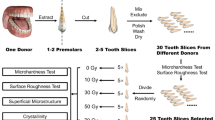
Abstract
Objectives
The aim of this study is to determine the effects of in vitro and in vivo high-dose radiotherapy on microhardness and associated indentation pattern morphology of enamel.
Materials and methods
The inner, middle, and outer microhardness of enamel was evaluated using three experimental groups: control (non-radiated); in vitro irradiated; in vivo irradiated. In vitro specimens were exposed to simulated radiotherapy, and in vivo specimens were extracted teeth from oral cancer patients previously treated with radiotherapy. Indentations were measured via SEM images to calculate microhardness values and to assess the mechanomorphological properties of enamel before and after radiotherapy.
Results
Middle and outer regions of enamel demonstrated a significant decrease in microhardness after in vitro and in vivo irradiation compared to the control group (p < 0.05). Two indentation patterns were observed: pattern A—presence of microcracks around indent periphery, which represents local dissipation of deformation energy; pattern B—clean, sharp indents. The percentage of clean microindentation patterns, compared to controls, was significantly higher following in vitro and in vivo irradiation in all enamel regions. The highest percentage of clean microindentations (65%) was observed in the in vivo irradiated group in the inner region of enamel near the dentin-enamel junction.
Conclusions
For the first time, this study shows that in vitro and in vivo irradiation alters enamel microhardness. Likewise, the indentation pattern differences suggest that enamel may become more brittle following in vitro and in vivo irradiation.
Clinical relevance
The mechanomorphological property changes of enamel following radiation may be a contributory component of pathologic enamel delamination following oral cancer radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy is one of the common oral cancer therapies which may affect dental and oral health. High-energy X-ray radiotherapy that is used to kill the cancer cells can cause some oral complications including xerostomia, loss of taste, jaw trismus, radiation-related caries, and dentition breakdown [1,2,3,4,5]. While some of the complications are transient, dentition breakdown typically starts within the first year post-radiotherapy and becomes more severe with time, negatively impacting the patient’s quality of life [1]. Post-radiation dental lesions are different than caries in non-radiated patients. The lesions develop with initial loss of enamel near the dentin-enamel junction (DEJ) leading to partial to total enamel delamination resulting in exposed dentin that is more vulnerable to subsequent decay [6, 7].
Until recently, salivary gland damage and subsequent xerostomia were considered the most likely etiologies for post-radiation dentition breakdown [1, 2, 5]. However, our clinical research has indicated the severity of dentition breakdown is also linked to the individual tooth dose with three tiers of tooth dose-response [8]. Below 30 Gy, there is minimal tooth damage, between 30 and 60 Gy, there is a two to three times increased of breakdown likely linked to salivary gland damage, and at > 60 Gy, there is a ten times increased risk of tooth breakdown suggesting there is radiation-induced damage to the tooth in addition to salivary gland indirect effects. Thus, it is important to understand radiotherapy-induced tooth structure changes that might lead to dentition breakdown.
A dramatic increase in the number of oral cancer patients [9] has led to studies which attempt to understand the potential impact of therapeutic radiation on the mechanical properties [10,11,12,13,14] and chemical composition of mineralized tooth substrates [15,16,17]. It has been noted that dentin is weakened after in vitro radiation and loses its ability to support the covering enamel [13]. This could be one of the possible explanations for the clinical observation of enamel loss [14, 17]. At the same time, under normal occlusal loads, the dentin-enamel junction (DEJ) destabilizes in caries-resistant regions, i.e., incisal/occlusal and cervical. This likely results in microcrack formation of enamel or gap formation at DEJ, leading to extreme bacterial colonization and severe caries [14]. It is also reported that in vitro irradiation affects the mechanical and chemical properties of enamel [10,11,12,13, 16]. To date, the impact of radiotherapy on mechanomorphological properties of dental enamel in oral cancer patients remains unclear and the in vivo effects of high dose therapeutic radiation on teeth are lacking within the literature. Recently, our group reported type VII and type IV collagen as constituents of the enamel organic matrix that plays a role in the stability of the DEJ [18,19,20,21]. Likewise, we also reported that type IV collagen immunoreactivity within the enamel organic matrix was reduced following radiotherapy in oral cancer patients [21]. As a continuation of our research focus, we designed the in vitro and in vivo studies to evaluate therapeutic radiation effects on the mechanomorphological properties of dental enamel to better understand the mechanism of enamel delamination associated with oral cancer radiotherapy. In this study, enamel microhardness and associated indentation pattern morphology was analyzed at three regions (inner, middle, outer) of enamel by using Vickers hardness test and scanning electron microscopy (SEM). The hypothesis tested was that the mechanomorphological properties of dental enamel would change following in vivo and in vitro irradiation.
Materials and methods
Non-carious posterior teeth (n = 6) previously extracted from individuals 18–23 years old were collected according to a protocol approved by an institutional review board (IRB13-924NHS). Because these extracted teeth were not associated with any patient identifiers, their use in the project was categorized as not human subject research by the IRB. Half the teeth were assigned to the non-radiated control group and the other half to the in vitro irradiated group. Using an in vitro oral cancer radiotherapy model, we exposed the in vitro group to a cumulative dose of 70 Gy (2 Gy/day × 5 days/week × 7 weeks). In vitro radiation exposure was done using a Varian 2100iX linear accelerator with an energy of 6 MV photons (Kansas City Cancer Centers, Kansas City, KS).
Additionally, another group of posterior extracted teeth (in vivo irradiated group) was collected from three patients, who had previously undergone radiotherapy for oral cancer. The tooth collection protocol, which was approved by an institutional review board (IRB13–143), included informed consent explaining to the patient how their extracted teeth and radiotherapy records could potentially be used in a research project to better understand the effects of radiotherapy on tooth structure and dentition breakdown. Based on radiotherapy treatment records, tooth-level radiation doses were calculated for the crown of each tooth with doses > 60 Gy. For all extracted teeth, excess soft tissue was removed and the teeth were stored at 4 °C in phosphate buffered saline (PBS, pH 7.4) with 0.002% sodium azide to inhibit microbial contamination prior to any testing.
A slow-speed water-cooled diamond saw (Buehler Ltd., Lake Bluff, IL, USA) was used to remove the roots approximately 5–6 mm below the cementoenamel junction (CEJ). Teeth were sectioned buccolingually and then mesiodistally to generate quarters. A portion of the previously sectioned root surface of each quarter section was removed at a 45° angle with respect to the tooth axis leaving approximately 1–2 mm of the remaining root below the CEJ. Each quarter was then sectioned into a 2-mm thick enamel section running diagonally from the CEJ to the cusp tip (Fig. 1). This detailed tooth specimen sectioning protocol was used to ensure that the enamel rods were uniformly oriented perpendicular to the enamel cut surface and the direction of microhardness testing. Sections were embedded with a cold-curing epoxy resin (EpoxiCure 2, Buehler, Lake Bluff, IL, USA) and were then polished using a motorized polishing wheel (Metaserv, Buehler, Lake Bluff, IL, USA) with silicon carbide paper sequentially from 400 to 1200 grit. Specimens were rinsed with distilled water and sonicated for 1–2 min between each grade of SiC paper and examined at ×20 magnification to confirm their smoothness.
Tooth specimen preparation for microhardness testing. a After the root of each tooth was removed 5–6 mm below the CEJ, each tooth was sectioned buccolingually and then each half was sectioned mesiodistally resulting in four quarter sections. b A portion of the previously sectioned root surface of each quarter section was removed at a 45° angle with respect to the tooth axis leaving approximately 1–2 mm of the remaining root below the CEJ. c Each quarter was sectioned diagonally starting approximately 2 mm from the CEJ to the cusp tip. d A 2-mm thick specimen was obtained by sectioning again diagonally at the CEJ
It is reported that enamel microhardness values measured either by Vickers or Knoop microhardness testers are within the same range [22]. However, Vickers hardness number (VHN) values of enamel are not affected by variation of indentation loads while Knoop hardness of enamel shows load dependency [22]. In this study, to compare with reported enamel microhardness, which were obtained at different indentation loads, Vickers microhardness tester was used. A microhardness tester (MO Tukon, Wilson Instrument Division, Bridgeport, CT, USA) with a pyramidal diamond indenter at constant load of 500 gf with a 12-s dwell time was used to perform Vickers indentation. Microhardness of enamel was measured in three regions, namely inner enamel, middle enamel, respectively 50 μm and 200 μm from the DEJ, and outer enamel with a distance 200 μm from outer surface of enamel. At least 17 indentations with a minimum distance of 200 μm between indents were made at each of the three regions per specimen in each experimental group.
Following microindentation testing, scanning electron microscopy (SEM) images (FEI-XL30, FEI Company, Hillsboro, OR) were made of the uncoated tooth sections using 15 kV at ×1000 magnification and gaseous secondary electron detector (GSE). SEM images were used to generate hardness values, confirm enamel rod orientation (perpendicular to the cut surface), and characterize microindentation patterns. Vickers hardness number (VHN) was calculated as the load divided by the surface area of indentation:
Where F is the applied load (kg), α is apex angle (136°), and d is the average length of the diagonal (mm) and which was measured with the image analysis software Image J (National Institutes of Health; Bethesda, MD). For each experimental group, overall mean microhardness and standard deviation (SD) were calculated at each region (inner, middle, outer). Microhardness data was compared using a univariate analysis of variance and Tukey’s post hoc analyses with statistical significance set at α = 0.05. For any significant differences, the associated power was also reported. The energy dissipation which reflects the material toughness [23] was interpreted based on the comparison of microindentation patterns and analyzed using non-parametric Kruskal-Wallis and Mann-Whitney post hoc tests at a significance level of 0.05.
Results
Enamel microhardness
Means and standard deviations of Vickers hardness test are shown in Fig. 2. In all three groups, microhardness increased gradually from inner to outer enamel. At all enamel locations, microhardness values decreased following in vitro and in vivo irradiation as compared to the control group; however, this reduction was significant (p < 0.05) only in middle and outer enamel. Moreover, the microhardness in outer enamel was significantly lower (p < 0.05) in the in vivo irradiated group as compared to the in vitro irradiated. With the detected significant reductions in microhardness, associated study power ranged from 0.96 to 1.0, which is considered very good power [24].
Vickers microhardness test results of control (non-radiated), in vitro irradiated, and in vivo irradiated groups in three regions of enamel: 50 μm from DEJ (inner enamel), 200 μm from DEJ (middle enamel), and 200 μm from outer edge of enamel (outer enamel). Mean and standard deviation values were calculated based on at least 17 indents per region of each specimen of control, in vitro radiated, and in vivo radiated groups. Error bars represent SD. Different letters indicate statistically significant differences between groups within each enamel region (p < 0.05)
Morphology and structural characterization
SEM micrographs of uncoated specimens revealed throughout the section from inner to outer enamel that rods were oriented uniformly perpendicular to the surface and parallel to the axial section (Fig. 3). SEM analysis of Vickers indentations revealed two different patterns: pattern A demonstrating the presence of microcracks plus microdamage around the indent periphery; and pattern B showing clean, sharp indents (Fig. 3). Microcracks and uncracked ligament bridging are the common toughening mechanisms in biocomposite materials such as bone and tooth [25, 26]. Throughout enamel, in the control group, pattern A was demonstrated in approximately 70% of the indents and pattern B was observed in about 30%, as shown in Table 1. Following either in vitro or in vivo radiation, all regions showed a significantly higher (p < 0.05) percentage of pattern B as compared to the percentage of pattern B in the control group.
SEM micrographs of microindentation patterns. Inner enamel 50 μm from DEJ (a–a”), middle enamel 200 μm from DEJ (b–b”), and outer enamel 200 μm from outer surface (c–c”). Within the control group (a, b, c), across enamel regions ~ 70% of the microindentations were pattern A—with microcracks around the indent periphery (white arrows) and uncracked ligament bridging (black arrows). In the in vitro irradiated (a’, b’, c’) and in vivo irradiated (a”, b”, c”) groups, there was a higher percentage of pattern B—sharp, clean microindentations at all three enamel regions of enamel as compared to the control group pattern B percentages (see Table 1). As noted across images, enamel rods were oriented perpendicular to the cut surface in all three regions
Discussion
To evaluate the impact of high-dose oral cancer radiotherapy on the mechanomorphological properties of human enamel, this is the first study to include in vivo irradiated specimens (tooth radiation dose > 60 Gy) in addition to in vitro irradiated specimens (70 Gy dose). Results of this study show that enamel microhardness decreased following in vivo and in vivo irradiation, which is in good agreement with other in vitro studies [11, 12, 27].
In contrast, it has also been reported that enamel microhardness increased following in vitro irradiation [13]; however, this could be related to the type of teeth evaluated, i.e., deciduous teeth were used in that study. In two other studies, enamel microhardness did not change after in vitro irradiation [16, 28], which could be related to differences in irridation or testing protocols. For example, in the current study, a cumulative dose of 70 Gy (2 Gy/day × 5 days/week × 7 weeks) was used to mimic the clinical situation, in contrast to a single tooth dose of 35 to 70 Gy of Co irradiation [28]. Similary, there are testing protocol differences as compared to another study [16] in which enamel specimens were sectioned perpendicular to their oral surface and the hardness of the exposed external surface was tested; while in the current study, specimens were sectioned differently and microhardness of enamel was tested at three different depths.
As already suggested, contrary reports about the effect of radiotherapy on enamel hardness may be partially due to the lack of a standard methodology in microhardness testing. The differences could potentially be related to variations in testing methods or anisotropic mechanical properties. Nanoindentation studies indicate that the mechanical properties of enamel are dependent upon rod orientation. It was observed that hardness and Young’s modulus increased when rods were perpendicular to the direction of testing [29,30,31]. Despite the growing recognition of enamel rod orientation in mechanical properties, the effect of this parameter has not yet been considered in microhardness testing. In previous studies, it has been reported rods were oriented parallel to the cut surface by sectioning the teeth buccolingually or mesiodistally [10, 13, 32, 33]. However, if enamel measurements are to be made across that cut surface, rods are relatively parallel to one another at the occlusal surface but become decussated and oriented irregularly as they progress toward the DEJ [34]. Thus, the rod orientation at the surface is not homogenous and measurements at varying locations across enamel may not be internally comparable. To overcome this limitation, our group focused on developing a novel protocol to obtain homogenous microstructure of enamel specimens where all rods across enamel appeared perpendicular to the cut surface (Fig. 3). It was found that when the rods were oriented uniformly perpendicular to the cut surface, there is no or little enamel dislodgement from the surface after the indentation, which could affect the Vickers indentation pattern and microhardness measurements [35].
In the current study, following in vitro and in vivo irradiation, there was a significant decrease (p < 0.05) in microhardness in middle and outer enamel as compared to the non-radiated control group. The post-radiotherapy microhardness reduction is in agreement with previous in vitro studies [11, 12, 27]; however, those studies did not specify the location where measurements were done within enamel. As a complex bioceramic tissue, enamel has a hierarchical structure with a naturally graded micro-composition resulting from the unique molecular and cellular activities occurring during organogenesis. Functionally graded properties of enamel can be attributed to its elemental composition [36]. Enamel is a highly mineralized tissue [37] consisting predominantly of carbonated hydroxyapatite (96%) with 3% water and a very small amount of organic matrix (~ 1% wt.) [38]. Organic matrix [18, 39, 40] and water [41, 42] are more abundant at the DEJ zone near inner enamel while mineral contents gradually decrease from outer enamel toward the DEJ [43]. Thus, the effect of radiotherapy may vary from outer enamel to inner enamel and is dependent upon the localized micro-composition. As mentioned previously, following in vitro and in vivo irradiation, the hardness of enamel was decreased. This trend was similar throughout enamel in all three regions; however, this reduction was not statistically significant in enamel close to the DEJ where organic content is more prominent.
The reduction of enamel hardness following irradiation can potentially be explained by decarboxylation and demineralization that was reported in a previous study that developed a molecular level model to explain detailed changes in mineralized tissue following irradiation [44]. It was shown that protein matrix side chain carboxylation groups involved with the coordination of apatite bound calcium ions are partially removed by decarboxylation following irradiation [44]. It is believed that the interaction between the organic matrix and apatite crystals is electrostatic in nature involving protein side chain carboxylate groups and apatite phosphate groups via calcium ion bridges. High-dose radiotherapy exposure of teeth, > 60 Gy, could provide sufficient excitation energy to induce decarboxylation and a loss of acidic phosphate groups. The mineral-organic interaction between protein and collagen is reduced, and the development of carbon dioxide may induce microcracks in the hydroxyapatite mineral. The decarboxylation-related changes on a molecular level may affect the mineral content, composition, structure, and subsequently enamel mechanical properties.
Furthermore, the fact that the organic content in enamel is restricted to the innermost part of the tissue, while middle and outer enamel are composed more of mineral content, suggests that decarboxylation cannot be the only reason for reduction of hardness. As shown in Fig. 2, while outer enamel microhardness was significantly lower than the non-radiated control group in both the in vitro and in vivo irradiated groups, outer enamel microhardness was significantly (p < 0.05) lower in the in vivo group as compared to the in vitro group. While these results suggest that the lower enamel microhardness of the in vitro irradiated specimens is related to a direct effect of radiation, the even lower microhardness of the in vivo irradiated specimens is likely multifactorial with direct radiation effects plus indirect effects from radiation-induced xerostomia, which also plays a role in dentition deterioration [1,2,3,4]. Following xerostomia, pH levels in the mouth change and create an acidic environment which leads to an increased potential for demineralization.
In our study, while the hardness of inner enamel 50 μm from the DEJ decreased following in vitro and in vivo irradiation, this reduction was not significant as compared to the control group. A previous study reported that in addition to the effect on the organic content of enamel, radiation also decreased the water content in the inner enamel [45]. It is known that dehydration has an effect on mechanical properties and increases enamel hardness [46, 47]. The contradictory effects of radiation resulting from decarboxylation and dehydration in inner enamel might potentially be one of the reasons why the hardness reduction was not statistically significant in enamel closest to the DEJ. Another possible reason may be due to the high standard deviation of the Vickers hardness measurements in the inner enamel region. The higher standard deviations may due to a high intrinsic variability of enamel near the DEJ. As a broad transitional and anatomic region [48], the DEJ zone is a few micrometers wide [49,50,51] and the Vickers indenter which produces a relatively big footprint may not be sensitive enough to detect differences in detailed information from the complex region of enamel near the DEJ.
To better understand post-radiotherapy changes linked to pathologic enamel delamination, the mechanomorphological properties of enamel were also evaluated by analyzing the microindentation patterns. SEM imaging revealed that microindentation induced microcracks around the indentation periphery in non-radiated specimens (Fig. 3a–c), and interestingly, these microcracks were not visible following in vitro and in vivo irradiation in a majority of the specimens (Fig. 3a’–c”). As shown in Table 1, following in vitro and in vivo irradiation, the percentage of sharp clean indentations (pattern B) was higher (p < 0.05) in all enamel regions as compared to the control group. The highest percentage of pattern B, 65%, was observed in inner enamel close to the DEJ following in vivo radiation. A biocomposite mineralized material such as enamel might be compared to bone because of their structure and directional properties [52]. Like bone, microstructural and compositional features of enamel have a great impact on sustaining mechanical properties. Microcrack generation and uncracked ligament bridging as noted in Fig. 3a–c control (non-radiated) specimens are mechanisms to dissipate energy and are known key contributors to toughening biomaterials such as bone [9, 26, 53,54,55] and tooth [25, 32]. It was found that the fracture toughness of bone increased as microcracks grew [56, 57]. However, it should be emphasized that although the initiation of microcracks is a way of dissipating energy, the suppression of crack growth has to be considered in order to prevent failure [58]. The presence of sharp clean microindentations with the absence of microcracks following radiation suggests that enamel loses energy dissipation efficiency and becomes more brittle. Likewise, it was reported that embrittlement increased in mineralized tissue following X-ray irradiation by the removal of calcium-mediated electrostatic binding of collagen side chain carboxylate groups to apatite phosphate groups [44]. Moreover, as stated before, water plays a critical role in sustaining the mechanical properties as well as toughness of bone and enamel. Dehydration following radiation could lead to a decrease in toughness of teeth [29, 30] and bone [59, 60], and it could be associated with an increased embrittlement of the enamel. However, to confirm the reduction of the toughness following radiation, future quantitative fracture toughness measurement is needed.
Our results suggest the determinant effect of in vitro and in vivo radiotherapy on the mechanomorphological properties of enamel is dependent upon the localized micro-composition of enamel and resulted in changing the natural graded properties of enamel. It appears that following in vitro and in vivo irradiation, inner enamel may be more affected due to dehydration and decarboxylation of tissue making inner enamel more brittle, while the middle and outer edges of enamel may be more affected by demineralization causing these regions to become softer. Moreover, our group previously reported that following radiation, there was a decrease in the enamel protein/mineral ratio near the DEJ [15] as well as a reported post-radiotherapy decrease in immunoreactivity of type IV collagen that is localized near the DEJ [21]. Collectively, radiotherapy appears to play a role in the alteration of the enamel microcomposition and its subsequent graded properties. Therefore, the hypothesis that mechanomorphological properties of dental enamel would change following in vivo and in vitro irradiation could be accepted.
As always, there are limitations with all studies. For example, although the age of the non-radiated and in vitro irradiated tooth specimens was from patients within the same age range, we could not control for the age of the in vivo irradiated teeth from oral cancer patients. In order to maintain equal evaluation groups for statistical testing, the sample size in this study was also limited due to the significant limitation of collecting in vivo irradiated teeth from oral cancer patients. Typically, due to potential complications such as post-extraction radionecrosis of the jaws, teeth are not extracted until they are not restorable and as a result, those teeth/fragments cannot be evaluated. However, despite the limited sample size, statistically significant differences were detected in conjunction with good study power ranging from 0.96 to 1.0. Nevertheless, further studies are needed to elucidate the mechanism whereby radiation alters the ultra-structure of enamel.
Conclusions
Radiation-related caries is a multifactorial problem with contributory factors that include indirect radiotherapy effects on the salivary glands leading to xerostomia as well as previously reported direct radiotherapy effects on mineralized tooth structure. In this study, we evaluated and compared the effects of both in vivo and in vitro irradiation on the mechanomorphological properties of enamel. Our results showed enamel microhardness decreased in all regions of enamel following in vivo and in vitro irradiation; however, this reduction was significant (p < 0.05) only in middle and outer enamel. Likewise, following in vivo and in vitro irradiation, significant changes in the microindentation patterns throughout the enamel sections suggest that enamel loses energy dissipation efficiency and becomes more brittle. Based on the current results, we speculate that high-dose oral cancer radiotherapy induces a negative impact on enamel mechanomorphological properties, which could be one of the contributing factors for post-radiotherapy pathologic enamel delamination that initiates near the DEJ leading to resultant radiation-related caries.
Abbreviations
- DEJ:
-
Dentin-enamel junction
- CEJ:
-
Cementoenamel junction
- Gy:
-
Gray
- PBS:
-
Phosphate buffered saline
- VHN:
-
Vickers hardness number
SEM
Scanning electron microscopy
- GSE:
-
Gaseous secondary electron detector
- ANOVA:
-
Analysis of variance
- SD:
-
Standard deviation
References
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14(3):199–212. https://doi.org/10.1177/154411130301400305
Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Luckel H (2006) Radiation-related damage to dentition. Lancet Oncol 7(4):326–335. https://doi.org/10.1016/S1470-2045(06)70658-1
Karmiol M, Walsh RF (1975) Dental caries after radiotherapy of the oral regions. J Am Dent Assoc 91(4):838–845. 10.14219/jada.archive.1975.0493
Anneroth G, Holm LE, Karlsson G (1985) The effect of radiation on teeth: a clinical, histologic and microradiographic study. Int J Oral Surg 14(3):269–274. https://doi.org/10.1016/S0300-9785(85)80038-7
Silva AR, Alves FA, Antunes A, Goes MF, Lopes MA (2009) Patterns of demineralization and dentin reactions in radiation-related caries. Caries Res 43(1):43–49. https://doi.org/10.1159/000192799
Jongebloed WL, Gravenmade EJ, Retief DH (1988) Radiation caries. A review and SEM study. Am J Dent 1(4):139–146
Jansma J, Vissink A, Jongebloed WL, Retief DH, Johannes’s-Gravenmade E (1993) Natural and induced radiation caries: a SEM study. Am J Dent 6(3):130–136
Walker MP, Wichman B, Cheng A-L, Coster J, Williams KB (2011) Impact of radiotherapy dose on dentition breakdown in head and neck cancer patients. Pract Radiat Oncol 1(3):142–148. https://doi.org/10.1016/j.prro.2011.03.003
Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ (2002) Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer 2:37. https://doi.org/10.1186/1471-2407-2-37
Goncalves LM, Palma-Dibb RG, Paula-Silva FW, Oliveira HF, Nelson-Filho P, Silva LA, Queiroz AM (2014) Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J Dent 42(8):986–992. https://doi.org/10.1016/j.jdent.2014.05.011
Franzel W, Gerlach R (2009) The irradiation action on human dental tissue by X-rays and electrons—a nanoindenter study. Z Med Phys 19(1):5–10. https://doi.org/10.1016/j.zemedi.2008.10.009
Franzel W, Gerlach R, Hein HJ, Schaller HG (2006) Effect of tumor therapeutic irradiation on the mechanical properties of teeth tissue. Z Med Phys 16(2):148–154. https://doi.org/10.1078/0939-3889-00307
de Siqueira MT, Palma-Dibb R, de Oliveira H, Garcia Paula-Silva F, Nelson-Filho P, da Silva R, da Silva L, de Queiroz A (2014) The effect of radiation therapy on the mechanical and morphological properties of the enamel and dentin of deciduous teeth—an in vitro study. Radiat Oncol 9(1):1–7. https://doi.org/10.1186/1748-717x-9-30
Kielbassa AM, Beetz I, Schendera A, Hellwig E (1997) Irradiation effects on microhardness of fluoridated and non-fluoridated bovine dentin. Eur J Oral Sci 105(5 Pt 1):444–447. https://doi.org/10.1111/j.1600-0722.1997.tb02142.x
Reed R, Xu C, Liu Y, Gorski JP, Wang Y, Walker MP (2015) Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine. Arch Oral Biol 60(5):690–697. https://doi.org/10.1016/j.archoralbio.2015.02.020
Kielbassa AM, Wrbas KT, Schulte-Monting J, Hellwig E (1999) Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch Oral Biol 44(3):243–251. https://doi.org/10.1016/S0003-9969(98)00123-X
Kielbassa AM, Munz I, Bruggmoser G, Schulte-Monting J (2002) Effect of demineralization and remineralization on microhardness of irradiated dentin. J Clin Dent 13(3):104–110
Dusevich V, Xu C, Wang Y, Walker MP, Gorski JP (2012) Identification of a protein-containing enamel matrix layer which bridges with the dentine–enamel junction of adult human teeth. Arch Oral Biol 57(12):1585–1594. https://doi.org/10.1016/j.archoralbio.2012.04.014
McGuire JD, Walker MP, Dusevich V, Wang Y, Gorski JP (2014) Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction. Connect Tissue Res 55(S1):33–37. https://doi.org/10.3109/03008207.2014.923883
McGuire JD, Walker MP, Mousa A, Wang Y, Gorski JP (2014) Type VII collagen is enriched in the enamel organic matrix associated with the dentin–enamel junction of mature human teeth. Bone 63:29–35. https://doi.org/10.1016/j.bone.2014.02.012
McGuire JD, Gorski JP, Dusevich V, Wang Y, Walker MP (2014) Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy. J Dent Res 93(10):1028–1034. https://doi.org/10.1177/0022034514548221
Chuenarrom C, Benjakul P, Daosodsai P (2009) Effect of indentation load and time on Knoop and Vickers microhardness tests for enamel and dentin. Mater Res 12(4):473–476. https://doi.org/10.1590/S1516-14392009000400016
Yao H, Dao M, Imholt T, Huang J, Wheeler K, Bonilla A, Suresh S, Ortiz C (2010) Protection mechanisms of the iron-plated armor of a deep-sea hydrothermal vent gastropod. PNAS 107(3):987–992. https://doi.org/10.1073/pnas.0912988107
Cohen J (1988) Statistical power analysis for the behavioral sciences (2nd ed). Lawrence Erlbaum Associates, Inc, Hillsdale, p 52–56
Kruzic JJ, Nalla RK, Kinney JH, Ritchie RO (2003) Crack blunting, crack bridging and resistance-curve fracture mechanics in dentin: effect of hydration. Biomaterials 24(28):5209–5221. https://doi.org/10.1016/S0142-9612(03)00458-7
Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO (2005) Mechanistic aspects of fracture and R-curve behavior in human cortical bone. Biomaterials 26(2):217–231. https://doi.org/10.1016/j.biomaterials.2004.02.017
Qing P, Huang S, Gao S, Qian L, Yu H (2015) Effect of gamma irradiation on the wear behaviour of human tooth enamel. Sci Rep 5(1):11568. https://doi.org/10.1038/srep11568
Markitziu A, Gedalia I, Rajstein J, Grajover R, Yarshanski O, Weshler Z (1986) In vitro irradiation effects on hardness and solubility of human enamel and dentin pretreated with fluoride. Clin Prev Dent 8(4):4–7
Staines M, Robinson WH, Hood JAA (1981) Spherical indentation of tooth enamel. J Mater Sci 16(9):2551–2556. https://doi.org/10.1007/BF01113595
Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP (2002) Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol 47(4):281–291. https://doi.org/10.1016/S0003-9969(02)00006-7
Habelitz S, Marshall SJ, Marshall GW Jr, Balooch M (2001) Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol 46(2):173–183. https://doi.org/10.1016/S0003-9969(00)00089-3
An B, Wang R, Arola D, Zhang D (2012) The role of property gradients on the mechanical behavior of human enamel. J Mech Behav Biomed Mater 9:63–72. https://doi.org/10.1016/j.jmbbm.2012.01.009
He LH, Swain MV (2009) Enamel-a functionally graded natural coating. J Dent 37(8):596–603. https://doi.org/10.1016/j.jdent.2009.03.019
Bajaj D, Arola D (2009) Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomater 5(8):3045–3056. https://doi.org/10.1016/j.actbio.2009.04.013
Xu C, Dusevich V, Reed R, Thiagarajan G, Gorski JP, Walker MP, Wang Y (2013) Enamel rod orientation effect on fracture toughness measurements. J Dent Res 92(Spec Iss B):842
Xu C, Reed R, Gorski J, Wang Y, Walker M (2012) The distribution of carbonate in enamel and its correlation with structure and mechanical properties. J Mater Sci Mater Med 47(23):8035–8043. https://doi.org/10.1007/s10853-012-6693-7
Fincham AG, Moradian-Oldak J, Simmer JP (1999) The structural biology of the developing dental enamel matrix. J Struct Biol 126(3):270–299. https://doi.org/10.1006/jsbi.1999.4130
Ten Cate AR (ed) (1998) Oral histology: development, structure, and function, 5th edn. Mosby-Year Book, Inc, St. Louis. https://doi.org/10.1016/0278-2391(90)90217-P
Lin CP, Douglas WH (1994) Structure-property relations and crack resistance at the bovine dentin-enamel junction. J Dent Res 73(5):1072–1078. https://doi.org/10.1177/00220345940730050901
Lin CP, Douglas WH, Erlandsen SL (1993) Scanning electron microscopy of type I collagen at the dentin-enamel junction of human teeth. J Histochem Cytochem 41(3):381–388. https://doi.org/10.1177/41.3.8429200
Zahradnik RT, Moreno EC (1975) Structural features of human dental enamel as revealed by isothermal water vapour sorption. Arch Oral Biol 20(5–6):317–325. https://doi.org/10.1016/0003-9969(75)90021-7
Dibdin GH, Poole DFG (1982) Surface area and pore size analysis for human enamel and dentine by water vapour sorption. Arch Oral Biol 27(3):235–241. https://doi.org/10.1016/0003-9969(82)90057-7
Robinson C, Weatherell JA, Hallsworth AS (1971) Variation in composition of dental enamel within thin ground tooth sections. Caries Res 5(1):44–57. https://doi.org/10.1159/000259731
Hubner W, Blume A, Pushnjakova R, Dekhtyar Y, Hein HJ (2005) The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: an FT-IR microscopic investigation. Int J Artif Organs 28(1):66–73
Poyton HG (1989) Oral radiology. B.C. Decker Inc, Philadelphia, p 17–22
He LH, Swain MV (2007) Influence of environment on the mechanical behaviour of mature human enamel. Biomaterials 28(30):4512–4520. https://doi.org/10.1016/j.biomaterials.2007.06.020
Baldassarri M, Margolis HC, Beniash E, Baldassarri M, Margolis HC, Beniash E (2008) Compositional determinants of mechanical properties of enamel. J Dent Res 87(7):645–649. https://doi.org/10.1177/154405910808700711
White SN, Paine ML, Luo W, Sarikaya M, Fong H, Yu Z, Li ZC, Snead ML (2000) Dentino-enamel junction is a broad transitional zone uniting dissimilar bioceramic composites. J Am Ceram Soc 83(1):238–240. https://doi.org/10.1111/j.1151-2916.2000.tb01181.x
Marshall GW Jr, Balooch M, Gallagher RR, Gansky SA, Marshall SJ (2001) Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res 54(1):87–95. https://doi.org/10.1002/1097-4636(200101)54:1<87::AID-JBM10>3.0.CO;2-Z
Habelitz S, Marshall SJ, Marshall GW Jr, Balooch M (2001) The functional width of the dentino-enamel junction determined by AFM-based nanoscratching. J Struct Biol 135(3):294–301. https://doi.org/10.1006/jsbi.2001.4409
Balooch G, Marshall GW, Marshall SJ, Warren OL, Asif SA, Balooch M (2004) Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J Biomech 37(8):1223–1232. https://doi.org/10.1016/j.jbiomech.2003.12.012
Mullins LP, Bruzzi MS, McHugh PE (2007) Measurement of the microstructural fracture toughness of cortical bone using indentation fracture. J Biomech 40(14):3285–3288. https://doi.org/10.1016/j.jbiomech.2007.04.020
Vashishth D, Tanner KE, Bonfield W (2000) Contribution, development and morphology of microcracking in cortical bone during crack propagation. J Biomech 33(9):1169–1174. https://doi.org/10.1016/S0021-9290(00)00010-5
Zioupos P (2001) Accumulation of in-vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microsc 201(Pt 2):270–278. https://doi.org/10.1046/j.1365-2818.2001.00783.x
Kahler B, Swain MV, Moule A (2003) Fracture-toughening mechanisms responsible for differences in work to fracture of hydrated and dehydrated dentine. J Biomech 36(2):229–237. https://doi.org/10.1016/S0021-9290(02)00327-5
Vashishth D, Behiri JC, Bonfield W (1997) Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech 30(8):763–769. https://doi.org/10.1016/S0021-9290(97)00029-8
Norman TL, Vashishth D, Burr DB (1995) Fracture toughness of human bone under tension. J Biomech 28(3):309–320. https://doi.org/10.1016/0021-9290(94)00069-G
Burr D (2003) Microdamage and bone strength. Osteoporos Int 14(Suppl 5):S67–S72. https://doi.org/10.1007/s00198-003-1476-2
Broz JJ, Simske SJ, Greenberg AR, Luttges MW (1993) Effects of rehydration state on the flexural properties of whole mouse long bones. J Biomech Eng 115(4a):447–449. https://doi.org/10.1115/1.2895510
Currey JD (1988) The effects of drying and re-wetting on some mechanical properties of cortical bone. J Biomech 21(5):439–441. https://doi.org/10.1016/0021-9290(88)90150-9
Acknowledgements
The authors want to acknowledge Steven Howard, (Kansas City Cancer Centers; Kansas City, KS) for his assistance with simulated radiotherapy treatment of tooth specimens.
Funding
This work was supported by the National Institute of Dental and Craniofacial Research grant R01DE021462.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For collection of any extracted teeth from oral cancer patients who had received previous radiotherapy, the tooth collection protocol that was approved by an institutional review board (IRB13-143) included informed consent explaining to the patient how their extracted teeth and radiotherapy records could potentially be used in a research project.
Rights and permissions
About this article
Cite this article
Seyedmahmoud, R., Wang, Y., Thiagarajan, G. et al. Oral cancer radiotherapy affects enamel microhardness and associated indentation pattern morphology. Clin Oral Invest 22, 1795–1803 (2018). https://doi.org/10.1007/s00784-017-2275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2275-z