Abstract
Objectives
The present study evaluated the effect of an enamel matrix derivative (EMD) and platelet-rich fibrin (PRF)-modified porcine-derived collagen matrix (PDCM) on human umbilical vein endothelial cells (HUVEC) in vitro.
Materials and methods
PDCM (mucoderm®) was prepared to 6 mm (±0.1 mm) diameter discs. PDCM samples were incubated with either EMD, PRF, or control solutions for 100 min at 4 °C before the experiments. Cell-inducing properties of test materials on HUVEC cells were tested with cell proliferation assays (MTT, PrestoBlue®), a cytotoxicity assay (ToxiLight®), a Boyden chamber migration assay, and a cell attachment assay. Scanning electron microscopy (SEM) imaging was performed to determine the surface and the architecture of the modified matrices.
Results
Cell proliferation was elevated in the EMD and PRF groups compared with control (p each ≤0.046). PRF modification increased HUVEC migration ability by 8-fold compared with both control and EMD groups (p each <0.001). Both treatments significantly promoted the cell attachment of HUVEC to PDCM, as assessed by direct cell counts on the matrices (p each <0.001).
Conclusions
HUVEC cell characteristics were overall improved by EMD- and PRF- modified PDCM. Adsorbed bioactive molecules to the PDCM surface may have contributed to a more preferable environment to surrounding cells.
Clinical relevance
The results may give evidence that PDCM modification with EMD or PRF, respectively, might be a useful approach to improve clinical outcomes, to prevent inflammatory reactions and wound-healing disturbances, and to expand the clinical application area of PDCM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A porcine-derived collagen matrix (PDCM; mucoderm®, Botiss Biomaterials, Berlin, Germany) has been introduced as a potential substitute for autogenous soft tissue grafts in periodontal plastic and implant surgery. It is mainly composed of natural types I and III collagen without any artificial cross-linking. The material properties of the collagen matrix, including the chemical and structural biocompatibility originated from the native tissue, are supposed to drive surrounding cells to attach easily and interact with the matrix, thereby promoting tissue regeneration [1].
PDCM as an alternative to autogenous connective tissue grafts (CTG) has long been a subject of interest in the field of periodontal plastic surgery. Major drawbacks of autogenous soft tissue grafting, such as limited availability, need for a second surgical site, and patient morbidity, seemed to be solved by using PDCM. Patients suffered less from gingival augmentation procedures using PDCM compared with CTG in terms of pain and discomfort level, apart from the time-saving aspect. However, PDCM demonstrated inferior clinical outcomes in root coverage procedures compared with CTG in terms of mean and complete root coverage, recession reduction, and gain of keratinized tissue [2].
The manufacturing process of PDCM includes the procedure of excluding cells and interfibril protein or non-protein substances from original porcine tissue, in order to avoid unwanted immunogenicity. Therefore, PDCM is devoid of tissue-regenerating and/or cell-inducing capabilities which, on the other hand, are well expected in CTG grafting procedures.
Interfibril substances refer to various extracellular matrix (ECM) components, such as glycosaminoglycans, proteoglycans, and glycoproteins. These molecules not only present cell-binding ligands providing sequence motifs for cell attachment but also trigger intracellular signal pathways and activate cell growth and differentiation [3]. Therefore, the addition of cell-inducible proteins may compensate for the absence of cell-activating molecules in PDCM, leading to better clinical outcomes.
Enamel matrix derivative (EMD) is a group of proteins extracted from porcine enamel matrix supplied in the form of purified acid extract. EMD was basically developed with an intention to regenerate lost periodontal tissues, since researchers discovered that Hertwig’s root sheath cells secret enamel proteins during the root formation 20 years ago [4,5,6]. A recently published meta-analysis demonstrated the beneficial effect of EMD in the treatment of intrabony defects [7]. EMD applied in root coverage procedures is able to increase the odds of complete root coverage nearly 3.5-fold compared with a coronally advanced flap (CAF) alone [7]. When comparing clinical results between EMD and CTG grafting, both combined with a CAF, no statistically significant differences were found after 10 years [8]. Different studies demonstrated the clinical benefits of EMD application in periodontal regeneration. The exact molecular mechanisms are not clarified in detail up to date. Further studies reported that EMD does not exclusively stimulate periodontal cells but also provides signals to cells responsible for early wound healing, such as different immune and endothelial cells [9, 10].
Platelet-rich fibrin (PRF) is a form of platelet concentrates developed more than 15 years ago by Choukroun’s group for the specific use in oral and maxillofacial surgery [11]. PRF clots obtained in glass tube after a centrifuge at 400×g contain platelets and their byproducts released during platelet activation. By virtue of the slow polymerization process, the fibrin network of the PRF clot features a homogeneously organized 3D structure with numerous substances within platelet granules. These include numbers of growth factors (GF), circulating cytokines, glycoproteins, and fibrin-associated glycanic chains [12, 13]. PRF clot passively releases these molecules in a sustained manner, promoting wound-healing process in vivo [14,15,16,17]. Originally, EMD is typically utilized for hard tissue mineralization (cementum formation) whereas PRF is primarily used for soft tissue regeneration.
The aim of the present study was to find out and evaluate a technique to reinforce the function of PDCM as an alternative to autogenous soft tissue grafts by adding the cell-activating substances EMD and PRF to PDCM. Since angiogenesis and revascularization play a major role in wound healing, we analyzed the effects of EMD- and PRF-pretreated PDCM on cell viability, migration ability, and cell attachment on human umbilical vein endothelial cells (HUVEC) in vitro.
Materials and methods
PDCM sample preparation
PDCM (mucoderm®; Botiss Biomaterials, Berlin, Germany; diameter 30 × 40 mm) was prepared to 6 ± 0.1 mm discs with a sterile and cylindrical surgical punch under sterile conditions. The diameter of each sample was controlled with a sterile caliper.
Test solution preparation
Emdogain® (Straumann, Basel, Switzerland) was diluted with serum-free medium (basal medium eagle (BME); Lonza, Basel, Switzerland) to a concentration of 100 μg/ml and stored at 4 °C before use. PRF was prepared from freshly drawn human peripheral venous blood collected in 10 ml glass-coated plastic tubes (Vacuette®; Greiner BioOne GmbH, Frickenhausen, Germany), centrifuged at 400×g for 12 min at room temperature (PC-02; Process, Nice, France) according to Choukroun’s protocol [13]. Blood was collected from one single volunteer of the research group by sterile venipuncture. The PRF clot was obtained by removing the red blood cell part with an extra care not to disclude the buffy coat area. The clot was taken to a 6-well plate, incubated with 4 ml of serum-free medium at 37 °C. After 24 h, medium that includes released exudates from PRF clot was collected to a new plastic tube and stored in a fridge at −20 °C before use. Serum-free medium served as control group.
Before each experiment, PDCM discs were rehydrated with three different solutions, either control, EMD or PRF (dependent of the experimental group they belong to), for 100 min at 4 °C. Four hundred microliters from each solution was used to rehydrate each PDCM sample. After 100 min of incubation, samples were washed with PBS (Dulbecco’s phosphate buffered saline; Sigma-Aldrich, Steinheim, Germany) rigorously for twice. All procedures were performed under sterile conditions.
Cell cultures
HUVEC (PromoCell, Heidelberg, Germany) were cultured in endothelial cell growth medium supplemented with hydrocortisone, 10% fetal calf serum (FCS), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), gentamicin sulfate, amphotericin-B, and bovine hypothalamic extract (PromoCell) according to standard cell culture protocols, under standard culture conditions (5% CO2 at 37 °C). Cells at passages three to sixwere used for all the experiments.
Cell proliferation assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, Steinheim, Germany) and PrestoBlue® assays (Invitrogen, Life Technologies GmbH, Darmstadt, Germany) were performed to analyze cell viability and proliferative activity of HUVEC under the influence of EMD and PRF-preconditioned PDCM.
MTT assay is based on the reduction of MTT compound to a water-insoluble formazan. This reaction happens inside the cells by the enzyme mitochondrial succinic dehydrogenases. Due to the water-insoluble property of the reduced end product, the dissolving process is necessary before colorimetric change measurement.
PrestoBlue® (PB) is a water-soluble, cell-permeable, resazurin-based compound. The reducing environment within viable cells converts PB to red-fluorescent dye. However, cell permeability of the end product makes it possible to measure the result without damaging cell integrity. Therefore, PB allows the continuous observation of cell growth.
PDCM samples were incubated with either control, EMD, or PRF solution for 100 min before the experiment. Cells were seeded on a new 24-well plate (5 × 104 cells/well) in HUVEC medium with supplements. Incubated and washed PDCM samples were then transferred to cell-seeded plates immediately after the seeding. MTT was measured after 1, 2, 3, and 7 days while PB was measured after 0, 1, 3, 6, 24, 48, 72, and 96 h. Synergy HT Multi-Mode Microplate Reader (Bio-Tek Instruments; Winooski, VT, USA) was used to measure absorbance and fluorescence for MTT and PB, respectively. Four wells were used for each group at each representative test. Whole experiments were repeated three times.
Cytotoxicity assay
ToxiLight® BioAssay Kit (Lonza Rockland Inc., Rockland, USA) was used to measure the release of adenylate kinase (AK) from dead cells. Cell necrosis occurs rapidly within a day or even within a few hours, losing their membrane integrity as they undergo rapid swelling. AK is one of the protein molecules released by cell necrosis. The ToxiLight® BioAssay utilizes the enzyme activity of AK, which phosphorylates ADP to form ATP. The amount of resultant ATP is then analyzed by measuring light intensity produced from bioluminescent firefly luciferase reaction.
Prepared samples were transferred to 24-well plates immediately after cells were seeded at 5 × 104 cells/well. Twenty microliters of supernatants were collected from each well at 1, 2, and 3 days after seeding and transferred to a luminescence-compatible 96-well plate. One hundred microliters of AK detection reagent (AKDR) were added to each well, and luminescence was measured after 5 min using a Synergy HT Multi-Mode Microplate Reader. Logarithmic signals were converted to a linear scale and expressed as relative luminescence units. Four wells were used for each group at each representative test. Experiments were repeated three times.
Cell migration assay
The chemotactic effect of EMD- and PRF-pretreated PDCM to HUVEC was evaluated by 24-well Boyden chamber assay system (Thin-Cert™; Greiner BioOne, Essen, Germany). The transwell inserts used included polyethylene membrane bottom with 8.0 μm pores. Test samples were placed in 24-well plate with 800 μl serum-free medium for 24 h at 37 °C before cell seeding. After 24 h, PDCM samples were removed from the wells to utilize only the conditioned medium as test solutions. Transwell inserts seeded with cells (1.0 × 105 cells/well) in 400 μl serum-free medium were seated above each well. After 24 h incubation, medium was removed from well plate and cells were stained with Calcein-AM fluorescent dye (Invitrogen). After 45 min incubation at 37 °C, transwell inserts were transferred to freshly prepared black 24-well plate containing 500 μl of trypsin–EDTA (Invitrogen) per well. After another 10 min of incubation at 37 °C, transwells were removed from black 24-well plate. Fluorescence was measured using Synergy HT Multi-Mode Microplate Reader to quantify Calcein-AM-stained migrated cells. Four wells were used for each group at each representative test. Experiments were repeated three times.
Cell attachment assay
To evaluate cell-material surface interaction, amounts of cells attached to PDCM were counted. Prepared samples were placed onto 24-well plate before cells were seeded. HUVEC were then seeded on the PDCM at 1.0 × 105 cells/well. After 24 h incubation, culture mediums were removed from each well. Cells on PDCM were stained with SYTO® Green Nucleic Stain-Kit (Invitrogen) to visualize them under an inverted microscope (Axiovert 40C; Carl Zeiss, Jena, Germany). Pictures were taken under 25-fold magnification at four different areas from each sample. Cells were counted automatically using ImageJ software (National Institutes of Health; Bethesda, Maryland, USA) (Suppl. 1). Four wells were used for each group at each representative test. Experiments were repeated three times.
Scanning electron microscopy
Samples were prepared like previously reported [1]. PDCM samples were treated with each test (EMD, PRF) and control solution for 100 min. After, they were washed twice with PBS, dehydrated in ethanol, and freeze dried. Samples were then sputtered with gold in an argon atmosphere and then examined by means of a Philips ESEM XL-30 scanning electron microscope (Philips, Eindhoven, Netherlands).
Statistical analysis
SPSS Statistics 18.0 software (SPSS Inc., Chicago, USA) was used to perform statistical analysis. Statistical difference was analyzed by analyses of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) post hoc test. If the data showed a significance with the normality test, Kruskal-Wallis H test was performed. p values <0.05 were considered to be statistically significant. Only the intergroup differences were taken into account.
Results
HUVEC cell proliferation activity
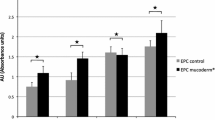
MTT assay showed significantly increased cell proliferation in EMD and PRF groups as compared with the control. The difference between EMD and control groups was observed only at 7 days (p = 0.018). PRF treatment significantly promoted cell activity at all different time points compared with both control and EMD groups (p each ≤0.01), except for 7 days measurement, which showed a significant difference only toward control group (p = 0.001) (Fig. 1a).
Effect of EMD- and PRF-pretreated PDCMs on HUVEC cell proliferation and cytotoxicity. Cells were cultured on PDCMs pretreated with either control (serum-free medium), EMD, or PRF solution. a MTT assay. Overall, EMD and PRF groups showed an increased cell activity at different timepoints. Asterisks represent significant differences of the PRF group compared with the EMD and the control groups at each timepoint of measurement. Number and section signs represent significant differences of the PRF and EMD groups compared with the control group at each timepoint of measurement. b PrestoBlue® assay. After 72 and 96 h, EMD and PRF groups revealed an increased cell activity compared with the control group. Asterisks represent significant differences compared with the control group. c ToxiLight® assay. Twenty microliters of supernatants were collected from each well at days 1, 2, and 3 after seeding and transferred into a 96-well plate. The amount of adenylate kinase (AK) in each supernatant was measured using a microplate reader. PRF group showed an increased cytotoxicity after 1 day compared with the EMD and the control groups. Asterisks represent significant differences of the PRF and EMD groups compared with the control group. Values expressed as means from three independent experiments. Error bars represent standard deviations (SD)
PrestoBlue® assay revealed significant increase of cell activity in EMD and PRF groups at 72 and 96 h compared with control group (p each ≤0.046) (Fig. 1b).
HUVEC cytotoxicity
ToxiLight® assay showed elevated level of AK at first 24 h in PRF group compared with both the control and EMD groups (p each ≤0.007). No increased cytotoxicity was observed by EMD treatment (p each >0.05) (Fig. 1c).
HUVEC cell migration
Conditioned medium with PRF-pretreated PDCM significantly increased the chemotactic migration ability of HUVEC approximately by 8-fold compared with both the control and EMD groups (p each <0.001) (Fig. 2). EMD group showed no additional effect on HUVEC cell migration ability (p each >0.05).
Effect of EMD- and PRF-pretreated PDCMs on HUVEC migration ability. Prepared PDCM samples (control, EMD, and PRF solutions) were incubated with serum-free medium for 24 h in the bottom of 24-well plates. PDCMs were then removed, and transwells (Boyden chambers) were seated upper them. Calcein-AM-stained HUVEC cells in the Boyden chambers migrated through the Boyden chambers. Cell migration was measured by using a microplate reader. Migration was significantly increased in the PRF group compared with both EMD and control groups. Asterisks represent significant differences of the PRF group compared with the EMD and the control groups. Values expressed as means from three independent experiments. Error bars represent standard deviations (SD)
HUVEC attachment
EMD and PRF pretreatments increased the cell affinity of PDCM showing significantly promoted HUVEC attachment to the PDCM surface compared with the control (p each <0.001) (Fig. 3a). No significant difference was observed between EMD and PRF groups (p > 0.05). Box plots reveal crude distribution of each data series (Fig. 3b). Data in PRF group showed relatively broad distribution compared with the other groups with more than 50% of data concentrated below the mean (168.6, 323.2, and 916.0% for 25 percentiles, median, and 75 percentile values, respectively). In EMD group, each percentile value from 25 to 75% indicated 316.9, 517.2, and 962.4, respectively. These numbers along with the representative microscopic appearances imply the irregularity and variance of data from PRF group (Suppl. 2). Cells on PRF-pretreated PDCM showed all-or-none appearance. Samples were often clearly divided to cell-present and non-present area while the cell-existing zones showed exceptionally densely packed cells.
Effect of EMD and PRF modifications of PDCM on HUVEC cell attachment. Cells were seeded on control, EMD, and PRF solution-pretreated PDCM samples. After 24 h, cells were stained with SYTO® Green and observed with an inverted microscope. Pictures were taken from each sample, and the attached cells were digitally counted with the software ImageJ. a PDCM-attached cell density was significantly increased in the EMD and PRF groups compared with the control group. Asterisks represent significant differences compared with the control group. Values expressed as means from three independent experiments. Error bars represent standard deviations (SD). b Box plots. Asterisks and circles represent outliers
Scanning electron microscopy
Scanning electron microscopy (SEM) imaging of the control and test matrices are shown in Fig. 4. No particular differences could be observed among the control and the two test groups concerning the surface layer and the cross-section architecture. Visualization of the surface layers showed hydrated surfaces with some macroscopic grooves and holes originated from original matrix surface topography and suitable for cell and blood vessel ingrowth (Fig. 4a). Cross-sectional views revealed internal architecture of each treated PDCM (PRF and EMD), demonstrating a highly porous structure composed of loosely packed collagen fibers, which was well preserved even after 100 min of each protein modification and overall very suitable for tissue regeneration (Fig. 4b).
Scanning electron microscopy (SEM). a Surface layer. Images in low magnification on the left side (a, c, e) and in high magnification on the right side (b, d, f) represent uniform surfaces with some macroscopic grooves and holes suitable for cell and blood vessel ingrowth. b Cross-sections. Images in low magnification on the left side (a, c, e) and in high magnification on the right side (b, d, f) represent the highly porous internal structures of the samples with loosely packed collagen fibers suitable for tissue regeneration. (a, b control group; c, d PRF group; e, f EMD group)
Discussion
To achieve surgical outcomes comparable with CTG grafts, PDCM should be immediately occupied by tissue-regenerating cells so that the newly formed tissues may intermingle with the scaffolds’ collagen fibers before the degradation of the scaffold has been completed.
Since the energy demanded to support new cell ingrowth at the site of tissue regeneration is delivered through blood vessels in the form of oxygen and metabolites [18], accelerated revascularization of PDCM forms the basis of solid and high-quality tissue regeneration.
In this context, angiogenesis starts with migration and proliferation of endothelial cells. Mature endothelial cells from pre-existing blood vessels migrate and proliferate by chemical cues like GFs and/or signals from the wound site [19, 20]. Once the cells migrate to the targeted site, they need to establish proper interaction with the underlying matrix surface to survive and for further differentiation. This interaction requires proper cell-matrix adherence as a prerequisite, since the focal adhesions that cells form toward the underlying surface to build a tight attachment, serve not only as a mechanical linkage but also as a biochemical signaling hub to direct signals to intracellular signaling pathways associated with various cellular behaviors [21, 22]. Therefore, proliferation, migration, and the affinity of cells to the matrix were tested to determine the biocompatibility of controls and modified PDCMs to endothelial cells.
In the present study, we used EMD and PRF as PDCM-modifying factors in order to provide additional signals to surrounding cells. As a result, PDCM modification with EMD increased the HUVEC proliferation compared with the control group when examined by MTT and PrestoBlue® assays. Cell migration was not enhanced nor prohibited by EMD-PDCM-conditioned medium. These results are inconsistent with the ones from previous in vitro studies where both cell proliferation and migration were increased by EMD treatment [23, 24]. The methodological difference may explain the discrepancy of the results. While diluted EMD solutions were added directly into the culture medium in other studies, PDCM acted as a form of EMD delivery device in the present study.
EMD is chemically soluble in acid pH level and in low temperature. However, as the pH and temperature rise, EMD starts to be adsorbed and precipitated to a hydroxyapatite or collagen surface [25]. Miron et al. coated demineralized freeze-dried bone allograft (DFDBA) particles at 4 °C with EMD solutions at a working concentration of 100 μg/ml overnight. SEM visualization showed a network of EMD protein fibers covering the surface of DFDBA particles with variable protein densities [26]. Therefore, it is likely that our pre-incubating method enabled EMD molecules to be adsorbed and precipitated into PDCM 3D architecture, thereby altering topographical and biochemical surface characteristics of the material.
Adsorbed and precipitated EMD molecules to PDCM have to be processed by cellular matrix metalloproteinases (MMPs) before they become active to surrounding cells [27]. This process could be delayed in our experimental conditions, owing to the affinity of EMD to collagen molecules and the absence of scaffold degradation activity. This partially may explain the delayed response in the cell proliferation assays and no response in migration assay.
PRF modification of PDCM promoted activity and migration of HUVECs compared with serum-free medium controls. Proliferative activity was significantly increased at all time points of measurement, while PRF-PDCM-conditioned medium enormously upregulated HUVEC migration ability.
PRF is a biological substitute with a high potency and a multitude of possibilities. It is perfectly biocompatible due to the origin, i.e., patients’ own blood, easy to produce and to handle, and carries multiple growth factors (GF) in high concentrations [28,29,30]. However, when it comes to the clinical application, the question is whether the amount of GF in the PRF clot is high enough to induce any additional effect in the targeted site. Recent clinical studies showed that PRF clots grafted in post-extraction sockets or palatal wound sites significantly promoted soft tissue-healing capacity [15, 16]. The pattern and amount of GF release from PRF clot investigated in vitro suggest a concrete evidence as to explain the effect of PRF grafting [14].
In this study, a PRF-24 h exudate to hydrate the PDCM was used. In order to determine how PDCM is biofunctionalized with the help of bioactive molecules achieved from PRF clot, we had to exclude the PRF clot from the cell culture medium. According to a previous study, PRF releases GF for a maximum of 28 days in virtue of organized fibrin network [14]. Therefore, the exudate we used in this study may contain partial amount of the substances that PRF releases in total but still large enough to stimulate the cells as proven in our study.
HUVEC attachment to the test material surfaces was remarkably promoted in EMD and PRF group compared with the control group. Since HUVECs’ attachment to the biomaterial surface is usually completed within several hours [31], our data present an attachment and cell growth on PDCM during the initial 24 h. Several factors could be considered to explain this phenomenon.
EMD molecules on PDCM surfaces may have elevated intracellular integrin signals, promoting attachment and proliferation of the cells. EMD increases the expression and the release of TGF-β in cells [7, 32], while TGF-β signaling enhances the activity of integrin signaling in various cell types including HUVEC [31, 33, 34]. Altered surface topography of the matrix due to the precipitation of EMD protein fibers could be another influencing factor to cell behavior [31, 35].
Beside GF, PRF also contains adhesive proteins, such as fibrinogen, vitronectin, and fibronectin [36]. These proteins carry sequences that bind to integrin receptors on the cell surface, promoting cell-matrix adherence. Especially, fibronectin (FN) is known to carry not exclusively integrin-binding domain but also GF-binding domain in the close proximity. This spatial proximity between two domains is proved to amplify the function of GF signaling by allowing the signal exchanges between integrin and GF pathways, which in turn leads to the synergistic co-activation [37, 38]. Therefore, it can be reasonably assumed that collagen-bound FN primarily assists in HUVEC attachment. Then it triggers the proliferative activity of cells in collaboration with GF signaling. This explains the odd distribution of cells, i.e., “all-or-none” pattern, on PRF-treated PDCM surface.
Once cells are migrated and attached to the matrix surface, it is important that cells penetrate the internal structure efficiently to allow microvessel ingrowth and tissue regeneration. Our SEM analysis demonstrated that the EMD and PRF modifications of PDCM does not affect the 3D architecture of the matrices of its own, implying the strong possibility of well-induced microvessel ingrowth subsequently after endothelial cell colonization on the matrix surface.
In conclusion, EMD and PRF pretreatments successfully promoted cell affinity of PDCM and upregulated HUVEC cell viability as well as proliferation and migration ability in vicinity. Angiogenesis is the most important step in early stage of tissue regeneration. Our results give evidence that the co-application of cell-inducing proteins, such as EMD or PRF, may enhance the formation of new blood vessels around and through the implanted collagen tissue matrix, which in turn may contribute to improved surgical results. Well-designed animal and clinical studies are required to verify the clinical benefits.
Recently, a liquid EMD carrier system has been developed (Osteogain®, Straumann AG, Switzerland). This new EMD liquid system enhanced protein adsorption to bone grafting materials compared with conventional EMD gel system (Emdogain®, Straumann AG, Switzerland) [39, 40]. It seems to work well with collagen matrices as well [41]. Our further research would be to test PDCM with EMD liquid in order to optimize clinical application in soft tissue grafting procedures.
References
Pabst AM, Happe A, Callaway A, Ziebart T, Stratul SI, Ackermann M, Konerding MA, Willershausen B, Kasaj A (2014) In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J Periodontal Res 49(3):371–381. doi:10.1111/jre.12115
Atieh MA, Alsabeeha N, Tawse-Smith A, Payne AG (2015) Xenogeneic collagen matrix for periodontal plastic surgery procedures: a systematic review and meta-analysis. J Periodontal Res. doi:10.1111/jre.12333
Kim Y, Ko H, Kwon IK, Shin K (2016) Extracellular matrix revisited: roles in tissue engineering. Int Neurourol J 20(Suppl 1):S23–S29. doi:10.5213/inj.1632600.318
Hammarstrom L (1997) Enamel matrix, cementum development and regeneration. J Clin Periodontol 24(9 Pt 2):658–668
Gestrelius S, Andersson C, Lidstrom D, Hammarstrom L, Somerman M (1997) In vitro studies on periodontal ligament cells and enamel matrix derivative. J Clin Periodontol 24(9 Pt 2):685–692
Miron RJ, Sculean A, Cochran DL, Froum S, Zucchelli G, Nemcovsky C, Donos N, Lyngstadaas SP, Deschner J, Dard M, Stavropoulos A, Zhang Y, Trombelli L, Kasaj A, Shirakata Y, Cortellini P, Tonetti M, Rasperini G, Jepsen S, Bosshardt DD (2016) 20 years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol. doi:10.1111/jcpe.12546
Koop R, Merheb J, Quirynen M (2012) Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol 83(6):707–720. doi:10.1902/jop.2011.110266
McGuire MK, Scheyer ET, Nunn M (2012) Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue: comparison of clinical parameters at 10 years. J Periodontol 83(11):1353–1362. doi:10.1902/jop.2012.110373
Bosshardt DD (2008) Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol 35(8 Suppl):87–105. doi:10.1111/j.1600-051X.2008.01264.x
Miron RJ, Dard M, Weinreb M (2015) Enamel matrix derivative, inflammation and soft tissue wound healing. J Periodontal Res 50(5):555–569. doi:10.1111/jre.12245
Choukroun J, Adda F, Schoeffler C, Vervelle A (2001) Une opportunite′ en paro-implantologie: le PRF. Implantodontie 42:55–62 French
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e45–e50. doi:10.1016/j.tripleo.2005.07.009
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e37–e44. doi:10.1016/j.tripleo.2005.07.008
Schar MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D (2015) Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res 473(5):1635–1643. doi:10.1007/s11999-015-4192-2
Marenzi G, Riccitiello F, Tia M, di Lauro A, Sammartino G (2015) Influence of leukocyte- and platelet-rich fibrin (L-PRF) in the healing of simple postextraction sockets: a split-mouth study. Biomed Res Int 2015:369273. doi:10.1155/2015/369273
Femminella B, Iaconi MC, Di Tullio M, Romano L, Sinjari B, D'Arcangelo C, De Ninis P, Paolantonio M (2016) Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol 87(2):103–113. doi:10.1902/jop.2015.150198
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20(9):2353–2360. doi:10.1007/s00784-016-1719-1
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Germain S, Monnot C, Muller L, Eichmann A (2010) Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol 17(3):245–251. doi:10.1097/MOH.0b013e32833865b9
Michaelis UR (2014) Mechanisms of endothelial cell migration. Cell Mol Life Sci 71(21):4131–4148. doi:10.1007/s00018-014-1678-0
Zaidel-Bar R, Cohen M, Addadi L, Geiger B (2004) Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 32(Pt3):416–420. doi:10.1042/BST0320416
Giancotti FG (1997) Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 9(5):691–700
Kasaj A, Meister J, Lehmann K, Stratul SI, Schlee M, Stein JM, Willershausen B, Schmidt M (2012) The influence of enamel matrix derivative on the angiogenic activity of primary endothelial cells. J Periodontal Res 47(4):479–487. doi:10.1111/j.1600-0765.2011.01456.x
Bertl K, An N, Bruckmann C, Dard M, Andrukhov O, Matejka M, Rausch-Fan X (2009) Effects of enamel matrix derivative on proliferation/viability, migration, and expression of angiogenic factor and adhesion molecules in endothelial cells in vitro. J Periodontol 80(10):1622–1630. doi:10.1902/jop.2009.090157
Gestrelius S, Andersson C, Johansson AC, Persson E, Brodin A, Rydhag L, Hammarstrom L (1997) Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J Clin Periodontol 24(9 Pt 2):678–684
Miron RJ, Bosshardt DD, Laugisch O, Dard M, Gemperli AC, Buser D, Gruber R, Sculean A (2013) In vitro evaluation of demineralized freeze-dried bone allograft in combination with enamel matrix derivative. J Periodontol 84(11):1646–1654. doi:10.1902/jop.2013.120574
Lyngstadaas SP, Wohlfahrt JC, Brookes SJ, Paine ML, Snead ML, Reseland JE (2009) Enamel matrix proteins; old molecules for new applications. Orthod Craniofac Res 12(3):243–253. doi:10.1111/j.1601-6343.2009.01459.x
Philippart P, Daubie V, Pochet R (2005) Sinus grafting using recombinant human tissue factor, platelet-rich plasma gel, autologous bone, and anorganic bovine bone mineral xenograft: histologic analysis and case reports. Int J Oral Maxillofac Implants 20(2):274–281
Mazor Z, Peleg M, Garg AK, Luboshitz J (2004) Platelet-rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: patient series study. Implant Dent 13(1):65–72
Thor A, Wannfors K, Sennerby L, Rasmusson L (2005) Reconstruction of the severely resorbed maxilla with autogenous bone, platelet-rich plasma, and implants: 1-year results of a controlled prospective 5-year study. Clin Implant Dent Relat Res 7(4):209–220
Yang D, Lu X, Hong Y, Xi T, Zhang D (2013) The molecular mechanism of mediation of adsorbed serum proteins to endothelial cells adhesion and growth on biomaterials. Biomaterials 34(23):5747–5758. doi:10.1016/j.biomaterials.2013.04.028
Okubo K, Kobayashi M, Takiguchi T, Takada T, Ohazama A, Okamatsu Y, Hasegawa K (2003) Participation of endogenous IGF-I and TGF-beta 1 with enamel matrix derivative-stimulated cell growth in human periodontal ligament cells. J Periodontal Res 38(1):1–9
Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN (2011) Lumican regulates osteosarcoma cell adhesion by modulating TGFbeta2 activity. Int J Biochem Cell Biol 43(6):928–935. doi:10.1016/j.biocel.2011.03.008
Warstat K, Meckbach D, Weis-Klemm M, Hack A, Klein G, de Zwart P, Aicher WK (2010) TGF-beta enhances the integrin alpha2beta1-mediated attachment of mesenchymal stem cells to type I collagen. Stem Cells Dev 19(5):645–656. doi:10.1089/scd.2009.0208
Curtis A, Wilkinson C (1997) Topographical control of cells. Biomaterials 18(24):1573–1583
Ranganathan AT, Chandran CR (2014) Platelet-rich fibrin in the treatment of periodontal bone defects. J Contemp Dent Pract 15(3):372–375
Pankov R, Yamada KM (2002) Fibronectin at a glance. J Cell Sci 115(Pt 20):3861–3863
Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA (2011) Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 3(100):100ra189. doi:10.1126/scitranslmed.3002614
Miron RJ, Bosshardt DD, Buser D, Zhang Y, Tugulu S, Gemperli A, Dard M, Caluseru OM, Chandad F, Sculean A (2015) Comparison of the capacity of enamel matrix derivative gel and enamel matrix derivative in liquid formulation to adsorb to bone grafting materials. J Periodontol 86(4):578–587. doi:10.1902/jop.2015.140538
Zhang Y, Jing D, Buser D, Sculean A, Chandad F, Miron RJ (2016) Bone grafting material in combination with Osteogain for bone repair: a rat histomorphometric study. Clin Oral Investig 20(3):589–595. doi:10.1007/s00784-015-1532-2
Miron RJ, Fujioka-Kobayashi M, Zhang Y, Sculean A, Pippenger B, Shirakata Y, Kandalam U, Hernandez M (2016) Osteogain(R) loaded onto an absorbable collagen sponge induces attachment and osteoblast differentiation of ST2 cells in vitro. Clin Oral Investig. doi:10.1007/s00784-016-2019-5
Acknowledgments
Special thanks to Kerstin Bahr for SEM imaging. This work contains substantial parts of the dissertation to DMD of Jung Soo Park. Free samples of the tested matrix were allocated free of charge from Botiss (Botiss Biomaterials, Berlin, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No funding was received for this study. The work was supported by the Department of Operative Dentistry and Periodontology, University Medical Center, Augustusplatz 2, 55131 Mainz, Germany. Free samples of the tested matrix were allocated free of charge from Botiss (Botiss Biomaterials, Berlin, Germany).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Suppl. 1
Automatic cell counting using ImageJ software. The original RGB image (a) is inverted and converted to gray-scale (8 bits) (b). “Image-based Tool for Counting Nuclei (ITCN)” command is then used to count cells. Counted particles are identified as red marks on the result picture (c). Sixteenfold magnified view of the area marked on Fig. 1c (d). (GIF 292 kb)
Suppl. 2
Microscopic images of SYTO® 11-stained HUVEC cells on PDCM samples. Cells were seeded on control, EMD, and PRF solution-pretreated PDCM samples. After 24 h, cells on matrices were stained with SYTO® Green, observed with an inverted microscope. Images were taken under 25-fold magnification from four different areas of each sample. Whereas cells are sparsely distributed on control sample (a), EMD- and PRF-treated PDCMs demonstrate an enormous amount of cells attached on the surface (b, d). In the PRF group, there were wide variations concerning the density of attached cells on the samples (c, d). Cell distributions were variable even within the same sample (c). a Control group; b EMD group; c, d PRF group. (GIF 412 kb)
Rights and permissions
About this article
Cite this article
Park, J.S., Pabst, A.M., Ackermann, M. et al. Biofunctionalization of porcine-derived collagen matrix using enamel matrix derivative and platelet-rich fibrin: influence on mature endothelial cell characteristics in vitro. Clin Oral Invest 22, 909–917 (2018). https://doi.org/10.1007/s00784-017-2170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2170-7