative materials. J Dent 26:177–182
Wasson EA, Nicholson JW (1993) New aspects of the setting chemistry of glass-ionomer cements. J Dent Res 72:481–483
Dickey B, Price R, Boyd D (2016) Evidence of a complex species controlling the setting reaction of glass ionomer cements. Dent Mater 32:596–605. https://doi.org/10.1016/j.dental.2016.01.012
Zainuddin N, Karpukhina N, Hill RG, Law RV (2009) A long-term study on the setting reaction of glass ionomer cements by (27) Al MAS-NMR spectroscopy. Dent Mater 25:290–295. https://doi.org/10.1016/j.dental.2008.07.008
de Amorim RG, Leal SC, Mulder J, Creugers NHJ, Frencken JE (2014) Amalgam and ART restorations in children: a controlled clinical trial. Clin Oral Investig 18:117–124. https://doi.org/10.1007/s00784-013-0955-x
Cefaly DFG, Tapety CMC, Mondelli RFL, Lauris JRP, Phantumvanit P, Navarro MFL (2006) Three-year evaluation of the ART approach in class III and V restorations in permanent anterior teeth. Caries Res 40:389–392
da Mata C, Allen PF, McKenna G, Cronin C, O’Mahony D, Woods N (2015) Two-year survival of ART restorations placed in elderly patients: a randomized controlled clinical trial. J Dent 43:405–411. https://doi.org/10.1016/j.jdent.2015.01.003
Faccin ES, Ferreira SH, Kramer PF, Ardenghi TM, Feldens CA (2009) Clinical performance of ART restorations in primary teeth: a survival analysis. J Clin Pediatr Dent 33:295–298
Frencken JE (2010) The ART approach using glass-ionomers in relation to global oral health care. Dent Mater 26:1–6. https://doi.org/10.1016/j.dental.2009.08.013.
Frencken JE, van 't Hof MA, van Amerongen WE, Holmgren CJ (2004) Effectiveness of single-surface ART restorations in the permanent dentition: a meta-analysis. J Dent Res 83:120–123
Rutar J, Mcallan L, Tyas MJ (2002) Three-year clinical performance of glass ionomer cement in primary molars. Int J Paediatr Dent 12:146–147
Burrow MF, Tyas MJ (2007) Clinical evaluation of three adhesive systems for the restoration of non-carious cervical lesions. Oper Dent 32:11–15
Fagundes TC, Barata THE, Bresciani E, Santiago SL, Franco EB, Lauris JRP, Navarro MF (2014) Seven-year clinical performance of resin composite versus resin-modified glass ionomer restorations in noncarious cervical lesions. Oper Dent 39:578–587. https://doi.org/10.2341/13-054-C
McComb D, Erickson RL, Maxymiw WG, Wood RE (2002) A clinical comparison of glass ionomer, resin-modified glass ionomer and resin composite restorations in the treatment of cervical caries in xerostomic head and neck radiation patients. Oper Dent 27:430–437
Qvist V, Manscherb E, Teglers PT (2004) Resin-modified and conventional glass ionomer restorations in primary teeth: 8-year results. J Dent 32:285–294
van Dijken JWV, Pallesen U (2008) Long-term dentin retention of etch-and-rinse and self-etch adhesives and a resin-modified glass ionomer cement in non-carious cervical lesions. Dent Mater 24:915–922
Friedl K, Hiller KA, Friedl KH (2011) Clinical performance of a new glass ionomer based restoration system: a retrospective cohort study. Dent Mater 27:1031–1037. https://doi.org/10.1016/j.dental.2011.07.004
Mickenautsch S, Yengopal V, Leal SC, Oliveria LB, Bezerra AC, Bonecker M (2009) Absence of carious lesions of glass-ionomer and amalgam restorations: a meta-analysis. Eur J Paediatr Dent 10:41–46
Türkün LS, Kanik Ö (2016) A prospective six-year clinical study evaluating reinforced glass ionomer cements with resin coating on posterior teeth: Quo Vadis? Oper Dent 41:587–598. https://doi.org/10.2341/15-331-C
Billington R, Williams J, Pearson GJ (1990) Variation in powder/liquid ratio of a restorative glass-ionomer cement used in general dental practice. Br Dent J 168:164–167
Dowling AH, Fleming GJP (2009) Are encapsulated anterior glass-ionomer restoratives better than their hand-mixed equivalents? J Dent 37:133–140. https://doi.org/10.1016/j.jdent.2008.10.006
Gonzalez-Bonet A, Kaufman G, Yang Y, Wong C, Jackson A, Huyang G, Bowen R, Sun J (2015) Preparation of dental resins resistant to enzymatic and hydrolytic degradation in oral environments. Biomacromolecules 16:3381–3388. https://doi.org/10.1021/acs.biomac.5b01069
ISO 9917-2:2010. Water based cements–Part 2: resin-modified cements. International Organization for Standardization, Geneve
Prosser HJ, Powis DR, Brant P, Wilson AD (1984) Characterization of glass-ionomer cements. 7. The physical properties of current materials. J Dent 12:231–240
Ferracane JW (2013) Resin-based composite performance: are there some things we can’t predict? Dent Mater 29:51–58. https://doi.org/10.1016/j.dental.2012.06.013
Hu J, Du X, Huang C, Fu D, Ouyang X, Wang Y (2013) Antibacterial and physical properties of EGCG-containing glass ionomer cements. J Dent 41:927–934. https://doi.org/10.1016/j.jdent.2013.07.014
Zoergiebel J, Ilie N (2013) Evaluation of a conventional ionomer cement with new zinc formulation: effect of coating, aging and storage agents. Clin Oral Investig 17:619–626. https://doi.org/10.1007/s00784-012-0733-1
Bonifácio CC, Kleverlaan CJ, Raggio DP, Werner A, de Carvalho RCR, van Amerongen (2009) Physical-mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust Dent J 54:233–237
Yamazaki T, Schricker SR, Brantley WA, Culbertson BM, Johnston WJ (2005) Viscoelastic behavior and fracture toughness of six glass-ionomer cements. J Dent Res 96:266–272
Xie D, Brantley WA, Culbertson BM, Wang G (2000) Mechanical properties and microstructures of glass-ionomer cements. Dent Mater 16:129–138
Lucksanasombool P, Higgs WAJ, Higgs RJED, Swain MV (2002) Time dependence of the mechanical properties of GICs in simulated physiologic conditions. J Mater Sci Mater Med 13:745–750
Xie D, Yang Y, Zhao J, Park JG, Zhang JT (2007) A novel comonomer-free light-cured glass-ionomer cement for reduced cytotoxicity and enhanced mechanical strength. Dent Mater 23:994–1003
Weng Y, Howard L, Xie D (2014) A novel star-shaped poly (carboxylic acid) for resin-modified glass-ionomer restoratives. J Biomater Sci Polym Ed 25:1076–1090. https://doi.org/10.1080/09205063.2014.920169
Culbertson BM (2006) New polymeric materials for use in glass-ionomer cements. J Dent 34:556–565
Price RBT, Labrie D, Rueggeberg FA, Sullivan B, Kostylev I, Fahey J (2014) Correlation between the beam profile from a curing light and the microhardness of four resins. Dent Mater 30:1345–1357. https://doi.org/10.1016/j.dental.2014.10.001
Al-Angari SS, Hara AT, Chu T, Platt J, Eckert G, Cook NB (2014) Physicomechanical properties of a zinc-reinforced glass ionomer restorative material. J Oral Sci 56:11–16. https://doi.org/10.2334/josnusd.56.11
De Moor RJG, Verbeeck RMH (1998) Changes in surface hardness of conventional restorative glass ionomer cements. Biomaterials 19:2269–2275
Tyas MJ (1990) Correlation between fracture properties and clinical performance of composite resins in class IV cavities. Aust Dent J 35:46–49
Ferracane JL, Condon JR (1999) In vitro evaluation of the marginal degradation of dental composites under simulated occlusal loading. Dent Mater 15:262–267
Baig MS, Lloyd CH, Fleming GJP (2015) Fracture toughness testing: a discriminatory mechanical testing performance indicator for glass-ionomer restoratives? Dent Mater 31:877–886. https://doi.org/10.1016/j.dental.2015.04.014
Ilie N, Hickel R, Valceanu AS, Huth KC (2012) Fracture toughness of dental restorative materials. Clin Oral Investig 16:489–498. https://doi.org/10.1007/s00784-011-0525-z
Kanchanavasita W, Anstice HM, Pearson GJ (1997) Water sorption characteristics of resin-modified glass-ionomer cements. Biomaterials 18:343–349
Small ICB, Watson TF, Chadwick AV, Sidhu SK (1998) Water sorption in resin-modified glass-ionomer cements: an in vitro comparison with other materials. Biomaterials 19:545–550
Versluis A, Tantbirojn D, Lee MS, Tu LS, DeLong R (2011) Can hygroscopic expansion compensate polymerization shrinkage? Part I. Deformation of restored teeth. Dent Mater 27:126–133. https://doi.org/10.1016/j.dental.2010.09.007
Gurgan S, Kutuk ZB, Ergin E, Oztas SS, Cakir FY (2015) Four-year randomized clinical trial to evaluate the clinical performance of a glass ionomer restorative system. Oper Dent 40:134–143. https://doi.org/10.2341/13-239-C
Acknowledgments
The opinions offered in the work are those of the authors only and do not reflect the official opinion of the United States Air Force, Department of Defense, or the United States Government.
Funding
This work was supported by 81 Medical Group Protocol FKE20150010N.FI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Supplemental Figure 1
Header: GIC Mean Flexure Strength 12 Month Results (MPa). Footer: n = 12 (PNG 78 kb)
Supplemental Figure 2
Header: RMGI Mean Flexure Strength (MPa). Footer: n = 12 (PNG 169 kb)
Supplemental Figure 3
Header: GIC Mean Flexural Modulus (GPa). Footer: n = 12 (PNG 218 kb)
Supplemental Figure 4
Header: RMGI Mean Flexural Modulus (GPa). Footer: n = 12 (PNG 180 kb)
Supplemental Figure 5
Header: GIC Mean Flexural Toughness (mJ/mm3). Footer: n = 12 (JPG 566 kb)
Supplemental Figure 6
Header: RMGI Mean Flexural Toughness (mJ/mm3). Footer: n = 12 (JPG 489 kb)
Supplemental Figure 7
Header: GIC Mean Hardness Results (KHN). Footer: n = 12 (PNG 72 kb)
Supplemental Figure 8
Header: RMGI Mean Hardness Results (KHN). Footer: n = 12 (PNG 148 kb)
Supplemental Figure 9
Header: GIC Mean Fracture Toughness (MPa √m). Footer: n = 12 (JPG 490 kb)
Supplemental Figure 10
Header: RMGI Mean Fracture Toughness (MPa √m). Footer: n = 12 (JPG 451 kb)
Supplemental Table 1
(DOCX 22 kb)
Supplemental Table 2
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Moberg, M., Brewster, J., Nicholson, J. et al. Physical property investigation of contemporary glass ionomer and resin-modified glass ionomer restorative materials. Clin Oral Invest 23, 1295–1308 (2019). https://doi.org/10.1007/s00784-018-2554-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2554-3