Abstract
This chapter outlines the physical and chemical properties of glass-ionomer (GIC) and resin-modified glass-ionomer cements. The latter part proceeds to summarise various aspects of their clinical performance.
It is noted that these materials are brittle in nature when fully matured or set. Glass-ionomer cements, due to the process of the setting reaction, reach their full strength about 24 h after the initial mixing. The resin-modified materials have an additional hydrophilic resin included that improves early strength and aesthetics but importantly reduces the initial sensitivity to water, allowing early finishing shortly after placement.
Application of a resin coating on the surface of GICs has shown some improvement in the fracture strength, but seems to be material dependent based on current evidence. The improvement in strength is thought to be due to the resin-filling surface defects and cracks where fracture may be initiated. Not all materials or studies have shown consistent outcomes for this coating method. There is limited evidence to suggest that the wear resistance may also be enhanced with the resin coating.
Ion release is also described in this chapter. This part shows that the initial release of ions, in particular fluoride, is high but tapers off to steady low-level release. The clinical benefits are still not well understood.
The latter part of the chapter summarises various aspects of the clinical performance of GICs. Studies of retention in non-carious cervical lesions are described, as well as recent work using the atraumatic restorative treatment (ART) technique. The last part outlines results from fissure sealant studies that tend to show poor retention of GIC sealants. However, even though retention may be limited, it appears that GICs can afford some long-lasting anticariogenic effects to the fissure system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glass-ionomer cement
- Fracture toughness
- Flexural strength
- Ion release
- Physical properties
- Clinical evaluation
- Fissure sealant
2.1 Introduction
As we move away from the age of silver amalgam restorations, the need for durable and biologically compatible long-lasting materials is becoming necessary. Never before has the situation been more important to have a dental material that can bond reliably to tooth structure, can potentially reduce biofilm formation and can also inhibit dental diseases as well as protect the tooth. The broad group of glass polyalkenoate (ionomer) cements is showing signs of being able to fulfil many of these qualities. In view of their properties and ease of use, they can be developed even further to become an increasingly useful group of materials to assist with overcoming the problems of dental disease management in such groups as paediatric, special-needs and elderly patients as well as routine caries management.
2.2 Composition and Classification
Glass-ionomer cements (GICs) are termed polyelectrolyte cements. The concept was first introduced in 1962 with the development of the zinc polycarboxylate cements made from a mixture of zinc oxide and polyacrylic acid. Polyacrylic acid was chosen as it was known to complex with calcium and potentially form hydrogen bonds with collagen (Smith 1998).
Glass-ionomer cement can be regarded as a composite material. Essentially, GICs are made up of a cross-linked polyacid matrix in which the fillers are the glass particles in the cement. In the resin-modified version of GIC (RM-GIC), the matrix also contains a polymer network of resinous materials throughout the set cement. The detailed differences will be described later in this chapter. The major categories in GICs are essentially the ‘conventional’ and ‘resin-modified’ GICs; the only difference is that the latter contains a polymerisable resin. It is generally accepted that RM-GICs have a higher toughness and better aesthetics than conventional materials (Xu and Burgess 2003). In addition, it appears that the greater resin content gives rise to a higher Weibull modulus compared with conventional GICs with regard to strength (McCabe 1998). Traditionally, GICs have been classified based on the publication of Wilson and McLean (1988). A modified version of the classification is outlined below, demonstrating the diversity of the GICs; hence, nowadays they can be better regarded as a ‘group’ of materials. In broad terms, the original classification is centred on the viscosity of the material and therefore its clinical uses. In 2009, Mount et al. proposed a revised classification of direct tooth-coloured restorative materials. This revision was brought about by the changes occurring in tooth-coloured filling materials where manufacturers blurred the boundaries between RM-GICs and resin composites due to further modifications of the materials. This revision was centred more towards how the setting cements reacted with tooth tissues. An emphasis was placed on the fact that glass-ionomer-based materials should have a distinct acid–base setting reaction that also incorporates an ion-exchange reaction with the underlying tooth structure. If this was not evident, then such materials should, ideally, not be called glass-ionomer cements even though the glass fillers may be almost identical to those found in glass-ionomer cements (Mount et al. 2009).
The generally accepted classification for GICs is outlined below, and it still relates closely to the viscosity of the unset cement.
2.2.1 Type 1: Luting and Bonding (RM-GIC Adhesive) Materials
These materials are used for the cementation of indirect restorations including crowns, bridges and orthodontic brackets. They are delivered as either conventional or resin-modified materials. The resin-modified materials can set with or without light polymerisation. These materials are either delivered as separate powder and liquid systems, encapsulated materials or nowadays even a paste/paste system (only the resin-modified materials). The luting cements are able to achieve a good thin film thickness in the order of 20 μm. The powder/liquid ratio can be in the order of 1.7:1 or increased to as much as 3.8:1 when the acid has been dehydrated to a powder form.
The other material in this group can be termed a RM-GIC adhesive. This material has only been available since the mid-1990s and has shown to be a useful alternative for bonding resin composite to tooth structure instead of with resin-based adhesives (Burrow and Tyas 1998). Only a couple of these adhesives are commercially available (Fuji Bond LC, GC Corporation, Japan; Riva Bond LC, SDI Ltd, Australia). Delivery can now be in the form of a hand-mixed powder and liquid or encapsulated. The mixed material is applied to the tooth as a thin layer using a micro-brush, similar to a resin-based adhesive.
2.2.2 Type 2: Restorative Materials
This group was originally classified into ‘aesthetic’ and ‘nonaesthetic’ materials and either conventional or resin-modified. More recently, many of these materials could be classified as being only Type 2, with the exception of the so-called reinforced materials that contain silver and are not tooth-coloured. The aesthetic and nonaesthetic subgroups are now almost eliminated,
The powder/liquid (P:L) ratio varies slightly amongst the currently available materials ranging from about 3.1:1 up to 3.6:1. The capsulated materials tend to have a higher powder/liquid ratio. The P:L ratio shows little variation whether the GIC is a conventional or resin-modified material.
The older classification included a second subgroup of a higher P:L ratio. These cements were stronger, set faster and could be trimmed and polished immediately after setting. It could be argued that some of the current GICs should still be placed in this subgroup. However, there are now other materials that set quickly and can be finished early and have good aesthetics but also a higher viscosity. Hence, the divisions of this classification have become less well defined. The lower P:L ratio conventional GICs have all but been replaced by the high-viscosity materials or by RM-GICs which provide the best aesthetics and acceptable strength. All of the restorative cements are radiopaque.
2.2.3 Type 3: Lining or Base Cements
These are either conventional or resin-modified and are either auto- or light-cured. More recent materials are presented in a paste/paste form for easier dispensing and application. These paste/paste materials are usually light-cured and resin-modified.
The original powder/liquid materials still exist and are widely available. These lining/base cements are most often used as a thin layer beneath restorations and serve as a thermal insulator or dentine replacement. However, the recent trend for dentine replacement is to use a restorative material (Type 2) in larger cavities due to their greater strength in association with the fast set allowing the restoration to be completed quickly.
Exceptions to the above materials now exist. Fissure sealant cements are usually low-viscosity, fast-setting and are typically conventional auto-cure materials. One manufacturer produces a high fluoride-releasing conventional GIC (Fuji VII, GC Corp, Japan) recommended for what has been termed as ‘fissure protection’ and has been reported in several research papers (Ganesh and Tandon 2006; Chen and Liu 2013). It has a powder/liquid ratio of 2:1. This GIC has also been recommended for use as a base due to its low viscosity and relatively high strength. Its initial introduction was aimed at stabilising caries lesions in patients with high risk of caries, i.e. as a ‘temporary’ cement.
A number of the newer materials that are claimed to be RM-GICs tend to have a very high resin content. Hence, the debate continues on what constitutes a true GIC, which led to the Mount et al. (2009) paper. The general consensus is that the mixed cement must be able to set in a dark environment to demonstrate the existence of an acid–base reaction which forms the matrix of the set cement. Those that do not meet this criterion are more like a resin composite with GIC glass particles and probably a little different for a polyacid-modified resin composite (PAMRC/Compomer).
Another variation of RM-GICs has been the modification and incorporation of different filler particles. This came about after the successful application of using very small nanofillers in resin composite materials. In the case of resin composite materials, the use of nanotechnology was able to improve aesthetic outcomes without affecting the physical properties. This same concept was applied to an RM-GIC produced by 3M-ESPE, St Paul, MN, USA. It has been marketed as Ketac Nano or Ketac N-100 (Falsafi et al. 2014). This material has also been referred to as a ‘nano-ionomer’. It still contains the fluoroaluminosilicate glass found in all GICs, with the addition of nanofillers which are not associated with the GIC setting reaction, but which have been coated and bond to the resin component of the cement. Debate continues whether this material is a ‘true GIC’ since it would seem that there is no typical acid–base reaction occurring. This material is delivered as a paste/paste system and relies on light polymerisation for setting to occur. However, it is claimed that there is also polycarboxylic acid copolymer present to contribute to the acid–base reaction with the fine aluminosilicate glass particles (Falsafi et al. 2014).
Another classification of GICs has also been used. This simplifies the materials into either conventional GICs or RM-GICs – the latter containing resin.
Within the conventional GICs, there are subgroups of the older materials that contain less reactive and larger glass particles and the newer more viscous and quicker setting cements that have more highly reactive and smaller glass particles. This latter group can be used as base or restorative materials and has a shorter setting time and increased strength. Some are now even being promoted for load-bearing restorations; however, the evidence still remains limited for other than small restorations.
Within the conventional GIC grouping are those materials that have been modified by the addition of a metal, typically silver. These materials can also be called admix GICs. Possibly, the most widely known material in this group is Ketac Silver (3M-ESPE, USA). This material is a ‘cermet’ where the silver and glass have been sintered together during the manufacturing process and then incorporated into the GIC powder. Other materials tend to have the silver separate from the fluoroaluminosilicate glass. They are not aesthetic materials, but have the advantage of increased wear resistance, but all other properties are a little different from other older conventional GICs. Typically, these GICs were used for posterior teeth or cores beneath crowns where there was sufficient tooth structure to support the GIC. With the advent of the high powder/liquid ratio viscous GICs that have improved strength and wear, the metal-modified materials are slowly being relegated to becoming a historical material.
The RM-GICs have tended not to vary greatly and have typically been the material of choice where aesthetics is important. Recently there has been the introduction of a more viscous, higher powder/liquid ratio RM-GIC, Fuji VIII (GC Corp, Tokyo, Japan), which chemically cures without the need of photoactivation. This latter material is regarded as an alternative to the high-viscosity conventional GICs where a slightly less soluble material is useful, e.g. in deep proximal box restorations.
2.3 Method of Delivery
The original GICs were delivered or dispensed as a separate powder and liquid. A scoop of specific volume and a bottle with a tip designed to dispense the correct drop size of liquid are still available in many parts of the world for both conventional GICs and RM-GICs (Fig. 2.1). The major disadvantages of this method is the potential variation that can occur if the powder is either packed too firmly into the scoop or if it too much air is incorporated due to shaking the bottle prior to dispensing. This will then give a powder/liquid ratio that is not ideal, thus leading to less than ideal handling and physical properties.
Due to the inconsistency of mix caused by dispensing the powder and liquid separately, the effect of varying mixing time and environmental influences such as ambient temperature and relative humidity, manufacturers developed capsulated GICs for both conventional and resin-modified materials. This provided practitioners with a high level of consistency of mix, the ideal viscosity for insertion into cavities and best physical and aesthetic properties and reduced the effects of temperature and humidity. Typically, each manufacturer has developed its own capsule design (Figs. 2.2 and 2.3), but essentially the method of use is very similar, i.e. activation of the capsule followed by mechanical mixing. The one great advantage of capsule use is the ease of inserting the viscous cement into cavity preparations in almost any part of the oral cavity.
Three different types of GICs delivered in capsule form: (a) a resin-modified GIC, (b) a silver-reinforced GIC and (c) a resin-modified GIC adhesive. Note that the last material is much more fluid and is applied to the tooth surface as a thin film to bond resin composite to the tooth surface. The material is mixed in an amalgamator which ensures a good consistency due to the manufacturer-controlled powder/liquid ratio
More recently, there has been a further development by manufacturers to develop paste/paste systems. This is especially useful for those materials that are used as a thin lining over a cavity surface. The GIC is usually a RM-GIC but can be light-cured or chemically (self-)cured. By having a paste/paste system, again the best physical properties can be attained as well as being able to be dispensed in small quantities. Each manufacturer has developed its own system with some of those materials that were originally dispensed as a powder and liquid being modified to a paste/paste system, making it simpler to mix and use (Figs. 2.4 and 2.5).
2.3.1 Setting Reaction
The setting of GICs is via the attack of the glass filler particles by the acid liquid. Surface dissolution of glass particles releases metal ions such as calcium, strontium and aluminium into the newly created matrix which is formed by cross-linking with the polyacid (Cook 1983). This setting reaction is dependent on the component parts of the powder and liquid.
The filler portion is made up of a fluoroaluminosilicate glass which can range from 40 to 75 % by weight in the cement mix. The proportion of filler relates to the qualities required for the cement, for example, low-viscosity luting cements or fissure protection materials have less powder compared with high-strength and high-viscosity cements used as restorative materials that are likely to bear occlusal loading (Frankenberger et al. 1997). The set cement becomes a composite comprising unreacted glass fillers which are surrounded by a siliceous gel which is embedded in a matrix made up of the polyacid salt that is responsible for holding the set cement together (Watson et al. 2014). The component parts of the cement are described in more detail below.
2.3.1.1 Glass
The glass particles in GICs are more reactive than those found in resin composite materials. This has been achieved by incorporating fluorine into the glass. The original glass was based on the composition of SiO2–AlO3–AlPO4–NaAlF6 (Smith 1998). The early work by Wilson and McLean (1988) showed a ratio of 1:2 (or more) of Al2O3/SiO2, and fluoride of up to 23 % was required for a viable GIC.
The original work when developing GICs was to use a glass made of calcium, fluorine, aluminium, silicon and oxygen. Further developments substituted the calcium with strontium and more recently zinc. Essentially, all GIC glasses have been based on a similar formula of calcium or strontium fluoroaluminosilicate glass (Shin-ichi et al. 2000; Zimehl and Hannig 2000; Nicholson 1998). The cements also contain other ions such as sodium, phosphorus, lanthanum, barium, boron and zinc. Strontium has been used to replace calcium and lanthanum to partially replace the aluminium, which gives the cement greater radiopacity. The composition of glasses has been extensively investigated but is beyond the scope of this book (De Barra and Hill 1998, 2000; Griffin and Hill 1999, 2000a, b). It would seem, however, that the Al:Si ratio is important to the glass composition, and this may influence the fluoride release (Akinmade and Nicholson 1994; Griffin and Hill 1999).
The glass in glass-ionomer has an amorphous structure in which microcrystalline structures seem to be present. The work by Schwieger et al. (2000)) using a model of the GIC setting reaction showed that the CaF2 phase seemed to be preferentially leached to establish a silicon-rich surface on the glass which was greater than the size of the glass particles.
The inclusion of fluorine in GICs was not originally to impart an anticariogenic effect, but to aid the setting reaction of the GIC. It acts as a network disrupter allowing ion release necessary for the setting reaction. The final cement is formed by the cross-linking of the polyalkenoate matrix with strontium, calcium, aluminium and lanthanum ions. Silicon tends to remain nonreactive in the GIC reaction; it gives strength and stability to the set cement.
A recent paper showed that when the particle size of the glass is reduced, the reactivity of the particles is greatly increased with the same glass composition (De Caluwé et al. 2014). This also tends to decrease the setting time when ‘nanogranular’ particles replace the ‘macrogranular particles’. Additional fluoride in the glass tends to decrease the setting time (De Caluwé et al 2014). The compressive strength increases as more nanogranular particles containing more fluoride are used.
2.3.1.2 Liquid
The other important component of the GIC is the liquid. The liquid is a polymeric acid having a carboxylate group(s), but it must be a lower molecular weight to prevent gelation. There are a broad range of acids that can be used, and each will provide some (Smith 1998) variation in the potential application of the set GIC (Mount 1990). The liquid can also contain water or tartaric acid. In addition, by increasing the number of carboxyl groups into the polymer chains, it also helps prevent gelation during setting, which imparts greater reactivity due to a greater number of carboxyl groups. This may also lead to increased cross-linking, thus enhancing the properties of the cement such as strength (Smith 1998). It appeared that larger-molecular-weight acids seemed to improve the overall physical strength of the cement (compressive strength, fracture toughness, etc.) (Fennel and Hill 2001a, b, c). In general, however, to achieve the ideal properties of the cement, some compromise must be made to the concentration of the liquid, which is usually limited to 50 % w/w.
The role of tartaric acid was investigated as the GICs were just starting to emerge as a clinically useful material (Wilson et al. 1976; Crisp and Wilson 1976). The inclusion of tartaric acid was shown to initially reduce the viscosity of the cement then rapidly increase it, almost leading to a ‘snap’ set (Prosser et al. 1982a, b). It seems that this action is due to the chelation of ions from the glass powder over the short term, which delays the formation of the gel stage of the cement. This leads to faster cross-linking of the polyacrylic acid component (Nicholson et al. 1988); hence, most GICs contain tartaric acid to improve the working time but reduce the setting time (Young et al. 2000).
It has also been shown that water is an important component in the setting reaction of GICs. It seems that the primary role of the water is to influence the acid–base reaction. The carboxylate groups dissociate allowing them to become active for ion transfer, and the water provides the medium for ion movement to the glass powder surface (Prentice 2005).
During the setting of the GIC, the amount of bound water in the matrix also increases. The matrix contains a degree of unbound water, hence the necessity for avoiding dehydration or desiccation (Small et al. 1998). This water transfer affords GICs the advantage of movement of ions (such as fluoride ions) that may reduce the damage caused in early caries attack. There is also some evidence indicating that ion uptake may occur from saliva leading to a surface hardening of the set cement surface (Okada et al. 2001).
2.3.2 Strength
Set glass-ionomer cements tend to be brittle materials. The final strength can be affected by a number of variables. The inclusion of resin into the cement seems to show improved strength but also tends to make the cement slightly less brittle in nature. This brittleness is one of the major reasons for GICs not being well suited for larger posterior load-bearing restorations.
During setting, GICs are sensitive to both moisture loss and uptake. Loss of water leads to dehydration of the cement that causes subsequent surface crazing and increased opacity. The consequences can thus be a weaker cement, a decrease in wear resistance and a loss of aesthetics. Recently, the high powder/liquid (P:L) ratio cements have shown some improvement in water sensitivity. These cements need to be protected during the 2 to 7 min of the initial set, depending on the product and whether it has been classified as a ‘fast’ or ‘normal’ set material. After this initial set, the high P:L ratio materials (e.g. Fuji IX GP, Fuji IX GP Fast, Ketac Molar, Ketac Molar Quick, Riva Self Cure Regular/Fast) can be trimmed under water spray without loss of strength or aesthetics. For the original conventional GICs, it was necessary to coat the cement with a waterproof material to prevent water uptake and weakening of the setting cement for 24 h. It would seem that over time, the physical properties of conventional GICs tend to improve. The recent work by Shiozawa et al (2014) showed that five different GICs all tended to show an initial increase in compressive strength and surface hardness over the first 1–3 months of storage in deionised water. They then remained reasonably constant although slight decreases were observed (Table 2.1); this was product dependent. They showed that the surface quantities of Si, Sr, Na and F decreased over the period of 1 year. This demonstrated that there was some decrease in the surface integrity and hardness of the cements due to storage in the water. The decrease in surface hardness is contrary to that found by Okada et al (2001), who stored their samples in saliva and distilled water and used a time period limited to 40 days. This is equivalent to the early times noted for the increase in strength and hardness in Shiozawa et al.’s (2014) study. It can be useful to further assess the phenomenon of hardness or microhardness as it may have clinical implications for restorations subjected to occlusal loading. It can give some indication of the overall strength of the cement as well as surface changes when exposed to the various fluids GICs come in contact with during function.
Interestingly, one study examined Knoop hardness of a high-viscosity GIC that had been harvested from 10-year-old restorations (Zanata et al. 2011). The hardness was compared with the same material stored in water for up to 720 days. The outcomes for this study showed a similar increase in hardness which was no different from the laboratory-stored samples. The 10-year-old samples were similar in hardness to the water-stored specimens after 180 days. Energy dispersive X-ray diffraction (EDX) analysis showed that Ca was present in the cement which seemed to indicate that Ca from the oral cavity was able to diffuse into the cement.
The introduction of RM-GICs helped to overcome some of the initial dehydration problems of the conventional GICs. However, once set, the RM-GICs also show moisture sensitivity. If allowed to dehydrate, the surface crazes and becomes opaque. Hence, it is critical to maintain the water balance within the matrix of all GICs to ensure that the maximum strength possible is ensured as well as the best aesthetics.
One of the commonly mistaken concepts is that GICs do not shrink (Kim and Hirano 1999; Bryant and Mahler 2007). Both conventional GIC and RM-GIC shrink during setting. The shrinkage from the acid–base reaction portion of the set is slower, but not necessarily less than the shrinkage of the resin portion. In RM-GICs, shrinkage occurs more rapidly during the light-curing phase (Cheetham et al. 2014). In this case, the shrinkage from the acid–base component is minimal.
When it comes to the stress of the bond to the tooth, it is likely that GICs can resist some of these forces better when a resin composite restoration is placed. The cement goes through a rubbery gel stage during its set. This may assist with countering some of the stresses occurring from a light-polymerising resin composite which can be rapid and high depending on the type of composite used.
2.3.3 Fracture Toughness
As GICs are regarded as brittle and failure is related to material fracture, some researchers have focused on the fracture toughness of these and other tooth-coloured filling materials. This approach helps to characterise fracture resistance and provides some indication as to how much energy of loading is required to cause a material to fail. Several other studies have looked at this characteristic. In an approach to improve fracture toughness of GICs, resin coatings have been applied to the surface of GICs. Both conventional and resin-modified materials have been tested. It was previously shown that the application of a resin coating on the resin-modified materials generally showed an increase in toughness (Mitchell et al. 1999; Mitsuhashi et al. 2003; Ilie et al. 2012). In comparison with resin composite materials, several studies have shown that the conventional materials have the lowest fracture toughness, whereas the RM-GICs have ‘comparable toughness’ to the microfilled, flowable and nano-hybrid resin composites (Ilie et al. 2012) (Table 2.2).
Mitsuhashi et al. (2003) showed that there was a high correlation between the P:L ratio of conventional GICs and fracture toughness, whereas this pattern was not observed for RM-GICs. It seems that the resin component is able to increase toughness and perhaps fill in spaces where a crack may propagate more easily. One of the problems for fracture toughness measurement and comparison amongst research groups, however, is the test methodology used. The test method can lead to different outcomes as seen in Table 2.2.
2.3.4 Shear Punch Strength
Shear punch strength is another test method that has been used for comparison of materials and evaluation of coatings of GICs. This method is quite simple and gives some indication of how a cement behaves when it is loaded during function (Nomoto et al. 2001). Although not widely used, it has shown some interesting outcomes. The first shear punch strength evaluation was published in 1996 (Mount et al. 1996). This comprehensive study included conventional GIC and RM-GIC as well as a number of resin composite materials. The study was interesting in that it investigated 2-h strengths compared with 5-day strengths. They showed that for the conventional GICs, the strength showed a significant increase from the 2-h test to that of 5 days. In the case of the RM-GICs, which could be either light- or auto-cured, again it was noted that the strength increased significantly over the 5 days. If allowed to only auto-cure (i.e. no light exposure), the strength tended to be less at 5 days; hence, it would seem the light-curing aspect to curing is an essential step to achieve maximum strength. In fact, the Photac-Fil material (3M-ESPE, St Paul, MN, USA) tested at 5 days showed a lower strength than the 2-h light-cured strength. For the resin composite materials, the strength tended to increase by about 5–10 %, depending on the material, over the 5 days (Mount et al. 1996). This study was conducted prior to the introduction of the high-viscosity/high P:L ratio cements. Two later studies compared the strengths of GICs with and without resin coating (Bonifácio et al. 2012; Bagheri et al. 2013). These studies showed that in some cases, the coating was beneficial, whereas for other materials, there was little change. The interesting outcome from the study by Bagheri et al. (2013), where strengths were tested at 24 h, 4 and 8 weeks, was that irrespective of resin coating, the shear punch strengths were all observed to steadily increase over the 8 weeks of the study. In most materials, the shear punch strengths almost doubled. The effect of the coating seemed to be greater at the longer time periods after the cement had matured.
The influence of food-simulating solutions on the shear punch strength has also been evaluated (Bagheri et al. 2007; Kaur and Nandlal 2013). The solutions used were lactic acid, NaOH and coffee in one study (Bagheri et al. 2007) and citric acid, ethanol and heptane in another (Kaur and Nandlal 2013). In the first study, a RM-GIC was compared with other resin-based restorative materials, whilst in the latter study, a high-viscosity conventional GIC was assessed. The RM-GIC was shown to be strongly affected by the food-simulating solutions compared with the other resin-based materials. The same effect was also noted for the conventional GIC. It would therefore seem that GICs are more susceptible to the influence of various dietary solutions of varying pH, and this may result in some surface deterioration and weakening during clinical service.
Due to the brittle nature of GICs and their early sensitivity to water, an additional step is needed to protect the materials from water exposure in order to achieve their ‘highest’ strength. It was noted that the original cements had a soft ‘opaque’ surface layer if exposed to water too early; this was easily abraded and was quite unaesthetic (Norman et al. 1969). Other reports suggest that the newer high-strength conventional GICs may benefit from early water exposure (Leirskar et al. 2003; Wang et al. 2006).
2.3.5 Erosion
One of the important properties any restorative material must have is the ability to resist degradation from exposure to various fluids that will contact the set material in the oral cavity. The oral environment is very harsh, with restorative materials being exposed to a wide variation of temperatures and changes in acidity and alkalinity. In recent years, the loss of tooth structure due to erosive or acidic materials has become a significant issue (Kitasako et al. 2015). The matrix of the GIC is the most susceptible part of the set cement when exposed to acids. Acids such as acetic, citric and lactic have all been used to evaluate erosion (Crisp et al. 1980; Matsuya et al. 1984; Fukuzawa et al. 1987). One of the important aspects for preventing the effects of erosion is when laminate or sandwich restorations, which fill the gingival portion of deep posterior approximal restorations, are placed (van Dijken et al. 1999). The study by Scholtanus and Huysmans (2007) showed the erosive degradation of approximal lesions restored with a GIC.
As noted above, the conventional GICs are sensitive to water exposure shortly after placement. The water will damage and erode the surface of the GIC if it is left unprotected on insertion (Oilo 1984; Gemalmaz et al. 1998).
In the case of sandwich/laminate restorations, if a patient’s oral hygiene is not adequate, then the biofilm may produce acid which can damage the surface of the set cement. This problem is exacerbated when the quantity of saliva is compromised and does not wash the acids away or have adequate buffering capacity. It has been shown (Nicholson et al 2000), however, that when GICs are exposed to lactic acid, the surrounding pH decreases initially, but as the GIC dissolves, the pH increases. Hence, GICs seem to exhibit a ‘side effect’ of being able to reduce the effects of acid attack by their own dissolution (Nicholson et al 2000). The Scholtanus and Huysmans (2007) study showed that this dissolution of conventional GIC has the beneficial effect of preventing caries initiation on susceptible adjacent tooth surfaces. It is most likely due to the constant exposure of a ‘fresh’ GIC surface that is able to release ‘maximum’ levels of fluoride ions into the surrounding environment and tooth structure. When erosion does occur, it is the matrix of the set cement that is most susceptible to damage (De Moor and Verveeck 1998; Patel et al. 2000).
RM-GICs are also susceptible to erosion. Water has been demonstrated to have an erosive effect on the surface of RM-GICs (Cattani-Lorente et al. 1999; Fano et al. 2004). It would appear that the amount of light irradiation, i.e. the duration of light curing, may have an influence on the degree of erosion of RM-GICs (Fano et al. 2004). They showed that an exposure time of less than 15 s resulted in cracking of the cement and a greater degree of erosion. The same study showed that the pH of the immersion solution also influenced the degree of erosion, which was also noted in the study by Czarnecka and Nicholson (2006). However, like the conventional GICs, when the degree of erosion does increase, the release of fluoride also increases (Carey et al. 2003), thus affording some benefit to assist with controlling demineralisation around cavity margins and adjacent teeth.
A recent study using pH cycling over a 35-day period using a cola drink and artificial saliva showed that both the conventional GIC and RM-GIC displayed greater amounts of erosion compared with amalgam and resin composite (Honório et al. 2008).
A study investigated the effects of erosion of a resin composite, conventional GIC and RM-GIC placed into root dentine cavities (Soares et al. 2012). It was observed that the acid erosion severely degraded the GIC surfaces but afforded the dentine at the cavity margins some protection against the erosive solution due to the ions released from the degrading GIC (Soares et al. 2012). Ion release has also been demonstrated in the study by Zalizniak et al. (2013) where GICs were exposed to various acid solutions. It was observed that ion release, particularly phosphate ions, seemed to be dependent on the type of acid the GICs were exposed to. The mechanism is still not well understood and needs further investigation.
Erosion also affects surface roughness. A study comparing resin composite and conventional GIC and RM-GIC showed large differences in surface roughness. Hence, it would seem that the addition of resin into RM-GICs may not provide a long-term benefit from the aspect of surface finish when exposed to an acidic solution (Hussein et al. 2014).
One of the areas which has so far not been investigated to any great extent is the influence of the new coating agents that are now used on GICs. These coating materials are resin-based and have been shown to increase the fracture toughness of the materials (Bagheri et al. 2010). However, little research has been conducted to determine whether these resin coatings or even the placement of a coat in the form of a resin-based adhesive or bonding resin will reduce the erosion. Unfortunately, this may also cause some reduction in the release of fluoride ions that may make the GICs less effective in reducing the caries experience or recurrence around margins or on adjacent teeth (Mazzaoui et al. 2000).
2.3.6 Abrasion
Often in association with erosion is abrasion of the softened GIC surface. Compared with resin composite materials, it has been reported that GICs have a much lower abrasion resistance. It is also known that due to the maturation of GICs during the setting process, their abrasion resistance is poor compared to the fully matured cement (Mount and Hume 2005). Although not aesthetic, it has been shown that the metal-reinforced GICs have a better abrasion resistance (Forss et al. 1991). With the introduction of the high powder/liquid ratio and small particle materials such as Fuji IX (GC Corp, Tokyo, Japan) or Ketac Molar (3M-ESPE, St Paul, MN, USA), it has been demonstrated that the wear (abrasion) can be reduced significantly (Kunzelmann et al. 2003).
Another means to increase abrasion resistance is also the concept of coating the GIC. To date, the research remains limited, similar to that for erosion resistance. One study using the resin glazing agent Bellfeel Brightener (Kanebo Ltd, Tokyo, Japan) when applied to a GIC surface showed a significant increase in surface hardness and thus more resistance to abrasion (Hotta and Hirukawa 1994). More recently, the use of proprietary resin coating agents, e.g. G-Coat Plus (GC Corp, Japan) in combination with the high-viscosity GIC, Fuji IX, has been marketed as EQUIA (GC Corp, Japan) or more recently as EQUIA Forte Fil (GC Corp). The application of the coating as described previously has been shown to increase the strength of the GIC and increase its abrasion resistance. It is believed the resin is able to infiltrate the GIC surface, thus filling cracks and porosities (Lohbauer et al. 2011).
For the RM-GICs, the incorporation of the resin was not shown to improve the abrasion resistance. In fact, a number of studies have reported that the abrasion resistance is decreased and that the RM-GIC materials will abrade more rapidly than conventional GICs (Pelka et al. 1996; Momoi et al. 1997; Peutzfeldt et al. 1997; Xie et al. 2000; Sunnegårdh-Grönberg et al. 2002). The reason for this reduction in abrasion resistance is thought to be due to the glass particles being bonded loosely to the matrix in association with a nonuniform distribution of the glass particles throughout the set cement (Xie et al. 2000). When a polyacid-modified resin composite (PAMRC) was compared with an RM-GIC clinically, it was also noted that the abrasion resistance was lower for the RM-GIC (Chinelatti et al. 2004).
2.3.7 Adhesion
One of the great advantages of GICs is that they have become known for their ability to adhere to the moist cut tooth surface. This group of materials is able to bond to all parts of the tooth and carious tooth structure with a high degree of reliability and low technique sensitivity. Interestingly, however, there has not been as wide an evaluation of adhesive tests compared with resin-based adhesives. The original glass-ionomer cements were applied to smear layer-covered dentine. In the mid-1980s, workers started to consider how the adhesion of GICs might be improved (Lacefield et al. 1985). It was known from the work with phosphoric acid on enamel that adhesion for resin-based materials could be greatly enhanced. Various treatments such as polyacrylic acid, H2O2, citric acid or surface cleaning alone were tested. It was reported that the polyacrylic acid showed improved adhesion (Hinoura et al. 1986). Around 1990, manufacturers introduced conditioners such as Ketac Conditioner (3M-ESPE, St Paul, MN, USA) and GC Conditioner (GC Corp, Tokyo, Japan) for conditioning the dentine and enamel. It was shown that the use of these polyacrylic acid-based materials greatly enhanced the adhesion (Joynt et al. 1990; Tanumiharja et al. 2001). After such work, it became a routine practice to condition the dentine prior to GIC placement. These studies showed that the conditioning removed the smear layer but did not remove the smear plugs which ‘protected’ the dentine from becoming very wet. The tooth surface is not etched in the same way as acids such as phosphoric acid. This was the commencement of routine conditioning of tooth surfaces prior to placement of a GIC lining or restoration as opposed to etching for enamel and dentine with resin-based adhesives. Later work showed that removal of the smear layer could lead to better adaptation and bonding of the cement to the tooth surface. Another early study showed that this improved adhesion could be achieved with the use of maleic acid conditioning of dentine prior to the placement of Vitrebond (3M-ESPE, St Paul, MN, USA), which was one of the first resin-modified GICs to become available commercially (Watson 1990). Later Tyas showed that the use of polyacrylic acid conditioning clinically seemed to have little effect on restoration survival (Tyas 1993). This same outcome was shown in another study comparing 10 % polyacrylic acid conditioned and nonconditioned non-carious cervical lesions over a 4-year period. However, a slightly better retention rate was observed for the conditioned group, 15.6 % loss compared with 21.9 % loss in the nonconditioned group (van Dijken 1996a). Slightly later, a further study investigated the adhesion of a ‘viscous’ conventional GIC, Chem-Flex (Dentsply DeTrey GmbH, Konstanz, Germany), to dentine using different conditioning agents. These included 10 % polyacrylic acid (PAA) without rinsing, 10 % PAA with water rinsing, 25 % PAA with rinsing and 32 % phosphoric acid and a control of smear layer-covered dentine (Tay et al. 2001). Further to the bond study, this group also investigated the interface using transmission electron microscopy (TEM). It was shown that bond strengths after conditioning were much higher compared to the control. The TEM observations showed that the acidity of the GIC during setting was not able to alter the smear layer-covered control surface. However, when 10 % PAA was used as the conditioning agent, it was observed that the smear layer was removed and also partial demineralisation occurred up to 0.8 μm deep. An ‘interphase’ was noted between the tooth surface and cement (Tay et al. 2001). With the stronger acids or longer conditioning times, the depth of demineralisation increased. This interphase layer was also reported in the study by Tanumiharja et al. (2001), who investigated the interface using field emission scanning electron microscopy. They observed that this layer was resistant to attack by acidic and basic solutions and could range between 2.8 and 3.4 μm thick. A very similar phenomenon was noted when an RM-GIC was bonded to conditioned dentine. This study showed that using a conditioner provided a significant improvement in bond strengths. The interface of the RM-GIC and dentine also showed the acid–base resistant layer described by Tanumiharja et al. (2001). Subsequently, others observed that a hybrid-like layer similar to that formed by resin-based adhesives also formed (Cardoso et al. 2010).
With respect to RM-GICs, Coutinho et al. (2007) investigated several RM-GIC materials including an RM-GIC adhesive (Fuji Bond LC, GC Corp, Tokyo, Japan) as well as direct restorative materials (Photac-Fil and Vitrebond, 3M-ESPE, St Paul, MN, USA). They characterised the interfaces using transmission, scanning and field emission electron microscopy and atomic force microscopy. The tooth surfaces were either not conditioned or conditioned and treated with either a 20 % acrylic–maleic acid copolymer or 25 % polyacrylic acid. For the Fuji Bond LC conditioned samples, a very thin gel phase layer (0.5–1.0 μm thick) was observed above a sub-micrometre hybrid layer (Coutinho et al. 2007). This was also noted for the Photac-Fil but not the case for Vitrebond.
When conditioning did not take place, the gel phase was not observed, but partial demineralisation still occurred as in the conditioned groups. It would seem that when RM-GICs adhere to the tooth surface, it is a dual-type adhesive process. A thin hybrid layer is formed onto a partially demineralised surface with hydroxyapatite still remaining present in the collagen fibre matrix. Above this hybrid layer is a thin gel phase layer which has been identified previously as an ‘absorption layer’ (Sidhu and Watson 1998). Coutinho et al. (2007) showed that in the case of Vitrebond, it did not react in the same manner as the other RM-GICs tested. There was no sign of a hybrid layer or gel phase formation, but it did not seem to affect the bond. The other part of the bond seemed to be due to the reaction of polycarboxylic acid copolymers interacting with hydroxyapatite to form a chemical bond (Yoshida et al. 2000). This ‘dual’ adhesion process provides an answer as to why many clinical studies show such a high success of RM-GIC restorations.
With respect to surface treatments, recently some have advocated that a short etch with phosphoric acid on dentine and enamel will enhance the bond to tooth structure when an RM-GIC is used. Whilst there may be some logic to this concept, it should be remembered that even a short etch with phosphoric acid has the potential to remove all of the hydroxyapatite from the tooth surface. Based on the work of Coutinho et al. (2007), it would appear that this would then prevent the polycarboxylate groups from interacting chemically with calcium and thus potentially remove one of the modes of adhesion of the RM-GICs, namely, the chemical portion. This may also have long-term implications on adhesion since the bond would tend to be essentially micromechanical and be subject to degradation of the collagen similar to resin-based adhesives. Recently, Hamama et al. (2014) investigated the effect of a 5-s etch with phosphoric acid compared to conditioning with polyacrylic acid-based conditioners. They showed that in the short term (24 h), the bond strengths were little different, but later unpublished work by this group showed that the bonding outcomes for the etched group tended to decrease over time or became more variable (Hamama et al., unpublished). This may be an indication that the bond using a polyacrylic acid-based conditioner can lead to a more stable bond over the long term.
2.3.7.1 Adhesion to Composite and Repair
With the advent of the sandwich or laminate technique to restore deep approximal cavities, many have questioned the ability of GICs to bond to resin composite. There is little or no problem of bonding to composite with the RM-GICs since the HEMA in the cement, being a methacrylate, can easily adhere to resin-based adhesives or resin composite, which are also methacrylate-based materials. However, concern has been expressed with respect to the ability of the resin portion of the RM-GIC to adequately polymerise in deep cavities. Although the depth of cure has been poorly studied, the few studies available seem to consistently demonstrate that depth of cure is indeed an issue that must be carefully considered. The first paper by Mount et al. (2002) concluded that ‘cavities more than 3 mm deep’ should be filled incrementally. The shade of the material was also an influencing factor for the depth of cure. A later study also made the same recommendation (Roberts et al. 2009). It would therefore seem that for cavities extending onto the root face, a conventional high powder/liquid ratio GIC is possibly the most reliable material to use. However, the issue remains of being able to achieve adhesion to the overlying resin composite.
The first study to investigate long-term adhesion of conventional GICs with recent etch-and-rinse and self-etch adhesives was that of Zhang et al. (2011). They showed that effective bonding could be achieved with any of the adhesives tested which included etch-and-rinse, self-etch and all-in-one systems. One potential issue was the effect of the phosphoric acid on the GIC surface causing microcracks that seemed to influence the long-term adhesion. The self-etch materials, however, showed quite stable adhesion over the 6 months of the test. Another study that included a conventional GIC was that of Navimipour et al. (2012). They investigated the adhesion of a conventional GIC and RM-GIC to resin composite using either phosphoric acid or Er,CR:YSGG laser for etching the GICs for 15 s. Both treatments showed improvements of bond strengths with the RM-GIC showing higher bond strengths compared with the conventional material. A further study compared RM-GIC and conventional GIC with an etch-and-rinse adhesive and acidic all-in-one adhesive (Pamir et al. 2012). This group concluded that both GICs bonded well to either adhesive, but an etch time of 30 s was recommended. The study by Zhang et al. (2011) contradicts this, as the impression was that stronger and longer etching can possibly damage the matrix of the underlying cement and weaken its cohesive strength. Generally, it was considered that the self-etch materials may provide a more reliable bond and reduce the possibility of damaging the underlying GIC. Recent studies on bonding an RM-GIC to resin composite with a variety of adhesives showed successful bonding (Kasraie et al. 2013; Boruziniat and Gharaei 2014). However, caution must still be exercised with respect to the depth of cure.
The other aspect in this section is the repair of GIC restorations. Very little work has been undertaken to determine if previously placed GIC restorations can be successfully repaired by the addition of new GIC. Only one study seems to have been published investigating the bonding of RM-GIC or resin composite to 4-day-old RM-GIC (Maneenut et al. 2010). The surfaces were treated with or without acid etch. The bond of the new RM-GIC was lower and slightly more variable in comparison with that of the resin composite. This study recommended that when addition to existing RM-GIC is warranted, the addition of resin composite is preferable (Maneenut et al. 2010). A very recent study (Welch et al. 2015) has investigated almost the same scenario as Maneenut et al. This latter study used roughening, roughening and etching or roughening, etching and the addition of a resin-based adhesive. It was concluded that the addition of the resin-based adhesive to a roughened and etched surface produced the best outcomes (Welch et al 2015). The conclusions concurred with the former study of preferably bonding resin composite to the RM-GIC when a repair is needed.
2.3.8 Ion Release
One of the major points all practitioners will mention about their reason for selection of a GIC material is the release of ions, particularly fluoride. Certainly this is a great advantage of GICs since it affords them the ability to alter the environment round the material. This allows potentially healing and/or preventing early caries attack, as well as aiding the healing of deep caries lesions in prepared cavities which have not had all the carious tissue excavated. Research is moving towards developing GICs to be able to release or supply other ions or compounds, and this is discussed elsewhere in this book. The first cements to show the effect of fluoride release and thus prevent re-initiation of caries were the silicate cements. The source of the fluoride from GICs is the glass particles. Unfortunately, it is still unclear how much fluoride is actually needed to prevent the initiation of caries. The paper by Randall and Wilson (1999) indicated that the clinical evidence was unclear with respect to the prevention of caries in teeth restored with GIC.
Some of the original work conducted on fluoride release from GICs was done by Forsten (1990, 1995). His work of 1990 was based on some of the original conventional GICs where fluoride release was evaluated from 24 h up to 8 weeks. This was the pioneering work on this important topic. It was shown that the release of fluoride was highest in the first 24 h whilst the cement was still maturing, with a large reduction in the first week, followed by a more steady decline over the 8 weeks of the study. Fluoride was still detectable at 8 weeks but at very low levels. When analysed from the aspect of cumulative release, it was noted that the amounts of fluoride detected was very much material dependent. Interestingly, Forsten also investigated the fluoride release after exposing the cements to running water for up to 22 months. Again even at 22 months, fluoride ions were still being released from the cements which were detected at about 1 part per million. The only material that did not show release of the fluoride ion was the cermet material, Ketac Silver (3 M-ESPE, USA). When exposed to an acidic solution, a greater level of fluoride was identified (Forsten 1990). Forsten followed this work up to 5 years later with an evaluation of RM-GIC fluoride release as well as uptake. This study initially observed fluoride release for up to 1 month. Again it was shown there was a high initial burst of fluoride at 24 h with a greatly reduced release by 1 month (Forsten 1995). Another aspect of the research was to determine whether 9-month-old RM-GIC specimens, which had been in running water, could take up fluoride if stored in a 50 ppm fluoride-containing solution for 1 week. He showed that even after 9 months, fluoride could still be detected, but specimens stored in the fluoride-containing solution showed a much greater release for the following week. This was probably the first paper to indicate that GICs could be ‘recharged’ with fluoride. Furthermore, it was also shown that in an acidic environment, the level of fluoride release increased (Forsten 1995). This fluoride release occurred due to erosion of the GIC, as discussed in the previous sections, where ‘fresh’ GIC that has F ions in abundance would be continually exposed. In a later review paper, Forsten noted that the fluoride release was a little different between the conventional GIC and RM-GIC. Forsten’s work also noted that the other fluoride-releasing materials such as PAMRC did not respond in the same way to the recharging process (Forsten 1998).
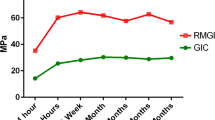
A more recent paper investigated the fluoride release from RM-GICs (Vitremer, 3M-ESPE; Fuji II LC, GC Corp), including an RM-GIC containing nanofillers (Ketac Nano, 3M-ESPE) and a flowable PAMRC (Dyract Flow, Dentsply), when exposed to solutions of different pHs (4, 5.5 and 7) (Moreau and Xu 2010). Fluoride release was measured up to 84 days post-setting. This study showed an initial high release of fluoride which was again material dependent. It was noted that the PAMRC and nanofilled RM-GIC released significantly less fluoride than the other two RM-GICs tested. During the first 2–3 weeks, the lower pH solutions led to greater release of fluoride, but by days 70–84, the rate of release was no different amongst the 3 different pH solutions. Figure 2.6 illustrates the typical pattern of cumulative fluoride ion release, whilst Fig. 2.7 shows the typical rate of fluoride release for these types of material.
This figure provides an illustration of the typical pattern shown for cumulative fluoride ion (F) release per specimen area (μg/cm2) from various tooth-coloured restorative materials: (a) Vitremer, (b) Fuji II LC, (c) Ketac Nano, (d) Dyract Flow and (e) Heliomolar. Each value is mean ± sd. The F release was higher in pH 4 solution than in pH 5.5 or pH 7 (Reprinted from Moreau and Xu (2010). With permission from Elsevier)
This figure illustrates the typical pattern of release of F ions over time: an initial high burst of ions with a rapid drop within the first week and then a steady low level thereafter. F release rate, which is the F release per specimen surface area per day, is shown for (a) Vitremer, (b) Fuji II LC, (c) Ketac Nano, (d) Dyract Flow and (e) Heliomolar. Each value is mean ± sd (Reprinted from Moreau and Xu (2010). With permission from Elsevier)
A previous study investigated the recharge and release of fluoride in a number of GICs at varying pH (Markovic et al. 2008). In this study, NaF solution was used for recharge, but there was no mention of surface deterioration as a function of the NaF exposure, but rather attributed recharging to the effects of the acidic environment. A very low pH of 2.5 was used as one of the test solutions. When specimens were placed in the NaF solution, it was noted that the surface fluoride content increased. They also concluded that fluoride release was related to the degradation of the cement, and the concentration of the fluoride at the surface was related to the surface media and pH. It seems that all the GICs tested could be recharged, and this was influenced by the pH, with better recharge occurring at a lower pH (Markovic et al. 2008). It would appear that the recharge process is a surface interaction as shown by Hadley et al. (2001) who investigated ion distribution in two GICs. They noted that when exposed to a KF solution, the GICs have a higher concentration of F on the surface of the GIC. Hence, it cannot be expected for these fluoride ions on the surface to diffuse into the deeper parts of the cement.
GICs not only release fluoride ions. It has been shown in a number of papers that other ions can also be released such as calcium, strontium, aluminium, phosphorus and silicon. The paper by Czarnecka and Nicholson (2006) showed that exposure of two RM-GICs to lactic acid caused a greater release of these ions compared with storage in water. The ion release did vary somewhat over the 6-week length of the study. The work by Zalizniak et al. (2013) showed that ion release also seemed to be dependent on the type of organic acid that the GIC was exposed to. Further work needs to be undertaken to explain why this is so, but it may relate to the valency of the acids used: lactic, citric and hydrochloric. Billington et al. (2006) undertook a comprehensive study analysing ion release and uptake. Part of their study showed that the mechanisms of uptake and release were still not well understood. They also noted that fluoride ions could disrupt the surface of fluoride-containing GICs (Billington et al. 2006). Hence, it would seem that further study is needed to better understand the dynamics of these processes of ion uptake and release.
2.3.8.1 Ion Release and Biofilm Formation
One of the benefits of the fluoride ion release is to assist with preventing demineralisation around cavity margins and adjacent teeth. Work has been done to determine the effect of the fluoride release and its effect on the biofilm that may develop on a restoration or at cavity margins (Al-Naimi et al. 2008; Chau et al. 2015). The study by Al-Naimi et al. (2008) compared a number of GICs, a PAMRC, a fluoride-releasing composite (Giomer, Shofu Dental Corp, Kyoto, Japan) and a resin composite. They measured fluoride release and showed that the GICs released more fluoride than the other materials. Biofilm was grown on the material surfaces at pH 3.8 or 7.1 in saliva. It was demonstrated that the biofilm growth was greatest in neutral conditions with much less growth in acidic conditions (Al-Naimi et al. 2008). This study showed that the higher fluoride-releasing GICs did not seem to alter biofilm growth. However, the confocal observations did not state if they used a live/dead staining technique, which would have provided better information as to whether the biofilm on the GICs was any different in characteristics compared with the other materials. The more recent study by Chau et al. (2015) shows different outcomes. Five different GICs, both conventional and resin-modified, were used together with a hydroxyapatite disc as the control material. This study used a mono-culture of S mutans to investigate a 94-h biofilm. Their results showed a negative correlation between acid production by the biofilm and fluoride release. It was also shown that the volume of the 94-h old biofilms was negatively correlated with the mean rate of fluoride release. Therefore, it appears from this work that if enough fluoride is released, it may ‘decrease the virulence of cariogenic biofilms’ (Chau et al. 2015).
Another interesting study investigated what happens to cavity margins around GIC restorations after the fluoride release is severely depleted. Several restorative materials, including a GIC, were placed in cavities and then were subjected to acid attack. The results showed that the cavity margins were indeed protected by uptake of the fluoride into the surrounding tooth tissue (Shiiya et al. 2012).
This uptake of fluoride in the surrounding tissues and the changes in biofilm growth indicate one of the potential advantages of GICs over most other tooth-coloured restorative materials in that placement of GICs may be one way of reducing initiation of breakdown of cavity-restoration margins through carious demineralisation. The review by Wiegand et al. (2007) describes the release and uptake of fluoride and its influence on caries and antibacterial activity. It was concluded that clinically, there remains a paucity of data from prospective clinical trials to provide a definitive answer to this issue. Hence, more clinical evaluation data are needed to better understand this point. This conclusion is similar to the findings 8 years previously of Randall and Wilson (1999).
2.4 Clinical Performance
The most important aspect of any material is how it performs clinically. More data have been published in recent years, but it still remains limited and not well standardised. There is now a lot of information from ART-based studies, but large prospective studies are scarce. The ART-based trials will be dealt with separately from those where GICs are used in a more ‘conventional’ manner.
The two reviews comparing the long-term survival of cervical restorations against resin-based adhesives and glass-ionomer cements both concluded that the GICs still achieve the highest survival rates (Peumans et al. 2005, 2014). Sidhu (2010) has also published a review of the clinical performance of RM-GICs. The paper by Hickel and Manhart (2001) investigated the longevity of materials in posterior teeth. They included GICs in this review although the authors stated that GICs are not considered for load-bearing restorations. They reported failure rates of between 1.4 and 14.4 % for GICs. The major reason for failure was noted to be due to caries and bulk failure. The studies reported in the Hickel and Manhart (2001) review are no longer contemporary and do not reflect current outcomes or materials.
2.4.1 Clinical Evaluation of GICs Placed in Non-carious Cervical Lesions
When testing the adhesive ability and longevity of GICs from the standpoint of clinical evaluations, it is the restoration of non-carious cervical lesions (NCCL) that has been the most common. The shape of NCCL is typically non-retentive, the lesions are quite prevalent and they occur in non-load-bearing regions. Hence, they are ideal for evaluating the adhesive qualities of a material. Table 2.3 summarises several studies over time with respect to retention of GICs and RM-GICs in NCCLs. Most studies investigated RM-GICs in this cavity configuration because of the improved aesthetics compared with many conventional GIC materials, particularly the earlier ones. The length of studies is quite variable with short studies of 12 months’ duration up to a few more comprehensive studies of 10 or more years in length. Many studies include a GIC, or more commonly an RM-GIC, for comparison or as a control material when evaluating new resin-based adhesives. This is due to the fact that restorations of NCCLs with a GIC or RM-GIC are often associated with good outcomes from the aspect of restoration retention. The figures in Table 2.3 show that the failure rates of these restorations remain quite low even for the longer-term studies. The studies of 10 and 13 years in length showed a failure of 76 % or annual failure rate of 2.7 %. This attests to the very good retention of GICs and RM-GICs when placed in NCCLs. The quality of the dentine of these lesions usually tends to be hypermineralised and sclerosed. This type of dentine is ideal for bonding of a GIC-based material as the mineral content is high, ensuring good chemical adhesion to the tooth surface.
Retention of a restoration is possibly the most important criterion. However, other factors such as marginal breakdown and surface characteristics of the material must also be evaluated as these will have a significant impact on the aesthetic quality of a restoration which, from a patient’s perspective, is the most important. The short study by Maneenut and Tyas (1995) showed marginal staining was beginning quite early in about five restorations of the 20 for each material they evaluated. They also noted darkening of the restorations. The 10-year study by Matis et al. (1996) indicated that about 87 % of the GIC restorations were rated alpha with no or minimal staining, whilst the resin-based material, Cervident (SS White Corp, Boston, USA), showed no discolouration. Interestingly though, when the parameter of marginal adaptation was examined, the GICs performed slightly better with an 81–87 % alpha rating compared with only 75 % for the resin-based material. These older GICs all showed surface roughness developing, with only 53–67 % of restorations showing an alpha rating after 10 years, compared with the resin-based material having a 100 % alpha rating. These changes did not appear to be tooth or arch position related. A slightly later study evaluated an RM-GIC and resin composite over 3 years (Özgünaltay and Önen 2002). At 3 years, only 59 % of the RM-GIC (Vitremer, 3M-ESPE) restorations had an alpha rating for marginal discolouration, whilst the resin composite, Z100 (3M-ESPE), had a 93 % alpha rating. Regarding marginal adaptation, it was almost the same for Vitremer at 95 % and the composite at 93 %. This study shows that resin composite seems to exhibit better long-term outcomes based on these two parameters. Another study of 5 years’ duration compared Vitremer with a resin composite (Franco et al. 2006). Interestingly, this study showed a combined 100 % alpha and bravo rating for marginal discolouration for both material types. However, marginal integrity was 76 % alpha and bravo rating for the resin composite compared with 85 % for the RM-GIC. The 3-year study by Burrow and Tyas (2007) compared two resin composites with an RM-GIC. This study showed minimal marginal staining amongst all of the materials tested. Although colour and shape of the restorations remained good, it was noted that the RM-GIC did show some loss of surface texture, but it was not enough to elicit concerns from patients. However, the RM-GIC had the greatest retention at 97 %. The most recent retrospective study (Namgung et al. 2013) indicated that the resin composite performed better than the GIC from the aspects of marginal discolouration and adaptation. Care is needed when interpreting these outcomes, as the number of resin composite restorations was much higher than the GIC restorations. It seemed, however, in this case, that the resin composites were performing better overall. The 2-year study evaluating restoration of carious lesions showed some slightly different outcomes to the non-carious cervical lesions (Folwaczny et al. 2000). This study compared a resin composite, PAMRC and two RM-GICs. They examined marginal integrity on both the enamel and cementum sides of the restoration. The resin composite showed alpha ratings of 88 % for enamel and 100 % for cementum, 73 and 85 %, respectively, for the PAMRC. As for the RM-GICs, the ratings were 70.6 and 58.8 for Fuji IILC (GC Corp) compared with 62.5 and 33.3 % for Photac-Fil (3M-ESPE). This study showed quite large variations, for example, marginal discolouration showed large variations between the two GICs. The GICs also did not perform particularly well with regard to surface integrity of the restorative material. Only 10 % of Photac-Fil and 23.5 % of Fuji II LC were given an alpha rating compared with 100 % for the resin composite and 95 % for the PAMRC (Folwaczny et al. 2000). These outcomes differ from the non-carious study outcomes and may reflect the different oral conditions of this group of patients. Little evidence exists relating to restoration survival and the oral environment, and it would seem important to know how a patient’s oral environment may influence the longevity and marginal quality of restorations.
2.4.2 Other Clinical Studies
The section above relates to a specific type of restoration, namely, NCCLs; however, GICs are not exclusively used for restoration of cervical lesions. They have also been recommended for the restoration of small approximal lesions in anterior teeth. The development of high powder/liquid ratio materials, also referred to as high-viscosity GICs, in association with the ART method for treatment of caries lesions has led to the use of GICs being extended to treatment of occlusal caries, as well as small posterior approximal load-bearing restorations. This section will summarise some of the work published in this area.
The original clinical work published on GIC use and survival was that of Mount (1997) where he outlined successful use of GICs by himself and others over 20 years since their inception.
One early retrospective study examined 42 restorations, half treated with resin composite and the other half with conventional GIC (de Araujo et al. 1998). This study examined restorations that had been placed for 24 months. Criteria such as aesthetics, anatomic form, staining and marginal leakage were classified into three groups. Most of the restorations were rated as either satisfactory or acceptable for all the criteria for both materials. By 24 months, the aesthetics remained acceptable for all the resin composite restorations, but in the case of the GIC, there was a steady decrease in acceptable restorations (equivalent to a beta rating), although it was only 23 % of the total. The outcome for staining was similar, but from the aspect of marginal leakage, both materials performed well (de Araujo et al. 1998). Another study comparing a total of 152 restorations of either resin composite, PAMRC or RM-GIC in anterior approximal restorations over 3 years reported that the resin composite and PAMRC ‘performed significantly better’ than the RM-GIC. The RM-GIC was observed to have changed colour slightly, but the quality of the surface of the RM-GIC decreased significantly (van Dijken 1996b).
A more recent practice-based study examined the performance of a ‘reinforced’ GIC (Fuji IX, GC Corp) in occlusal and 2-surface posterior approximal cavities (Burke et al. 2007). This study provides some insight into the clinical success centred in ‘real-world’ practices. Altogether, 67 occlusal and 102 posterior approximal restorations were evaluated over a mean restoration age of 25 months (range 5–56 months). Of all the restorations examined, 98 % were observed to be present and intact. No further caries was observed, and three had fractured and were replaced with another GIC restoration. The only factor amongst marginal adaptation, marginal discolouration and surface roughness that most notably changed with time was the surface roughness. It was concluded that over the 2 years of this study, the reinforced GIC restorations were ‘performing satisfactorily’ (Burke et al. 2007).
The coating of GICs for protection and increased fracture resistance has not been clinically evaluated widely. This method is now promoted as a technique to restore occlusal caries lesions. Recently a 3-year clinical trial reported the longevity of a high-viscosity GIC (Fuji IX, GC Corp) with and without the nanofilled resin coating G-Coat Plus (GC Corp) in comparison with a hybrid resin composite (Diem et al. 2014). Just over 80 restorations were initially placed for each method; however, at the end of the 3 years, only 69 GIC restorations, 65 coated-GIC and 64 resin composite restorations were evaluated. The results reported ‘moderate wear’ on 7 % of restorations with little difference between each method. Surface chipping and cracking was noted on 3 % of the coated-GIC and 2 % of resin composite restorations. With respect to wear, the GIC showed consistent wear more than the adjacent enamel over the 3 years. The coated-GIC showed slightly less wear but more than the resin composite which showed the least wear. In conclusion, the authors believed the coated-GIC was showing a trend of less wear than the uncoated-GIC. Their guarded conclusion was that the ‘G-Coat Plus gave some protection against wear’ (Diem et al. 2014). It is clear that more evidence is needed to determine whether this coating in association with the high powder/liquid GIC can be used in other than small occlusal restorations. If this is the case, careful monitoring for wear should be undertaken.
2.4.3 ART Restorations and Their Performance
As the ART method has developed and been studied, there are now an increasing number of studies evaluating the success of this commonly used public health method. Initially developed for caries management in countries where dental facilities may be limited, the technique is now seeing greater usage in many different clinical settings (Frencken 2010). One of the earlier studies over 12 months compared the ART method with conventional caries removal as well as amalgam restorations (Yip et al. 2002). This study evaluated 149 restorations in total, i.e. 60 restorations were placed for each of the GIC groups using either ART or conventional methods, and a further 29 restorations for dental amalgam. The GIC was also extended into any pits and fissures (as a sealant) not included in the small cavity preparations. This short study showed no failures in either the amalgam or GIC; the only issue noted was when GIC was extended into fissures, it was rapidly lost. They also noted some wear of the GIC with an increase in marginal discrepancies being noted in comparison with the amalgam. It was concluded that the high-viscosity GIC may be suitable for clinical use in small occlusal restorations (Yip et al. 2002).
Another recent study compared conventional rotary caries removal methods and restoration with resin composite to ART preparation using GIC. This study was also of 12 months’ duration, but the treatment was provided to patients with disabilities (Molina et al. 2014). A total of 298 carious lesions were restored in primary and permanent teeth, of which 182 used ART and the remaining 116 were conventional cavity preparations. The survival rates of the ART restorations in primary and permanent teeth were both 98 %. Interestingly, the ART restorations showed a better survival rate at 12 months compared with the conventional method using resin composite. The authors noted that longer-term data are now needed to further support the evidence base for the use of ART and GICs in the treatment of special-needs patients (Molina et al. 2014). This does, however, demonstrate where this technique has now extended beyond its original intention and seems to be proving a successful technique and philosophy.
Other studies have evaluated the success of using ART for restoration of not only occlusal but also posterior approximal restorations. A small study of only 6 months’ duration placed 60 ART restorations in children (Cefaly et al. 2005). There were 36 occlusal and 24 posterior approximal restorations. Over this short period of time, all occlusal restorations survived; however, the posterior approximal restorations showed a 100 % success rate for the RM-GIC, Fuji VIII (GC Corp), but only 92 % for the highly viscous GIC, Ketac Molar (3M-ESPE) (combined 96 %). This study was of a very short duration, but it does point to the potential problem and weakness of these GICs that when subjected to loading in a larger restoration not wholly supported by tooth structure, it does seem to lead to a higher failure rate. Hence, case selection is an important aspect to ensure restoration survival. Following on from this, a much larger and longer trial has reported the 3- and 6-year survival of ART restorations in small and large 1- and 2-surface restorations, although most restorations were placed in occlusal cavities (Holmgren et al. 2000; Lo et al. 2007). The study, as with most clinical evaluations, was not able to review all the restorations placed. At 3 years, 92 % of the small occlusal, 76 % of the large occlusal and 57 % of the approximal restorations were deemed satisfactory (Holmgren et al. 2000). By the end of 6 years, the survival outcomes were 76 % for the small occlusal and 59 % for the large restorations (Lo et al. 2007). This shows that the ART method for small restorations can provide a good outcome. However, when the restoration becomes larger, the evidence for GIC would seem to indicate that it is still not suitable for larger load-bearing situations (Lo et al. 2007).
Another study evaluated anterior approximal restorations using ART over 4 years with an RM-GIC in 117 restorations placed in 67 patients (Jordan et al. 2011). By the end of the study, only 76 of the original 117 restorations could be evaluated. At the 4-year recall, 13 restorations were lost with 10 restorations in 5 patients exhibiting secondary caries. In percentage terms, 28 % of the reviewed restorations did not need any intervention, 17 % needed minor intervention such as repair and 20 % were in need of replacement, with 35 % lost to follow-up (Jordan et al. 2011). The annual failure rate was determined at 4.9 %. For all the evaluation criteria of the restorations such as surface quality, marginal integrity and discolouration, all restorations showed deterioration over the life of the study.
A number of studies have been centred on using ART for paediatric patients and the treatment of primary teeth. The study by Hilgert et al. (2014) compared ART using a high-viscosity GIC with conventional treatment using dental amalgam. This study was quite large in that the initial numbers of restorations were 386 ART restorations (116 single surface, 270 multiple surface) compared with 364 amalgam (conventional) restorations (105 single surface, 259 multiple surface). The results showed that there was no significant difference between the ART or conventional methods of restoration. The 3-year survival rates were, however, better for the multiple surface amalgam (64.7 %) compared with the ART restorations (56.4 %). Furthermore, 20 % of the ART failures were due to marginal defects. From the aspect of overall cumulative survival, there was no significant difference between the two methods. However, the single-surface restorations lasted longer than the multiple surface restorations (Hilgert et al. 2014).
A further recent study on primary molars compared two techniques for approximal ART restorations over 18 months (Bonifácio et al. 2013). The methods here were the use of a single layer of the recommended P:L ratio compared with a 2-layer method which involved placing a 1:2 P:L ratio ‘flowable’ layer directly on the tooth surface which was then overlaid with the recommended P:L ratio material. Altogether, 208 cavities were restored. The cumulative survival of all restorations was calculated to be 68 % at 18 months (Bonifácio et al. 2013). There was no difference between the two groups. The authors concluded that the modification of the technique did not improve survival and further work was needed to enhance the long-term outcomes using ART for restoring primary teeth.
With the introduction of the nanofilled RM-GIC, two studies have reported the use of this material. The first used the ART method and compared the nanofilled material with a conventional high-viscosity GIC in primary molars (Konde et al. 2012). One hundred restorations in 50 patients were inserted and evaluated over 12 months. At 6 months, three patients were excluded due to secondary caries detected around the restorations. It was noted that slightly more patients had secondary caries in the conventional GIC group, but the numbers were quite small so these results should be viewed with some caution. It did show, however, that the new nanofilled material was performing as well as, if not slightly better than, the conventional GIC. Another study comparing the nanofilled material used conventional methods for cavity preparation and restoration. The nanofilled RM-GIC was compared with another RM-GIC for restoring single surfaces. Thirty restorations of each were placed and evaluated over 2 years (Abo-Hamar et al. 2015). In this study, the two materials performed in a similar manner. From the aspect of recurrent caries, the traditional RM-GIC performed marginally better at 2 years. Most other parameters showed little difference between the two materials. It was concluded that the nanofilled material was no better than the conventional RM-GIC (Abo-Hamar et al. 2015).
2.4.4 Sealants
The other clinical aspect of GIC use is as fissure sealants or fissure protection. More recently, GIC has been promoted as an excellent material for use as an ‘ART sealant’. Frencken (2014) recently published a ‘start-of-the-art’ piece on ART sealants. He outlined that meta-analyses had shown that there was no clear evidence suggesting either glass-ionomer or resin-based sealants were better than the other. The potential advantage of the ART sealant is that no specialised dental facilities are needed and the rate of dentine carious lesion development using this technique was approximately 1 % in the first 3 years (Frencken 2014).
A 3-year clinical evaluation of 400 GIC sealants compared a conventional and resin-modified GIC placed in first permanent molars (Pereira et al. 2003). The retention rates of the sealants were low by the end of the 3 years, but the caries incidence was much lower than the control teeth. The RM-GIC seemed to perform slightly better than the conventional material in terms of retention.
A small study compared the retention of GIC and resin sealants in teeth with and without preparation over 24 months (Dhar and Chen 2012). Twenty-five teeth were sealed with each of the techniques, i.e. resin or GIC, with or without preparation. For the glass-ionomer, 100 % sealant loss occurred in the no-treatment group compared with 60 % in the prepared teeth. In the resin-sealed group, 80 % of sealants were lost by 24 months compared with 32 % for the prepared group. With respect to caries incidence, the GIC group showed 4 % (prepared) and 8 % (unprepared) caries occurrence compared with 16 % (prepared) and 12 % (unprepared) for the resin group (Dhar and Chen 2012). This outcome shows that even though the GIC may be lost completely, there seems to be remaining benefit with respect to the prevention of caries. This study also showed that tooth preparation was not a wise choice as it increased the caries susceptibility.
With respect to caries risk, a 2-year study investigated the effect of a high fluoride-releasing conventional GIC as a sealant, comparing it with a resin-based sealant. One hundred and fifty teeth were sealed in 57 children (Chen and Liu 2013). The retention rate of the GIC at 2 years was 31 % for the low caries-risk group and 44.5 % for the high-risk group, compared with the resin sealant which was 77 % for the low-risk group and 63 % for the high-risk group. With regard to caries rates, no difference was noted between the two risk groups for either material (Chen and Liu 2013). Even though no difference was noted, it is perhaps interesting to note that even though the failure rate of the GIC was higher, this did not lead to a greater caries experience. A larger and longer study may provide more robust evidence.
Another 2-year study comparing a high fluoride-releasing GIC with an ormocer composite sealant in 200 teeth has been reported (Guler and Yilmaz 2013). Again, the retention rates for the resin-based sealant were much higher than the GIC. However, the caries experience for the GIC (16 %) was half that of the resin-based material (32 %). The outcomes of this study are contradictory to the previous study. This study concluded that ‘the GIC may be better for preventing occlusal caries’ (Guler and Yilmaz 2013).
A recent paper investigated the effect of a fluoride-releasing resin sealant and ART GIC sealant over 2 years in 280 children (383 molars) (Liu et al 2014). The results showed that the proportion of molars with dentine caries was 7.3 % for the ART sealant and 3.9 % for the resin-based sealant. Life table survival analysis at 2 years showed that the retention of the resin sealant was statistically higher at 73 % compared with the ART sealant at 50 %. From the aspect of caries not developing, there was no difference between the materials at 93 % (ART) and 96 % (resin). Liu et al. (2014) concluded that the effectiveness of both materials was no different and hence the ART sealant was a ‘good alternative’ where dental facilities are not available or limited. Hence again, even though retention is poorer for the GIC, there seems to be a growing pool of evidence that teeth treated with GIC sealants seem to have some preventive effect occurring even though the fissure system is exposed to the oral cavity.
2.5 Conclusions
GICs have now become a routine part of clinical practice for restorations, luting cements and lining materials. The strength and wear resistance of the recent materials has markedly improved over the last 10 or so years. The first major improvement was the incorporation of resins into the GIC; however, these materials were still not strong enough to withstand occlusal loading. The recent innovation of coating materials with a resin-based agent seems to have provided a major advance for use of higher powder/liquid ratio materials in the restoration of small load-bearing restorations.
The methods of delivery have also undergone major changes. Capsule systems have much improved allowing reliable mixing and dispensing directly into cavities in almost any location in the oral cavity. These systems have also reduced the porosity of the set cement, which has often been a problem with the older GICs. We are likely to see further modifications in delivery systems as manufacturers make further advances in both conventional GIC and RM-GICs.
GICs, particularly the resin-modified version, have been shown to be an excellent long-lasting restorative material for restoration of cervical lesions. In addition, because GICs are moisture tolerant and bond well to tooth structure, they remain an excellent material for restoring deep cavities where the dentine may be moist or adjacent to gingival tissues where moisture control is more difficult to achieve. The recent developments in conventional GICs with the resin coating agents are beginning to show that the GICs may become a good alternative for small, conservative occlusal restorations. However, the strength of GICs still limits their use for load-bearing restorations. Hence, they are still not recommended for larger restorations that are exposed to direct loading during function.
The release of ions such as fluoride and also the ability to probably slow down biofilm formation make the GICs useful materials for a variety of patient groups such as high caries-risk and special-needs patients. Further advances will also most likely improve treatment outcomes for these groups in the future years.
It is likely that as GICs are further developed, the potential exists for them to become a truly universal, tooth-coloured restorative material able to control caries initiation, heal early lesions and perhaps repair already damaged tooth structure from caries.
References
Abo-Hamar SE, El-Desouky SS, Abu Hamila AA. Two-year clinical performance in primary teeth of nano-filled versus conventional resin-modified glass-ionomer restorations. Quintessence Int. 2015;46:381–8.
Akinmade AO, Nicholson JW. Development of glasses for novel polyphosphonate dental cements. Br Ceram T. 1994;93:85–90.
Al-Naimi OT, Itota T, Hobson RS, McCabe JF. Fluoride release for restorative materials and its effect on biofilm formation in natural saliva. J Mater Sci Mater Med. 2008;19:1243–8.
Bagheri R, Tyas MJ, Burrow MF. Comparison of the effect of storage media on hardness and shear punch strength of tooth-colored restorative materials. Am J Dent. 2007;20:329–34.
Bagheri R, Azar MR, Tyas MJ, Burrow MF. The effect of aging on the fracture toughness of esthetic restorative materials. Am J Dent. 2010;23:142–6.
Bagheri R, Taha NA, Azar MR, Burrow MF. Effect of G-coat plus on the mechanical properties of glass-ionomer cements. Aust Dent J. 2013;58:448–53.
Bapna MS, Gadia CM, Drummond JL. Effects of aging and cyclic loading on the mechanical properties of glass ionomer cements. Eur J Oral Sci. 2002;110:330–4.
Billington RW, Williams JA, Pearson GJ. Ion processes in glass ionomer cements. J Dent. 2006;34:544–55.
Bonifácio CC, Kleverlaan CJ, Raggio DP, Werner A, de Carvalho RC, van Amerongen WE. Physical-mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust Dent J. 2009;54:233–7.
Bonifácio CC, Werner A, Kleverlaan CJ. Coating glass-ionomer cements with a nanofilled resin. Acta Odont Scand. 2012;70:417–7.
Bonifácio CC, Hesse D, de Oliveira RR, Bönecker M, Raggio DP, van Amerongen WE. Survival rate of approximal-ART restorations using a two-layer technique for glass ionomer insertion. Clin Oral Investig. 2013;17:1745–50.
Boruziniat A, Gharaei S. Bond strength between composite resin and resin modified glass ionomer using different adhesive systems and curing techniques. J Conserv Dent. 2014;17:150–4.
Brackett WW, Gilpatrick RO, Browning WD, Gregory PN. Two-year clinical performance of a resin-modified glass-ionomer restorative material. Oper Dent. 1999;24:9–13.
Bresciani E, Barata Tde J, Fagundes TC, Adachi A, Terrin MM, Navarro MF. Compressive and diametral tensile strength of glass ionomer cements. J Appl Oral Sci. 2004;12:344–8.
Bryant RW, Mahler DB. Volumetric contraction in some tooth-coloured restorative materials. Aust Dent J. 2007;52:112–7.
Burke FJ, Siddons C, Cripps S, Bardha J, Crisp RJ, Dopheide B. Clinical performance of reinforced glass ionomer restorations placed in UK dental practices. Br Dent J. 2007;203, E2. discussion 40–1.
Burrow MF, Tyas MJ. Clinical evaluation of a resin-modified glass-ionomer adhesive system. Oper Dent. 1998;23:290–3.
Burrow MF, Tyas MJ. Clinical evaluation of three adhesive systems for the restoration of non-carious cervical lesions. Oper Dent. 2007;32:11–5.
Cardoso MV, Delme KIM, Mine A, Neves AA, Coutinho E, De Moor RJG, Van Meerbeek B. Towards a better understanding of the adhesion mechanism of resin-modified glass-ionomers by bonding to differently prepared dentin. J Dent. 2010;38:921–9.
Carey CM, Spencer M, Give RJ, Eichmiller EC. Fluoride release from a resin-modified glass-ionomer cement in a continuous flow system. Effect of pH. J Dent Res. 2003;82:829–32.
Cattani-Lorente MA, Dupuis V, Payan J, Moya F, Meyer JM. Effect of water on the physical properties of resin-modified glass ionomer cements. Dent Mater. 1999;15:132–7.
Cefaly DF, Barata TJ, Tapety CM, Bresciani E, Navarro MF. Clinical evaluation of multisurface ART restorations. J Appl Oral Sci. 2005;13:15–9.
Chau NP, Pandit S, Cai JN, Lee MH, Jeon JG. Relationship between fluoride release rate and anti-cariogenic biofilm activity of glass ionomer cements. Dent Mater. 2015;31:e100–8.
Cheetham JJ, Palamara JE, Tyas MJ, Burrow MF. A comparison of resin-modified glass-ionomer and resin composite polymerisation shrinkage stress in a wet environment. J Mech Behav Biomed Mater. 2014;29:33–41.
Chen XX, Liu XQ. Clinical comparison of Fuji VII and a resin sealant in children at high and low risk of caries. Dent Mater J. 2013;32:512–8.
Chinelatti MA, Ramos RP, Chimello DT, Palma-Dibb RG. Clinical performance of a resin-modified glass ionomer and two polyacid modified resin composites in cervical lesion restorations; 1-year follow-up. J Oral Rehabil. 2004;31:251–7.
Cook WD. Degradative analysis of glass ionomer polyelectrolyte cements. J Biomed Mater Res. 1983;17:1015–27.
Coutinho E, Yoshida Y, Inoue S, Fukuda R, Snauwaert J, Nakayama Y, De Munck J, Lambrechts P, Suzuki K, Van Meerbeek B. Gel phase formation at resin-modified glass ionomer/tooth interfaces. J Dent Res. 2007;86:656–61.
Crisp S, Wilson AD. Reactions in glass ionomer cements: V. Effect of incorporating tartaric acid in the cement liquid. J Dent Res. 1976;55:1023–31.
Crisp S, Lewis BG, Wilson AD. Characterization of glass ionomer cements. 6. A study of erosion and water absorption in both neutral and acidic media. J Dent. 1980;8:68–72.
Czarnecka B, Nicholson JW. Ion release by resin-modified glass ionomer cements into water and lactic acid solutions. J Dent. 2006;34:539–43.
de Araujo MA, Araujo RM, Marsilio AL. A retrospective look at esthetic resin composite and glass-ionomer Class III restorations: a 2-year clinical evaluation. Quintessence Int. 1998;29:87–93.
De Barra E, Hill RG. Influence of alkali metal ions on the fracture properties of glass polyalkenoate (ionomer) cements. Biomaterials. 1998;19:495–502.
De Barra E, Hill RG. Influence of glass composition on the properties of glass polyalkenoate cements. Part III: Influence of fluorite content. Biomaterials. 2000;21:563–9.
De Caluwé T, Vercruysse CW, Fraeyman S, Verbeeck RM. The influence of particle size and fluorine content on aluminosilicate glass on the glass ionomer cement properties. Dent Mater. 2014;30:1029–38.
De Moor RJ, Verveeck RM. Effect of acetic acid on the fluoride release profiles of restorative glass ionomer cements. Dent Mater. 1998;14:261–8.
Dhar V, Chen H. Evaluation of resin based and glass ionomer based sealants placed with or without preparation – a two year clinical trial. Pediatr Dent. 2012;34:46–50.
Diem VT, Tyas MJ, Ngo HC, Phuong LH, Khanh ND. The effect of a nano-filled resin coating on the 3-year clinical performance of a conventional high-viscosity glass-ionomer cement. Clin Oral Invest. 2014;18:753–9.
Ermis RB. Two-year clinical evaluation of four polyacid-modified resin composites and a resin-modified glass-ionomer cement in class V lesions. Quintessence Int. 2002;33:542–8.
Falsafi A, Mitra SB, Oxman JD, Ton TT, Bui HT. Mechanisms of setting reactions and interfacial behaviour of a nano-filled resin-modified glass ionomer. Dent Mater. 2014;30:632–43.
Fano L, Fano V, Ma W, Wang X, Zhu F. Hydrolytic degradation and cracks in resin-modified glass-ionomer cements. J Biomed Mater Res Part B Appl Biomater. 2004;69:87–93.
Fennel B, Hill RG. The influence of poly(acrylic) acid molar mass and concentration on the properties o polyalkenoate cements. Part I – compressive strength. J Mater Sci. 2001a;36:5193–202.
Fennel B, Hill RG. The influence of poly(acrylic) acid molar mass and concentration on the properties of polyalkenoate cements. Part II: young’s modulus and flexural strength. J Mater Sci. 2001b;36:5177–83.
Fennel B, Hill RG. The influence of poly(acrylic) acid molar mass and concentration on the properties o polyalkenoate cements. Part III – fracture toughness and toughness. J Mater Sci. 2001c;36:5185–92.
Folwaczny M, Loher C, Mehl A, Kunzelmann KH, Hinkel R. Tooth-colored filling materials for the restoration of cervical lesions; a 24-month follow-up study. Oper Dent. 2000;25:251–8.
Forss H, Seppa I, Lappalainen WR. In vitro abrasion resistance and hardness of glass ionomer cements. Dent Mater. 1991;7:36–9.
Forsten L. Short- and long-term fluoride release from glass ionomers and other fluoride-containing filling materials in vitro. Scand J Dent Res. 1990;98:179–85.
Forsten L. Resin-modified glass ionomer cements: fluoride release and uptake. Acta Odont Scand. 1995;53:222–5.
Forsten L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effects. Biomaterials. 1998;19:503–8.
Franco EB, Benetti AR, Ishikiriama SK, Santiago SL, Lauris JRP, Jorge MFF, Navarro MFL. 5-year clinical performance of resin composite versus resin modified glass ionomer restorative system in non-carious cervical lesions. Oper Dent. 2006;31:403–8.
Frankenberger R, Sindel J, Kramer N. Viscous glass-ionomer cements: a new alternative to amalgam in the primary dentition? Quintessence Int. 1997;28:667–76.
Frencken JE. The ART, approach using glass-ionomers in relation to global oral health care. Dent Mater. 2010;26:1–6.
Frencken JE. The state-of-the-art of ART sealants. Dent Update. 2014;41:119–20. 122–4.
Fukuzawa M, Matsuya S, Yamane M. Mechanism of erosion of glass-ionomer cements in acidic buffer solution. J Dent Res. 1987;66:1770–4.
Ganesh M, Tandon S. Clinical evaluation of FUJI VII sealant material. J Clin Pediatr Dent. 2006;31:52–7.
Gemalmaz D, Yoruc B, Ozcab M, Alkumru HN. Effect of early water contact on solubility of glass ionomer luting cements. J Prosthet Dent. 1998;80:474–8.
Griffin SG, Hill RG. Influence of glass composition on the properties of glass polyalkenoate cements. Part I: Influence of aluminium to silicon ratio. Biomaterials. 1999;20:1579–86.
Griffin SG, Hill RG. Influence of glass composition on the properties of glass polyalkenoate cements. Part II: influence of phosphate content. Biomaterials. 2000a;21:399–403.
Griffin SG, Hill RG. Influence of glass composition on the properties of glass polyalkenoate cements. Part IV: influence of fluorine content. Biomaterials. 2000b;21:693–8.
Guler C, Yilmaz Y. A two-year clinical evaluation of glass ionomer and ormocer based fissure sealants. J Clin Pediatr Dent. 2013;37:263–7.
Hadley PC, Milella E, Gerardi C, Hill RG, Billington RW. Distribution of fluoride in glass ionomer determined using SIMS. Biomaterials. 2001;22:1563–9.
Hamama HH, Burrow MF, Yiu C. Effect of dentine conditioning on adhesion of resin-modified glass ionomer adhesives. Aust Dent J. 2014;59:193–200.
Hickel R, Manhart J. Longevity of restorations in posterior teeth and reasons for failure. J Adhes Dent. 2001;3:45–64.
Hilgert LA, de Amorim ZRG, Leal SC, Mulder J, Creugers NHJ, Frencken JE. Is high-viscosity glass-ionomer-cement a successor to amalgam for treating primary molars? Dent Mater. 2014;30:1171–8.
Hinoura K, Moore BK, Phillips RW. Influence of dentin surface treatments on the bond strengths of dentin-lining cements. Oper Dent. 1986;11:147–54.
Holmgren CJ, Lo ECM, Hu D, Wan H. ART restorations and sealants placed in Chinese school children – results after three years. Community Dent Oral Epidemiol. 2000;28:314–20.
Honório HM, Rios D, Fransisconi LF, Magalhães AC, Machado MA, Buzalaf MA. Effect of prolonged erosive pH cycling on different restorative materials. J Oral Rehabil. 2008;35:947–53.
Hotta M, Hirukawa H. Abrasion resistance of restorative glass-ionomer cements and a light-cured surface coating. Oper Dent. 1994;19:42–6.
Hussein TA, Bakar WZ, Ghani ZA, Mohamad D. The assessment of surface roughness and microleakage of eroded tooth-colored dental restorative materials. J Conserv Dent. 2014;17:531–5.
Ilie N, Hickel R, Valceanu AS, Huth KC. Fracture toughness of dental restorative materials. Clin Oral Invest. 2012;16:489–98.
Jordan RA, Hetzel P, Franke M, Markovic L, Gaengler P, Zimmer S. Class III atraumatic restorative treatment (ART) in adults living in West Africa – outcome after 48 months. Community Dent Oral Epidemiol. 2011;39:164–70.
Joynt RB, Davis EL, Wieczkowski G, Pierce L. Effect of dentinal pretreatment on bond strength between glass-ionomer and dentin. Oper Dent. 1990;15:173–7.
Jyothi NJ, Annapurna S, Kumar AS, Venugopal P, Jayashankara CM. Clinical evaluation of giomer and resin-modified glass ionomer cement in class V noncarious lesions: An in vivo study. J Conserv Dent. 2011;14:409–13.
Kasraie S, Shokripour M, Safari M. Evaluation of micro-shear bond strength of resin modified glass-ionomer to composite resins using various bonding systems. J Conserv Dent. 2013;16:550–4.
Kaur H, Nandlal B. Effect of dietary solvents on the strength of nanocomposite, compomer, glass ionomer cement: An in vitro study. J Conserv Dent. 2013;16:527–31.
Kim YG, Hirano S. Setting shrinkage and hygroscopic expansion of resin-modified glass ionomer in experimental cylindrical cavities. Dent Mater J. 1999;18:63–75.
Kitasako Y, Sasaki Y, Takagi T, Sadr A, Tagami J. Age-specific prevalence of erosive tooth wear by acidic diet and gastroesophageal reflux in Japan. J Dent. 2015;43:418–23.
Kleverlaan CJ, van Duinen RN, Felizer AJ. Mechanical properties of glass ionomer cements affected by curing methods. Dent Mater. 2004;20:45–50.
Konde S, Rais S, Jaiswal D. Clinical evaluation of a new art material: nanoparticulated resin-modified glass ionomer cement. J Int Prev Community Dent. 2012;2:42–7.
Kunzelmann KH, Burkle V, Bauer C. Two-body and three-body wear of glass ionomer cements. Int J Paediatr Dent. 2003;13:434–40.
Lacefield WR, Reindl MC, Retief DH. Tensile bond strength of glass-ionomer cement. J Prosthet Dent. 1985;53:194–8.
Leirskar J, Nordbø H, Mount GJ, Ngo H. The influence of resin coating on the shear punch strength of a high strength auto-cure glass ionomer. Dent Mater. 2003;19:87–91.
Liu BY, Xiao Y, Chu CH, Lo EC. Glass ionomer ART sealant and fluoride-releasing resin sealant in fissure caries prevention – results from a randomized clinical trial. BMC Oral Health. 2014;145:54.
Lo EC, Holmgren CJ, Hu D, van Palenstein HW. Six-year follow up of atraumatic restorative treatment restorations placed in Chinese school children. Community Dent Oral Epidemiol. 2007;35:387–92.
Loguercio AD, Reis A, Barbosa AN, Roulet JF. Five-year double-blind randomised clinical evaluation of a resin-modified glass ionomer and a polyacid-modified resin in noncarious cervical lesions. J Adhes Dent. 2003;5:323–32.
Lohbauer U, Krämer N, Siedschlag G, Schubert EW, Lauerer B, Müller FA, Petschelt A, Ebert J. Strength and wear resistance of a dental glass-ionomer cement with a novel nanofilled resin coating. Am J Dent. 2011;24:124–8.
Maneenut C, Tyas MJ. Clinical evaluation of resin-modified glass ionomer restorative cements in cervical ‘abrasion’ lesions: One –year results. Quintessence Int. 1995;26:739–43.
Maneenut C, Sakoolnamarka R, Tyas MJ. The repair of resin-modified glass-ionomer cements. Dent Mater. 2010;26:659–65.
Markovic DLJ, Petrovic BB, Peric TO. Fluoride content and recharge ability of five glass ionomer dental materials. BMC Oral Health. 2008;8–21.
Matis BA, Cochran M, Carlson T. Longevity of glass-ionomer restorative materials: results of a 10-year evaluation. Quintessence Int. 1996;27:373–82.
Matsuya S, Matsuya Y, Yamamoto Y, Yamane M. Erosion process of glass ionomer cement in organic acids. Dent Mater J. 1984;3:210–9.
Mazzaoui SA, Burrow MF, Tyas MJ. Fluoride release from glass ionomer cements and resin composites coated with a dentin adhesive. Dent Mater. 2000;16:166–71.
McCabe JF. Resin-modified glass-ionomers. Biomaterials. 1998;19:521–7.
Mitchell CA, Douglas WH, Cheng YS. Fracture toughness of conventional, resin-modified and composite luting cements. Dent Mater. 1999;15:7–13.
Mitsuhashi A, Hanaoka K, Teranaka T. Fracture toughness of resin-modified glass ionomer restorative materials: effect of powder/liquid ratio and powder particle size reduction on fracture toughness. Dent Mater. 2003;19:747–57.
Molina G, Faulks D, Mazzola I, Mulder J, Frencken JE. One year survival of ART and conventional restorations in patients with disability. BMC Oral Health. 2014;14:49.
Momoi Y, Hirosaki K, Kohno A, McCabe JF. In vitro toothbrush-dentifrice abrasion of resin-modified glass ionomers. Dent Mater. 1997;13:82–8.
Moreau JL, Xu HH. Fluoride releasing restorative materials: effects of pH on mechanical properties and ion release. Dent Mater. 2010;26:e227–35.
Mount GJ. Polyacrylic cements in dentistry. Am J Dent. 1990;3:79–84.
Mount GJ. Longevity in glass-ionomer restorations: review of a successful technique. Quintessence Int. 1997;28:643–50.
Mount GJ, Hume WR. Preservation and restoration of tooth structure. 2nd ed. Queensland: Knowledge Boos and Software; 2005.
Mount GJ, Makinson OF, Peters MC. The strength of auto-cured and light-cured materials. The shear punch test. Aust Dent J. 1996;41:118–23.
Mount GJ, Patel C, Makinson OF. Resin modified glass-ionomers: strength, cure depth and translucency. Aust Dent J. 2002;47:339–43.
Mount GJ, Tyas MJ, Ferracane JI, Nicholson JW, Berg JH, Simonsen RJ, Ngo HC. A revised classification for direct tooth-coloured restorative materials. Quintessence Int. 2009;40:691–7.
Namgung C, Rho YJ, Jin BH, Lim BS, Cho BH. A retrospective clinical study of cervical restorations: longevity and failure-prognostic variables. Oper Dent. 2013;38:376–856.
Navimipour EJ, Oskoee SS, Oskoee PA, Bahari M, Rikhtegaran S, Ghojazadeh M. Effect of acid and laser etching on shear bond strength of conventional and resin-modified glass-ionomer cements to composite resin. Lasers Med Sci. 2012;27:305–22.
Nicholson JW. Chemistry of glass-ionomer cements – a review. Biomaterials. 1998;19:485–94.
Nicholson JW, Brookman PJ, Lacy OM, Wilson AD. Fourier transform infrared spectroscopic study of the role of tartaric acid in glass ionomer cements. J Dent Res. 1988;67:1451–4.
Nicholson JW, Aggarwal A, Czarnecka B, Limanowska-Shaw H. The rate of change of pH of lactic acid exposed to glass-ionomer dental cements. Biomaterials. 2000;21:1989–93.
Nomoto R, Carrick TE, McCabe JF. Suitability of a shear punch for dental restorative materials. Dent Mater. 2001;17:415–21.
Norman RD, Swartz ML, Phillips RW, Virmani R. A comparison of the intraoral disintegration of three dental cements. J Am Dent Assoc. 1969;78:777–82.
Oilo G. Early erosion of dental cements Scan. J Dent Res. 1984;92:539–43.
Okada K, Tosaki S, Hirota K, Hume WR. Surface hardness change of restorative filling materials stored in saliva. Dent Mater. 2001;17:34–9.
Özgünaltay G, Önen A. Three-year clinical evaluation of a resin modified glass-ionomer cement and a composite resin in non-carious class V lesions. J Oral Rehabil. 2002;29:1037–41.
Pamir T, Sen BH, Evcin O. Effects of etching and adhesive applications on the bond strength between composite resin and glass-ionomer cements. J Appl Oral Sci. 2012;20:636–42.
Patel M, Tawfik H, Myint Y, Brocklehurst D, Nicholson JW. Factors affecting the ability of dental cements to alter pH of lactic acid solutions. J Oral Rehabil. 2000;27:1030–3.
Pelka M, Ebert J, Schneider H, Kramer N, Petschelt A. A comparison of two- and three-body wear of glass ionomers and composites. Eur J Oral Sci. 1996;104:132–7.
Perdigão J, Dutra-Corrêa M, Saraceni SHC, Ciaramicoli MT, Kiyan VH. Randomised clinical trial of two resin-modified glass ionomer materials: 1-year results. Oper Dent. 2012;37:591–601.
Pereira AC, Pardi V, Mialhe FL, Meneghim Mde C, Ambrosano GM. A 3-year clinical evaluation of glass-ionomer cements used as fissure sealants. Am J Dent. 2003;16:23–7.
Peumans M, Kanumulli P, De Munck J, Van Landuyt P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review. Dent Mater. 2005;21:864–81.
Peumans M, De Munck J, Mine A, Van Meerbeek B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent Mater. 2014;30:1089–103.
Peutzfeldt A, Garcia-Godoy F, Asmussen E. Surface hardness and wear of glass ionomers and compomers. Am J Dent. 1997;10:15–7.
Prentice LH. Investigations into the mechanical properties and curing characteristics of dental glass-ionomer cements. PhD thesis, University of Melbourne; 2005.
Prosser HJ, Jerome SM, Wilson AD. The effect of additive on the setting properties of a glass-ionomer cement. J Dent Res. 1982a;61:1195–8.
Prosser HJ, Richards CP, Wilson AD. NMR spectroscopy of dental materials. II. The role of tartaric acid in glass-ionomer cements. J Biomed Mater Res. 1982b;16:431–45.
Randall RC, Wilson NH. Glass-ionomer restoratives: a systematic review of secondary caries treatment effect. J Dent Res. 1999;78:628–37.
Roberts HW, Berzins DW, Charlton DG. Hardness of three resin-modified glass-ionomer restorative materials as a function of depth and time. J Esthet Restor Dent. 2009;21:262–72.
Santiago SL, Passos VF, Vieira AH, Navarro MF, Lauris JR, Franco EB. Two-year clinical evaluation of resinous restorative systems in non-carious cervical lesions. Braz Dent J. 2010;21:229–34.
Scholtanus JD, Huysmans MC. Clinical failure of class-II restorations of a highly viscous glass-ionomer material over a 6-year period: a retrospective study. J Dent. 2007;35:156–62.
Schwieger M, Groning P, Schlapbach L, Höland W, Rheinberger V. Thermal and chemical properties of a glass in the SiO2-CaOF system for dental applications. J Therm Anal Calorim. 2000;60:1009–18.
Shiiya T, Mukai Y, Ten Cate JM, Teranaka T. The caries-reducing benefit of fluoride-release from dental restorative materials after fluoride release has ended. Acta Odontol Scand. 2012;70:15–20.
Shin-ichi K, Atsuhiro T, Michiko H. GC Dental Ind Corp, assignee. Glass powder for dental glass ionomer cement. US Patent 6136737. 2000.
Shiozawa M, Takahashi H, Iwasaki N. Fluoride release and mechanical properties after 1-year storage of recent restorative glass ionomer cements. Clin Oral Investig. 2014;18:1053–60.
Sidhu SK. Clinical evaluations of resin-modified glass-ionomer restorations. Dent Mater. 2010;26:7–12.
Sidhu SK, Watson TF. Interfacial characteristics of resin-modified glass-ionomer materials: a study on fluid permeability using confocal fluorescence microscopy. J Dent Res. 1998;77:1749–59.
Small ICB, Watson TF, Chadwick AV, Sidhu SK. Water sorption in resin-modified glass-ionomer cements – an in vitro comparison with other materials. Biomaterials. 1998;19:545–50.
Smith DC. Development of glass- ionomer cement systems. Biomaterials. 1998;19:467–78.
Soares LE, Lima LR, Vieira LS, Do Espírito Santo AM, Martin AA. Erosion effects on chemical composition and morphology of dental materials and root dentin. Microsc Res Tech. 2012;75:703–10.
Sunnegårdh-Grönberg K, Peutzfeldt A, van Dijken JW. Hardness and in vitro wear of a novel ceramic restorative cement. Eur J Oral Sci. 2002;110:175–8.
Tanumiharja M, Burrow MF, Cimmino A, Tyas MJ. The evaluation of four conditioners for glass ionomer cements using field-emission scanning electron microscopy. J Dent. 2001;29:131–8.
Tay FR, Smales RJ, Ngo H, Wei SH, Pashley DH. Effect of different conditioning protocols on adhesion of a GIC to dentin. J Adhes Dent. 2001;3:153–67.
Tyas MJ. The effect of dentine conditioning with polyacrylic acid on the clinical performance of glass ionomer cement. Aust Dent J. 1993;38:46–8.
van Dijken JW. Four-year evaluation of the effect of 10% polyacrylic acid or water rinsing treatment on retention of glass polyalkenoate cement. Eur J Oral Sci. 1996a;104:64–6.
van Dijken JW. 3-year clinical evaluation of a compomer, a resin-modified glass ionomer and a resin composite in Class III restorations. Am J Dent. 1996b;9:195–8.
van Dijken JW, Pallesen U. Long-term retention of etch-and-rinse and self-etch adhesives and a resin-modified glass ionomer cement in non-carious cervical lesions. Dent Mater. 2008;24:915–22.
van Dijken JW, Kieri C, Carlén M. Longevity of extensive class II open-sandwich restorations with a resin-modified glass-ionomer cement. J Dent Res. 1999;78:1319–25.
Wang XY, Yap AU, Ngo HC. Effect of early water exposure on the strength of glass ionomer restoratives. Oper Dent. 2006;31:584–9.
Watson TF. A confocal microscope study of some factors affecting adaptation of a light-cured glass ionomer to tooth tissue. J Dent Res. 1990;69:1531–8.
Watson TF, Atmeh AR, Sajini S, Cook RJ, Festy F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry; biophotonics-based interfacial analyses in health and disease. Dent Mater. 2014;30:50–61.
Welch D, Seesengood B, Hopp C. Surface treatments that demonstrate a significant positive effect on the shear bond strength of repaired resin-modified glass ionomer. Oper Dent. 2015;40:403–9.
Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials – fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62.
Wilson AD, McLean JW. Glass-ionomer cement. Chicago: Quintessence Publishing; 1988.
Wilson AD, Crisp S, Ferner AJ. Reactions in glass-ionomer cements: IV. Effect of chelating comonomers on setting behavior. J Dent Res. 1976;55:489–95.
Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass ionomer cements. Dent Mater. 2000;16:129–38.
Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride releasing materials. Biomaterials. 2003;2451–61.
Yip KH, Smales RJ, Gaol W, Peng D. The effects of two cavity preparation methods on the longevity of glass ionomer restorations: an evaluation after 12 months. J Am Dent Assoc. 2002;133:744–51.
Yoshida Y, Van Meerbeek B, Nakayama Y, Snauwaert J, Hellemans L, Lambrechts P, Vanherle G, Wakasa K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res. 2000;79:709–14.
Young AM, Sherpa A, Pearson G, Schottlander B, Waters DN. Use of Raman spectroscopy in the characterisation of the acid–base reaction in glass-ionomer cements. Biomaterials. 2000;21:1971–9.
Zalizniak I, Palamara JE, Wong RH, Cochrane NJ, Burrow MF, Reynolds EC. Ion release and physical properties of CPP-ACP modified GIC in acid solutions. J Dent. 2013;41:449–54.
Zanata RL, Magalhães AC, Lauris JR, Atta MT, Wang L, Navarro MF. Microhardness and chemical analysis of high-viscous glass-ionomer cement after 10 years of clinical service as ART restorations. J Dent. 2011;39:834–40.
Zhang Y, Burrow MF, Palamara JE, Thomas CDL. Bonding to glass ionomer cements using resin-based adhesives. Oper Dent. 2011;36:618–25.
Zimehl R, Hannig M. Non metallic restorative materials based on glass ionomer cements – recent trends and developments. Colloid Surf A. 2000;163:55–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Burrow, M.F. (2016). Physicochemical Nature of Glass-Ionomer-Based Materials and Their Clinical Performance. In: Sidhu, S. (eds) Glass-Ionomers in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-319-22626-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-22626-2_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22625-5
Online ISBN: 978-3-319-22626-2
eBook Packages: MedicineMedicine (R0)