Abstract
Objectives
Post-radiation dental lesions affect mainly the cervical area of the tooth. Until now, there are quite few evidences regarding the effects of radiation exposure on root dentin breakdown. To better understand this effect, we used human root dentin specimens obtained from third molars from similarly aged individuals.
Materials and methods
Twenty specimens were analyzed by the surface hardness (SH), energy-dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD) to evaluate the baseline properties of their root dentin. Other six human teeth were prepared and analyzed by scanning electron microscopy (SEM). Then the specimens were randomly distributed between two groups (n = 13 per group) and irradiated with a total dose of 55 or 70 Gy in a linear accelerator. The percentage of EDX and surface hardness loss (%SHL) were determined based on measurements before and after irradiation. The specimens were also analyzed after irradiation by SEM and XRD. The Ca/P weight ratio was calculated.
Results
Based on SEM analysis, radiation exposure induced dehydration of the dentin. The Ca/P weight ratio decreased (p = 0.0045). The %SHL of specimens irradiated with 70 Gy was higher than that of the 55-Gy group (p < 0.05), although even the lower dose induced root dentin breakdown.
Conclusions
Overall, we can state that radiation exposure changes the composition and structure of human root dentin, which detrimentally affect its hardness.
Clinical relevance
The changes reported herein might influence the selection of the dental materials and will bring new knowledge in this field to prevent radiation-related caries in root dentin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy is an ionizing radiation-based therapeutic approach that is widely used for cancer patients. When used for head-and-neck cancer treatment, it may cause adverse effects in the oral cavity [1], including mucositis, hyposalivation, osteoradionecrosis, dentition breakdown, and radiation-related caries [2, 3]. It was previously thought that radiation-induced hyposalivation was the main cause of radiation-related caries development [4]. In contrast, recent investigations have suggested that radiation has direct effects on tooth destruction and post-radiation dental caries [5,6,7].
Post-radiation dental lesions affect mainly the cervical area of the tooth [8, 9], which include dentin root caries as a notable clinical complication. Human dentin is a complex tissue [10] that is highly soluble, possibly because of its less mineral content when compared to enamel and higher levels of carbonate and magnesium [11]. Therefore, dentin root caries rapidly progress, and this condition may lead to severe tooth destruction [9], which in turn also increases the risk of developing osteoradionecrosis [12] and negatively impacts the quality of life of the patient [13].
Whereas several studies have measured the mechanical properties of dental teeth after radiation exposure [3, 6, 14], there is a lack of knowledge concerning its effect on human dental structure and composition. In addition, most studies have focused on enamel [6, 7] or coronal dentin [14, 15] but not on root dentin. The structural pattern of coronal dentin differs from that of root dentin. The tubules run continuously from the dentin-enamel junction to the pulp in coronal dentin, and from the cementum-dentin junction to the pulp canal in the root [16]. This microstructure is related to the functional behavior of the tissue, as, for instance, the alignment of the tubules could affect its mechanical properties. Likewise, in the case of restorative procedures, changes in the substrate composition could potentially interfere with interactions with the restorative materials, especially during the adhesion process [17].
To date, no systematic study has been carried out relating the effect of radiation exposure on the chemical elements, structure, and mechanical properties of root dentin. With the combined use of energy-dispersive X-ray spectroscopy (EDX), microhardness, and X-ray diffraction (XRD), we evaluated these aspects of human root dentin before and after radiation exposure. In addition, scanning electron microscopy (SEM) was used to better understand the structure of radiated dentin. The null hypothesis was that radiation has no effect on root dentin.
Materials and methods
Experimental design
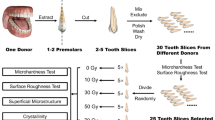
This in vitro study involved one factor: radiation exposure. Human root dentin specimens were obtained from fresh sound third molars. They were stored in a solution of 0.2% thymol for no longer than 1 month after extraction, then cleaned of gross debris and placed in distilled water for 24 h before the beginning of the experiment [18]. The samples with similar surface hardness (SH) and from individuals of similar age (18 to 25 years old) were randomly divided into two groups (n = 13 teeth per group) that were irradiated with 55 or 70 Gy, in which 6 of them (n = 3 per group) were used to evaluate SEM analysis before and after radiation exposure. The percentage surface hardness loss (%SHL) and EDX were determined based on measurements before and after irradiation. The Ca/P weight ratio was determined by weight. In addition, the teeth were analyzed before and after irradiation by XRD and SEM (this response variable used different specimens). The assessments done in this study was blinded performed by the examiner according to the measurements attributed to the groups exposed with 55 vs 70 Gy vs control doses for the different purposed analyses.
Ethical considerations
Ethical approval for this study involving human teeth was granted by the local Ethics Committee (No. 49812515.1.0000.5417). Sound human third molars free of caries, from individuals 18 to 25 years old, were collected with informed donor consent.
Specimen preparation
Buccal flattened and polished dentin specimens (4 × 6 × 3 mm) were obtained from the cervical roots of sound freshly extracted human molar teeth. The crowns were removed at the cemento-enamel junction using an ISOMET low-speed saw cutting machine (Buehler Ltd., Lake Bluff, IL, USA). The surfaces were ground flat and polished in a metallographic polisher (Aropol 2 V; Arotec, Cotia, SP, Brazil) using water-cooled carborundum discs (600 and 1200 grades of Al2O3 papers-CarbiMet paper discs; Buehler, Lake Bluff, IL, USA) and felt paper wet by diamond spray (1 μm; Buehler). All the specimens were ultrasonically cleaned in distilled water for 10 min to remove the debris.
The specimens were randomly divided (Excel 15.0, Microsoft, Redmond, WA, USA) into two groups: irradiated 55 Gy (55 Gy of radiation, n = 10) and irradiated 70 Gy (70 Gy of radiation, n = 10). All the specimens were analyzed before and after irradiation using EDX, XRD, and SH. The %SHL was calculated. In addition, other six human teeth were prepared for SEM. This analysis was conducted in different specimens before and after irradiation, due to the preparation of the specimens.
Gama irradiation exposure
The dentin specimens directly received a total dose of 55 or 70 Gy of radiation in a linear accelerator (Varian, Clinac 6EX, Palo Alto, CA, USA), to simulate clinical situations for the treatment for head-and-neck cancer patients [9, 13]. The irradiation used herein was based on intensity-modulated radiotherapy (IMRT). During the radiation exposure, the samples were mounted on stubs and remained completely submerged in water with 5 mL deep per specimen.
EDX analysis
For the analysis of the percentage component composition of the root dentin, EDX assessment was performed. The X-ray detector system was attached to a scanning electron microscope (FEI-Inspect S50, LNNano) operating at 20.0 kV, using a 5-nm spot size. This method allowed determining the relative amounts of calcium (Ca), phosphorus (P), oxygen (O), carbon (C), and magnesium (Mg) by volume percent.
SH tests
Baseline SH of the dentin specimens was determined by three indentations using a Knoop diamond indenter spaced 100 μm from each other. Assessments were made with 10-g load for 10 s, using a Buehler Ltd., MicroMet 6040 (Buehler, Lake Bluff, IL, USA). The specimens presenting baseline SH 60.07 ± 1.55 were selected for this study.
At the end of each radiation exposure, SH of the specimens was again determined at 100-μm distance from each other. The mean values of the baseline values were also averaged, and the percentage of surface hardness loss [%SHL = 100 (SH after radiation − baseline SH)/baseline SH] was calculated.
SEM analysis
SEM analysis was performed using the specimens from non-irradiated (control; n = 6) and irradiated teeth after the total dose of 55 Gy (n = 3) and 70 Gy (n = 3). The SEM-prepared specimens were cleaned for 10 min in an ultrasonic bath with purified water. The specimens were fixed on stubs with a double-sided adhesive carbon tape (Electron Microscopy Sciences, Washington, PA, USA) and were sputter-coated with gold in a vacuum metallizing machine (SDC 050; Bal-Tec AG, Balzers, Germany). The specimens were examined with a scanning electron microscope (Philips XL30 FEG, Eindhoven, The Netherlands). The images were observed by SEM at an accelerating voltage of 15 kV, a working distance of 20 mm, and with a magnification of × 10,000 and × 20,000.
Control and post-irradiation tissue morphological changes were analyzed using two score systems, in the magnification of × 1000. For dentin tubules, scores were attributed as follows, based on previous described criteria [15]: (0) Regular, (1) Partially obliterated, and (2) Totally obliterated. The presence of cracks and fissures was classified as (0) Absent or (1) Present.
XRD analysis
XRD was carried out using an X-ray diffractometer powder system (Rigaku Geigerflex, Woodland, TX) with Ni-filtered CuKα radiation and a source operating at 40 kV and 25 mA. The data were collected in the 2θ range from 10 to 70°.
Statistical analyses
Statistical analyses were performed with the Statistica software (SSP) version 10.0 (Statsoft®, Tulsa, Oklahoma, USA). Control and post-irradiation values were analysed for SH and EDX using two-way analysis of variance and Tukey’s tests at a level of significance of 5%. Qualitative analyses were performed for SEM and XRD.
Results
EDX and SEM
The atomic percentages of Ca, P, O, C, Mg, and the Ca/P weight ratio on the dentin were determined by EDX (Table 1 and Fig. 1). The atomic percent of C tended to decrease in all of the groups after irradiation with statistically significant difference (p = 0.03). O and Mg increased with significant difference in the groups (p = 0.000006 and p = 0.00061, respectively). Major changes in the chemical composition of dentin were observed in trace elements. Ca/P molar ratio decreased (p = 0.0045).
EDX/SEM results of studied groups. a, e, and i Non-irradiated root dentin (× 10,000 and × 20,000) with the absence of cracks and fractures in the dentinal structure (score 0). b, f, and j Irradiated 55 Gy showing the presence of cracks with dentin tubules partially obliterated (× 10,000 and × 20,000) (score 1). c, g, and k Irradiated 70 Gy showing presence of cracks and fractures with dentin tubules obliterated (× 10,000 and × 20,000) (score 1). Arrows mean the presence of cracks and fissures. d, h, and l are related to the spectra of EDX analyses of the main components of dentin surface
The morphological changes were observed using SEM images, which are present in Fig. 1. There were distinct differences between the morphologies of dentin before and after treatment in both irradiated specimens. SEM images suggested that the radiation induced a dehydration of the dentin indicated by the presence of cracks around of the tubules (Fig. 1). Before radiation, the score attributed for dentin tubules was (0) Regular (Fig. 1a). After 55 Gy, dentin tubules were partially obliterated (1) (Fig. 1b) and after 70 Gy, they were totally obliterated (2) (Fig. 1c). The presence of cracks and fissures was classified as Absent (0) for no-irradiated teeth (Fig. 1e, i) and Present (1) for irradiated teeth (Fig. 1f, j, g, and k).
X-ray diffraction
Figure 2 shows the XRD results of the dentin specimens’ surface, before and after radiation therapy with 55 and 70 Gy. The pattern of dentin produced peaks that were similar in their intensity and position in the baseline and irradiated with 55 Gy. When teeth were irradiated with 70 Gy, there was a remarkable disorganization of dentin apatite crystals.
Mechanical properties of root dentin—SH
The changes in the hardness values of root dentin and the %SHL are presented in Table 2. There was no significant difference regarding SH among dentin specimens before irradiation (p > 0.05). A significant reduction of SH mean was observed in all dentin specimens after irradiation (p < 0.05). The %SLH of the specimens irradiated with 70 Gy was higher than the specimens irradiated with 55 Gy (p < 0.05).
Discussion
The focus of our studies has been the root substrate dentin [3, 19]. Post-radiation dental lesions, which develop mainly in the cervical and incisal area, differ in their location and pattern when compared with caries lesions in patients who are not exposed to radiation [8, 9]. As a result, the root dentin of a patient undergoing radiotherapy might be directly exposed to radiation, which, in combination with reduced salivary flow because of radiotherapy or medication, might particularly predispose them to root caries. Based on the present results, radiation exposure affected the chemical composition, structural, and mechanical property (surface hardness) of root dentin. Therefore, our null hypothesis was rejected.
Our results showed a decrease in the Ca/P weight ratio (Table 1, p < 0.05), which indicates that radiation exposure altered the inorganic and organic components of human root dentin [20]. The Ca/P weight ratio and Ca/P molar ratio determine the rate of hydroxyapatite mineralization [20], an important parameter, as both the mechanical properties of the tooth substrate and its rate of biodegradation strongly depend on it [20]. This ratio was calculated for stoichiometric hydroxyapatite (HA; Ca/P weight ratio = 2.151 and Ca/P molar ratio = 1.67) [20], which varies according to the level of tissue mineralization. With regard to the changes in the inorganic components shown herein, the lower values of the Ca/P weight after radiation exposure in both the 55 and 70-Gy groups (p < 0.05) indicate that the irradiated root dentin structure was less mineralized with respect to Ca content than the sound one. This alteration might have decreased the permeability and solubility of the substrate [21], which also explains the reduction in SH and the increase in %SHL after irradiation. Our results are in agreement with the pioneer previous study of Kielbassa et al. [22] that evaluated the effects of irradiation on microhardness of dentin and concluded that dentin is severely affected by irradiation [22]. This study started a series of investigations by different research groups around the world regarding the influence on the establishment and development of root caries lesions undergone radiotherapy irradiation. In 2000, Kielbassa [23] also investigated the onset of initial demineralization in irradiated and non-irradiated human dentin, through an in situ design investigation, concluding that irradiated dentin is not more susceptible to caries than non-irradiated, if adequate oral hygiene techniques are implemented. According to our results, it is also important to evaluate the direct effect of irradiated teeth and dental caries using a biological in vitro model with biofilm in further studies, to see if the chemical alterations can be related with caries progression in root dentin.
Another important factor to be considered is the high water content of dentin [10]. The interaction between radiation and water is high [24]. When radiolysis occurs, H+ and OH− are released and then it can interact with other ions to produce new compounds. This explains the decrease in C ions and the lower values of the Ca/P weight after radiation exposure. We also observed the incorporation of O and Mg after irradiation with 55 and 70 Gy (Table 2, p = 0.000006 and p = 0.00061, respectively). Once more, the ions released by water after radiation exposure induced the formation of a secondary non-apatitic calcium phosphate phase, which likely would have made the HA more susceptible to degradation [21]. Furthermore, Mg as a substituent component inhibits crystal growth and strongly influences the lattice parameters, which might have made the apatite amorphous. This alteration may also contribute to cracks and the obliteration of dentin structure as shown by SEM images (Fig. 1b, c), as the presence of a less well-structured crystal arrangement increases the permeability and susceptibility of that substrate to cracks [25]. These structural defects can make the dentin dry and friable [15], which also impairs its mechanical resistance, which also could be related to the faster development of caries lesions in irradiated substrates. The formation of free radicals by radiolysis [24] within their structure can be present in irradiated teeth for long periods of time.
It is important to state that in clinical scenario, saliva is dramatically reduced in the patients undergoing radiotherapy treatment [2]. Therefore, the expected regular mineral changes between dental structure and oral environment are altered, which was for years considered the main concern for indirect-related root caries development (unprotection of teeth by saliva). In this way, the use of water in the present investigation was supported by the reason only to avoid dehydration, focusing on the impact of radiation dose itself on the dentin tissue. After these assessments, we can promote future investigations on the presence of saliva (artificial or natural).
All these changes in the substrate (i.e., reduced Ca mineralization or presence of free radicals within its structure) could also negatively interfere with the adhesion of the restorative dental materials that are commonly used to treat such lesions. The material of choice for root caries lesion restoration is the resin-modified glass ionomer cements, which involve in their chemical process the formation of ionic bonds between the carboxylate groups on the polyacid molecules and Ca ions in the tooth surface besides its fluoride rechargeable dynamic that could aid to prevent secondary caries [26]. However, more robust evidence still is necessary for the decision regarding the best restorative material. The established consensus is attributed to the use of adhesive materials. More investigations have been exploring this impact between irradiated substrate and restorative strategies in order to serve longer, even still controversial outcomes have been pointed out [27,28,29,30]. More recently, some adhesive systems that are based on functional monomers, such as 10-methacryloyloxidecyldihydrogen phosphate, must be used with caution, as this system promotes chemical bonding to hydroxyapatite Ca ions [31]. Current available information still impulses the researchers for continuing to investigate the substrate details attributed to radiotherapy influence [32].
With respect to the changes in the organic components of dentin, they could be partially explained by the induction and activation of enzymes that degrade collagens by radiation exposure, such as matrix metalloproteinases [32]. When collagen type IV is degraded, an instability in the substrate occurs [5], which explains the dentin breakdown presented herein (Fig. 1). Because collagen IV has a large biochemical/structural role in molecular bonding of enamel and dentin [5], irradiation of root dentin could negatively interfere with the adhesion process. When patients had undergone radiotherapy, more recently, not only the structural damage is of concern. In the light of the knowledge of enzymatic participation on formation and degradation of dentin in distinct circumstances, studies as purposed by Gomes-Silva et al. [33] run specific tests to evaluate any overexpression of MMP-20, a relevant dentin metalloproteinases related to the caries lesions. Based on the hypothesis that it could be over activated due to radiotherapy, immunohistochemically and morphological assessments were performed. However, no evidences pointed out regarding organic alterations.
In our study, X-ray diffractograms were used to analyze changes in the crystalline structure of the specimens. When X-rays interact with a crystalline substance, such as dental substrates, a diffraction pattern is obtained. Figure 2 shows a remarkable disorganization of dentin apatite crystals after irradiation with 70 Gy. However, irradiation did not induce a reduction in dentin crystallinity based on the XRD analysis, as the pattern of dentin before and after irradiation produced peaks that were similar in their intensity and position. These data indicate a comparable level of crystalline domains among the specimens before and after irradiation (Fig. 2). It is important to note that a new peak was observed between 20 and 25° after 70-Gy irradiation, but not after 55 Gy. This could be explained by the interaction between the high-energy X-ray irradiation and intrafibrillar mineral in root dentin and water which might have influenced its elastic behavior [34] and resulted in the observed pattern.
Based on our findings, we can state that root dentin is extremely vulnerable to the effects of radiation. However, a limitation of the present study should be noted. The irradiation used herein was based on intensity-modulated radiotherapy (IMRT) [35]. In a clinical situation, this method presents great advantages, mainly from the use of 360° rotation radiation therapy, which allows the primary target to receive the total amount of radiation necessary for treatment; whereas, the dose to the adjacent critical structures and organs at risk is limited [8, 13]. Despite the advantages of this method, it is quite expensive and in developing countries is not the method of choice [12]. Clinically, even with the use of the IMRT method to treat head-and-neck cancer, the teeth are located close to the targeted area and exposure of hard dental tissue cannot be prevented [13]. Because of the use of the IMRT method and its associated costs, the radiation exposure applied herein was not fractioned, as commonly indicated in clinical situations [13]. However, as the dose is cumulative, the final dose is the same and thus should be the effects. The literature shows that the fractionated doses is used to avoid alterations in the salivary glands and soft tissues and the dose is cumulative [36]; thus, once our model is not a physicochemical model and not biological, it is not necessary to use fractionated dose. Additionally, previous in vitro studies, without the involvement of cells or soft tissues, did not use fractionated dose either [37, 38]. We can also compare our results with the study that evaluated the effect of radiation on mechanical properties of root dentin [18]. Soares et al. [18] evidenced that the use of fractionated dose showed similar results in relation to the organic matrix, validating our results as well by means on the analysis of mechanical tests. In addition, it was expected the same effects in the final dose compared to the fractionated one, once clinically, the flow and the quality of the saliva in a patient undergoing radiotherapy are reduced, and the mineral deposition between each dose does not recover completely [36]. Also, fluoridation with acidic gels does not prevent softening due to radiation, when saliva is absent [22].
It is also important to note that when the specimens were subjected to radiation exposure, they were completely submerged in water (5.0 mL per specimen), minimizing dehydration of the irradiated area and energy absorption, to receive the required dose. This also maintained ideal conditions for the analysis and maintained the intrinsic water content of dentin. As dentin is a highly complex, hydrated biological tissue, changes in its microstructure, and composition could have influenced its mechanical properties [10]. In addition, we included similarly aged teeth in this analysis, to avoid difference in the chemical composition and microstructure of dentin.
An important finding from the present investigation is that—unlike the study of Liang et al. [39], in which a total dose of 50 Gy had little additional effect on dentin—our results showed damage in the teeth using about this same dose (Table 2 and Fig. 1). However, in agreement with this same study, we suggest that irradiation around 50 Gy could be the key dose that calls for anticipated preventive action against radiation-related caries (Tables 1 and 2 and Fig. 1).
The destructive properties of radiation in the head and neck region have many additional consequences to the oral tissues and oral function. One of the most known side effects is the alterations in the parotic and submandible salivary glands. This often provokes a reduction in quantity (hyposalivation) as well as a reduced quality of the saliva [13, 36]. Overall, this study also indicates that radiation exposure changes the composition and structural of human root dentin, which may contribute to deleterious changes in its mechanical properties.
We observed that a higher dose results in greater damage to the teeth. SEM analysis, as unique visual evaluation, was also in accordance to the responses noted by means of the other quantitative analyses of this study, aided to support the conclusions of this study.
In conclusion, the concern about the implications of radiotherapy on dental hard tissue has been increasing around the world, mostly stimulated by the increasing prevalence of head-and-neck cancers and the new possibilities of their treatment. Therefore, it is expected that investigations regarding the mechanism involved under the light of biological, physical, mechanical, and chemical reactions are explored, individually and adjunctively. We still are far away from the consensus for the best clinical approach, even adhesive materials and strategies are adequate for sure. Therefore, the comprehension about the substrate seems to be the key for its indication. As mostly of the investigations addressed for the structural changes [29, 32], in the present study, the notable alteration of chemical elements also observed by the SEM images is in accordance to previous studies that also pointed out for this alteration. This data support the observations reported by Kiebalssa et al. [22] who highlighted for the changes of mineral contents by means of microhardness assessment. As more fragile mineral arrangement is formed and based on the studies as performed by Springer et al. [32] who observed collagen structure compromising, respectively, researchers and clinicians may be looking for approaches that could conciliate the reinforcement of the collagen frame as the maintenance of the mineral arrangement. Studies regarding biomimetic action of collagen and the role of dentin enzymatic content sound to be interesting to join the information for these investigations, likely been the next step for this research field.
References
Spetch L (2002) Oral complications in the head and neck irradiated patient. Introduction and scope of the problem. Support Care Cancer 10:36–39
Craddock HL (2006) Treatment and maintenance of a dentate patient with ‘radiation caries’. Dent Update 33(8):462–468. https://doi.org/10.12968/denu.2006.33.8.462
Campos Velo MMA, Farha ALH, da Silva Santos PS, Shiota A, Sansavino SZ, Souza ATF, Honório HM, Wang L (2017) Gamma radiation increases the risk of radiation-related root dental caries. Oral Oncol 71:184–185. https://doi.org/10.1016/j.oraloncology.2017.06.007
Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14(3):199–192. https://doi.org/10.1177/154411130301400305
McGuire JD, Gorski JP, Dusevich V, Wang Y, Walker MP (2014) Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy. J Dent Res 93(10):1028–1034. https://doi.org/10.1177/0022034514548221
Qing P, Huang SB, Gao SS, Qian LM, Yu HY (2015) Effect of gamma irradiation on the wear behavior of human tooth enamel. Sci Rep 23:1156
Madrid CC, de Pauli PM, Line SR et al (2017) Structural analysis of enamel in teeth from head-and-neck cancer patients who underwent radiotherapy. Caries Res 51(2):119–128. https://doi.org/10.1159/000452866
Jongebloed WL, Gravenmade EJ, Retief DH (1998) Radiation caries. A review and SEM study. Am J Dent 1:139–146
Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Luckel H (2006) Radiation-related damage to dentition. Lancet Oncol 7(4):326–335. https://doi.org/10.1016/S1470-2045(06)70658-1
Mjör IA (1972) Human coronal dentin: structure and reactions. Oral Surg Oral Med Oral Pathol 33(5):810–813. https://doi.org/10.1016/0030-4220(72)90451-3
Hoppenbrouwers PM, Driessens FC, Borggreven JM (1987) The mineral solubility of human tooth roots. Arch Oral Biol 32(5):319–322. https://doi.org/10.1016/0003-9969(87)90085-9
Silva AR, Alves FA, Berger SB, Giannini M, Goes MF, Lopes MA (2010) Radiation-related caries and early restoration failure in head and neck cancer patients: a polarized light microscopy and scanning electron microscopy study. Support Care Cancer 18(1):83–97. https://doi.org/10.1007/s00520-009-0633-3
Lieshout HF, Bots CP (2014) The effect of radiotherapy on dental hard tissue—a systematic review. Clin Oral Investig 18(1):17–24. https://doi.org/10.1007/s00784-013-1034-z
Qing P, Huang S, Gao S, Qian L, Yu H (2016) Effect of gamma irradiation on the wear behavior of human tooth dentin. Clin Oral Investig 20(9):2379–2386. https://doi.org/10.1007/s00784-016-1731-5
Gonçalves LM, Palma-Dibb RG, Paula-Silva FW, Oliveira HF, Nelson-Filho P, Silva LA et al (2014) Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J Dent 42(8):986–992. https://doi.org/10.1016/j.jdent.2014.05.011
Kinney JH, Marshall SJ, Marshall GW (2003) The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med 14(1):13–19. https://doi.org/10.1177/154411130301400103
Ryou H, Niu LN, Dai L, Pucci CR, Arola DD, Pashley DH, Tay FR (2011) Effect of biomimetic remineralization on the dynamic nanomechanical properties of dentin hybrid layers. J Dent Res 90(9):1122–1128. https://doi.org/10.1177/0022034511414059
Soares CJ, Castro CG, Neiva NA, Soares PV, Santos-Filho PC, Naves LZ et al (2010) Effect of gamma irradiation on ultimate tensile strength of enamel and dentin. J Dent Res 89(2):159–164. https://doi.org/10.1177/0022034509351251
Tan H, Richards L, Walsh T et al (2017) Interventions for managing root caries—protocol. Cochrane Database Syst Rev 8:1–10
Slosarczyk A, Piekarczyk J (1999) Ceramic materials on the basis of hydroxyapatite and tricalcium phosphate. Ceram Int 25(6):561–565. https://doi.org/10.1016/S0272-8842(98)00019-4
Celik EU, Ergücü Z, Türkün LS, Türkün M (2008) Effect of different laser devices on the composition and microhardness of dentin. Oper Dent 33(5):496–491. https://doi.org/10.2341/07-127
Kielbassa AM, Beetz I, Schendera A, Hellwig E (1997) Irradiation effects on microhardness of fluoridated and non-fluoridated bovine dentin. Eur J Oral Sci 105(5P1):444–447. https://doi.org/10.1111/j.1600-0722.1997.tb02142.x
Kielbassa AM (2000) In situ induced demineralization in irradiated and non-irradiated human dentin. Eur J Oral Sci 108(3):214–221. https://doi.org/10.1034/j.1600-0722.2000.108003214.x
Cole T, Silver AS (1963) Production of hydrogen atoms in teeth by X-irradiation. Nature 16:700–701
Lindén LA, Björkman S, Hattab F (1986) The diffusion in vitro of fluoride and chlorhexidine in the enamel of human deciduous and permanent teeth. Arch Oral Biol 31(1):33–37. https://doi.org/10.1016/0003-9969(86)90110-X
Van Meerbeek B, Yoshida Y, Inoue S, De Munck J, van Landuyt K, Lambrechts P (2006) Glass-ionomer adhesion: the mechanisms at the interface. J Dent 34:615–617
Gernhardt CR, Kielbassa AM, Hahn P, Schaller HG (2001) Tensile bond strengths of four different dentin adhesives on irradiated and non-irradiated human dentin in vitro. J Oral Rehabil 28(9):814–820. https://doi.org/10.1046/j.1365-2842.2001.00758.x
Galetti R, Santos-Silva AR, Antunes AN, Alves Fde A, Lopes MA, de Goes MF (2014) Radiotherapy does not impair dentin adhesive properties in head and neck cancer patients. Clin Oral Investig 18(7):1771–1778. https://doi.org/10.1007/s00784-013-1155-4
Gupta N, Pal M, Rawat S, Grewal MS, Garg H, Chauhan D, Ahlawat P, Tandon S, Khurana R, Pahuja AK, Mayank M, Devnani B (2015) Radiation-induced dental caries, prevention and treatment—a systematic review. Natl J Maxillofac Surg 6(2):160–166. https://doi.org/10.4103/0975-5950.183870
Freitas Soares E, Zago Naves L, Bertolazzo Correr A et al (2016) Effect of radiotherapy, adhesive systems and doxycycline on the bond strength of the dentin-composite interface. Am J Dent 29:352–356
Tjäderhane L (2015) Dentin bonding: can we make it last? Oper Dent 40(1):4–18. https://doi.org/10.2341/14-095-BL
Springer IN, Niehoff P, Warnke PH, Böcek G, Kovács G, Suhr M, Wiltfang J, Açil Y (2005) Radiation caries—radiogenic destruction of dental collagen. Oral Oncol 41(7):723–728. https://doi.org/10.1016/j.oraloncology.2005.03.011
Gomes-Silva W, Prado-Ribeiro AC, Brandão TB, Morais-Faria K, de Castro Junior G, Mak MP, Lopes MA, Rocha MM, Salo T, Tjäderhane L, de Goes MF, Santos-Silva AR (2017) Postradiation matrix metalloproteinase-20 expression and its impact on dental micromorphology and radiation-related caries. Caries Res 51(3):216–224. https://doi.org/10.1159/000457806
Hübner W, Blume A, Pushnjakova R, Dekhtyar Y, Hein HJ (2005) The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: an FT-IR microscopic investigation. Int J Artif Organs 28(1):66–73. https://doi.org/10.1177/039139880502800111
Yu CX (1995) Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol 40(9):1435–1449. https://doi.org/10.1088/0031-9155/40/9/004
Hoebers F, Yu E, Eisbruch A, Thorstad W, O’Sullivan B, Dawson LA, Hope A (2013) A pragmatic contouring guideline for salivary gland structures in head and neck radiation oncology: the MOIST target. Am J Clin Oncol 36(1):70–76. https://doi.org/10.1097/COC.0b013e31823a538e
Joyston-Bechal S (1985) The effect of X-radiation on the susceptibility of enamel to an artificial caries-like attack in vitro. J Dent 13(1):41–44. https://doi.org/10.1016/0300-5712(85)90061-2
Pioch T, Golfels D, Staehle HJ (1991) An experimental study of the stability of irradiated teeth in the region of the dentinoenamel junction. Endod Dent Traumatol 8:241–244
Liang X, Zhang JY, Cheng IK, Li JY (2016) Effect of high energy X-ray irradiation on the nano-mechanical properties of human enamel and dentin. Braz Oral Res 30(1). https://doi.org/10.1590/1807-3107BOR-2016.vol30.0009
Acknowledgments
We thank FAPESP (São Paulo Research Foundation) for the concession of a scholarship to the first author (Proc. 2015/00817-2).
Funding
This work was supported by the FAPESP (São Paulo Research Foundation) (Proc. 2015/00817-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the local research ethics committee. All procedures performed in this study involving human teeth were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Velo, M.M.d.A.C., Farha, A.L.H., da Silva Santos, P.S. et al. Radiotherapy alters the composition, structural and mechanical properties of root dentin in vitro. Clin Oral Invest 22, 2871–2878 (2018). https://doi.org/10.1007/s00784-018-2373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2373-6