Abstract
Objectives
To prevent oral candidiasis, it is crucial to inactivate Candida-based biofilms on dentures. Common denture cleansing solutions cannot sufficiently inactivate Candida albicans. Therefore, we investigated the anticandidal efficacy of a physical plasma against C. albicans biofilms in vitro.
Materials and methods
Argon or argon plasma with 1 % oxygen admixture was applied on C. albicans biofilms grown for 2, 7, or 16 days on polymethylmethacrylate discs; 0.1 % chlorhexidine digluconate (CHX) and 0.6 % sodium hypochlorite (NaOCl) solutions served as positive treatment controls. In addition, these two solutions were applied in combination with plasma for 30 min to assess potential synergistic effects. The anticandidal efficacy was determined by the number of colony forming units (CFU) in log10 and expressed as reduction factor (RF, the difference between control and treated specimen).
Results
On 2-day-biofilms, plasma treatment alone or combined with 30 min CHX treatment led to significant differences of means of CFU (RF = 4.2 and RF = 4.3), clearly superior to CHX treatment alone (RF = 0.6). Plasma treatment of 7-day-or 16-day-old biofilms revealed no significant CFU reduction. The treatment of 7-day-old (RF = 1.7) and 16-day-old (RF = 1.3) biofilms was slightly more effective with NaOCl alone than with the combined treatment of NaOCl and plasma (RF = 1.6/RF = 1.9). The combination of CHX and plasma increased the RF immaterially.
Conclusion
The use of plasma alone and in combination with antiseptics is promising anticandidal regimens for daily use on dentures when biofilms are not older than 2 days.
Clinical relevance
Plasma could help to reduce denture-associated candidiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral candidiasis is the most common human fungal infection, which can be caused by an overgrowth of opportunistic Candida species on dentures [1]. Since 1936, the yeast has been associated with denture-related stomatitis, often termed Candida-associated denture stomatitis [2]. The oral cavity of 75 % of people wearing dentures is colonized by Candida albicans [3], which originates more often and in higher numbers from the denture surface covering the mucosa than from the mucosa itself, indicating that the denture acts as a reservoir of infection [4]. Additionally, the denture’s surface area correlates with the risk of contracting denture stomatitis [5]. The initial adhesion of commensal yeast cells of the genus Candida on dentures results in biofilm formation [2]. The basis for the biofilm matrix is extracellular polysaccharides secreted by C. albicans after adhesion [6]. This matrix protects the cells from different environmental influences and makes biofilms as opposed to planktonic C. albicans cells more resistant to antifungal agents [7].
To prevent denture stomatitis and maintain healthy supporting tissues, proper routine cleansing of dentures is necessary. Effective plaque removal requires a degree of manual dexterity that is often lacking, especially among the elderly [8]. Antimicrobial coatings of dentures help to decrease the risk of stomatitis, but cannot prevent biofilm development, so that a cleansing process is still necessary [1]. The conventional methods of denture plaque control, such as chemical cleansing baths with peroxides, hypochlorites, or chlorhexidine digluconate, are the first choice, but can cause discoloring of the denture [9, 10]. Furthermore, their ability to inactivate matured biofilms – especially those of Candida – is not sufficiently effective [11, 12]. Therefore, an alternative treatment to chemical disinfectant procedures is highly desirable.
Recently, cold atmospheric pressure plasmas have emerged as a promising technique in medicine [13, 14]. Among them, the volume dielectric barrier discharge (VDBD) method has been studied with respect to antimicrobial effects on C. albicans biofilms [15]. Different argon plasma sources were compared on C. albicans biofilms grown for 2 days on titanium discs, where the VDBD plasma source showed the highest anticandidal effect, but it was not investigated with an additional oxygen admixture [15].
In the present study, we investigated the antimicrobial potential of the VDBD plasma with argon or argon plus 1 % oxygen as the working gas on C. albicans biofilms grown for 2, 7, or 16 days on polymethylmethacrylate (PMMA) discs. With the admixture of oxygen, an increased ratio of antimicrobial active oxygen species in the plasma-gas-compound is expected. PMMA is the dominant material for the fabrication of denture bases.
Materials and methods
Biofilm formation
Biofilms were cultivated on PMMA discs with a diameter of 13 mm and a thickness of 3 mm. These discs were produced in the dental laboratory at the dental school of the University Medicine Greifswald, according to the manufacturer’s recommendations (SR Ivocap® Plus clear, Ivoclar Vivadent, Ellwangen, Jagst, Germany). The discs were washed in distilled water to remove any possible residues and steam-sterilized in a Varioclav (H + P Labortechnik GmbH, Hackermoos, Germany) for 2 h. For biofilm cultivation, the strongly biofilm-forming strain C. albicans ATCC (American Type Culture Collection, Rockville, MD, USA) 10,231 was used [16]. Before biofilm culturing, the discs were conditioned with dithiothreitol (DTT) saliva. For this purpose, 10 ml of saliva were collected from healthy individuals (approved by the ethics committee of University Medicine Greifswald, pooled and treated with 5 ml 1 M DL-dithiothreitol solution (DTT, Sigma-Aldrich, Steinheim, Germany) to reduce salivary protein aggregation and 5 ml distilled water. This DTT saliva mixture was then centrifuged (Mini Spin, Eppendorf, Hamburg Germany), and the supernatant was filtered through a 0.2-μm pore filter (HVM Filtramed, Rotenburg an der Fulda, Germany) and frozen at −20 °C until use [17]. C. albicans was suspended in YPD Broth (Yeast Extract Peptone Dextrose, Sigma-Aldrich, Steinheim, Germany). The sterile PMMA specimens were placed in 24-well microtiter plates (Techno Plastic Products AG, Trasadingen, Switzerland), covered with 500 μl DTT saliva, and incubated for 2 h at 37 °C. Afterwards, the DTT saliva was carefully removed, and 1 ml of microorganism suspension (concentration 106 cells/ml) was added and incubated aerobically at 37 °C. The medium was replaced every 24 h. After 2, 7, or 16 days, the medium was drawn off, the PMMA discs were washed with 0.9 % sodium chloride (NaCl) solution and transferred into a new, sterile microtiter plate.
After anticandidal treatment, the PMMA discs were placed in wells with 1 ml of 0.9 % NaCl solution; the biofilm was removed using an ultrasonic bath (Branson 2510, 130 W, 42 kHz, Dietzenbach, Germany) for 20 min and thereafter manually resuspended with a pipette to make serial dilutions of the resuspended biofilms. Subsequently, an aliquot portion of 0.1 ml from each dilution was plated on Sabouraud glucose (4 %) agar plates (Carl Roth, Karlsruhe, Germany) and incubated at 37 °C for 48 h. The colonies were counted and expressed as colony forming units (CFU)/ml in log10.
Plasma treatment
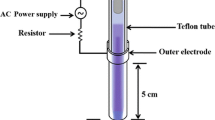
A VDBD plasma source, developed by the Leibniz Institute for Plasma Science and Technology e.V. (INP Greifswald, Germany), was used for biofilm treatment [15]. The VDBD source (Fig. 1) consisted of two flat, round metal electrodes. High voltage (40 kHz, 10 kV) was applied between the two electrodes to generate the plasma. A Peltier element was used for cooling. The electrodes were 15 mm apart, and the space between them was sealed air tight. Pure argon (Ar) was applied as the working gas for maintaining the discharge and flowed through the system at a rate of 50 standard cm3 per minute (sccm). In order to enable flow-through, the gas compound could escape through an exhaust tube (not shown in Fig. 1B). Additionally, 1 % of oxygen (0.5 sccm) was admixed to Ar. Accordingly, plasma treatments were termed “Ar plasma” and “Ar + 1 % O2 plasma.”
To distinguish the effect of plasma treatment from that of gas blowing, the biofilms were exposed to Ar gas and Ar + 1 % O2 gas flow without plasma ignition. These air-blowing treatments were termed “Ar gas” and “Ar + 1 % O2 gas.”
For biofilm treatment, a Petri dish (Techno Plastic Products AG, Trasadingen, Switzerland) with the biofilm-covered PMMA discs was located between the electrodes (without the cover plate). The plasma was applied on both sides of the discs. Up to four discs were treated simultaneously.
Antiseptic treatment
Chlorhexidine digluconate (CHX, Fagron GmbH & Co KG, Barsbüttel, Germany) as a 0.1 % aqueous solution was used for trials 2 and 3, and sodium hypochlorite (NaOCl, ApplyChem GmbH, Darmstadt, Germany) as a 0.6 % aqueous solution was additionally used for trial 3. The PMMA discs with the C. albicans biofilm were covered with 1 ml of the antiseptic solution and incubated for 30 min at room temperature. Then, the antiseptic solution was removed from each well.
For the combined treatment with plasma or gas in trials 2 and 3, the CHX- or NaOCl-covered biofilms were incubated for 10 min at room temperature. After removal of the solution, the antimicrobial effect of the remaining antiseptic was not neutralized by an inactivator until the plasma or gas treatment was finished, with a duration of 10 min per disc side. Hence, the biofilm was in contact with the residual antiseptic for 30 min, comparable with the sole CHX or NaOCl treatment.
The antiseptic effect of CHX was neutralized by adding 1 ml of inactivator, consisting of 40 g/l Tween 80, 30 g/l saponin, 4 g/l lecithin, 10 g/l SDS, and 1 g/l sodium thioglycolate, for 20 min at room temperature. An inactivator consisting of 30 g/l Tween 80, 3 g/l lecithin, 1 g/l histidine, and 5 g/l sodium thiosulfate was used to neutralize the antifungal effect of NaOCl. Neutralization was confirmed by the quantitative suspension test according to DIN EN 1040 (German Institute for Standardization) [18].
Experimental structure
Three different treatments were executed for stepwise evaluation of suitable parameters for treatment of strong biofilms on denture material with plasma:
-
First (trial 1), the anticandidal effects of argon plasma or argon gas with and without oxygen admixture were tested against 2-day-old biofilms. Each side of the disc was treated with plasma or gas for 1, 5, or 10 min (n = 8).
-
Second (trial 2), the interaction between argon plasma or argon gas with and without oxygen admixture combined with CHX solution was tested. The biofilms were treated for 10 min with plasma (n = 6). Treatment with CHX alone for 10 min served as the positive control.
-
Third (trial 3), the efficacy of argon plasma or argon gas against 7- and 16-day-old biofilms was investigated, alone and in combination with CHX or NaOCl. The biofilms were treated with plasma for 10 min (n = 6). Treatment with CHX or NaOCl alone served as positive controls.
After treatment, the antiseptically active residues were neutralized for 20 min by the respective inactivator solution. As a negative control, 0.9 % NaCl solution was used for all three trials.
Statistical analysis
Means and standard deviations (SD) were determined. To compare multiple groups, the unpaired t test with a subsequent Bonferroni correction of the calculated p values was used (GraphPad Prism® 4.02, La Jolla, CA, USA). Differences of means were considered statistically significant at p < 0.05.
The calculated reduction factor (RF) was the difference between the mean log10 (CFU/ml) values of the control specimen minus the mean log10 (CFU/ml) values of treated specimen.
Results
Anticandidal treatments
-
Trial 1
Two-day-old biofilms were treated with Ar plasma and Ar + 1 % O2 plasma for 1, 5, or 10 min (Table 1).
Table 1 Logarithm of the CFU/ml means (±standard deviation, SD) and the reduction factors (RF) of Candida albicans biofilms after 1, 5, or 10 min treatment with argon (Ar) or argon with 1 % oxygen admixture (Ar + 1 % O2) plasma as well as after the respective gas treatment without plasma ignition according to trial 1. The gray background marks the value, which reached the threshold of 4 log10 reduction steps for yeast disinfectants The RF increased with longer treatment times. For each treatment time, Ar plasma achieved the highest reduction of the mean CFU values, which were significantly different compared to the control (RF1min = 1.27, p < 0.05; RF5min = 2.84, p < 0.001; RF10min = 4.12, p < 0.001). By comparison, Ar + 1 % O2 plasma treatment showed less reduction of mean CFU values vs the control (RF1min = 0.61; RF5min = 0.64, RF10min = 0.92), which were statistically significant after 5 (p < 0.01) and 10 min (p < 0.005) of treatment. The means of the results after Ar plasma were statistically significantly different compared to the means after Ar + 1 % O2 plasma after 5 (p < 0.001) and 10 min (p < 0.005) treatment time.
To distinguish plasma treatment effects from that of gas blowing, the biofilms were exposed to Ar gas and Ar + 1 % O2 gas flow for the different treatment periods without plasma ignition. The gas flow of Ar gas and Ar + 1 % O2 gas reduced means of CFUs, which were statistically significantly different from the control only for Ar gas after 10-min treatment time (p < 0.005) (Ar gas: RF1min = 0.31, RF5min = 0.48, RF10min = 1.53; Ar + 1 % O2 gas: RF1min = 0.05, RF5min = 0.46, RF10min = 0.35).
-
Trial 2
Two-day-old biofilms were treated with CHX alone and in combination with Ar plasma, Ar + 1 % O2 plasma, and Ar gas (Table 2). The results of all antiseptic treatments were statistically significantly different from those of the control (p < 0.05), except after Ar gas and CHX + Ar gas treatment (p > 0.05).
Table 2 Logarithm of the CFU/ml means (±standard deviation, SD) and the reduction factor (RF) of Candida albicans biofilms after treatment with the antiseptics CHX or NaOCl and after 10 min treatment with argon gas (Ar gas), argon plasma (Ar plasma), and argon with 1 % oxygen admixture plasma (Ar + 1 % O2 plasma) alone or in combination with the antiseptics according to trials 2 and 3. The gray background marks the values, which reached the threshold of 4 log10 reduction steps for yeast disinfectants The means after treatment with Ar plasma (4.39 log10 CFU/ml) and CHX + Ar plasma (4.24 log10 CFU/ml) showed the strongest decrease in CFU values compared to the control with 8.56 log10 CFU/ml (RF = 4.17 and RF = 4.32), and also differed statistically significantly from the treatment results after using Ar gas (8.26 log10 CFU/ml) and CHX + Ar gas (7.99 log10 CFU/ml) as well as Ar + 1 % O2 plasma (7.41 log10 CFU/ml) and CHX + Ar + 1 % O2 plasma (7.83 log10 CFU/ml).
-
Trial 3
Seven- and 16-day-old biofilms were treated with CHX or NaOCl separately and in combination with Ar plasma (Table 2).
For 7-day-old biofilms, the mean value of 7.89 log10 CFU/ml after Ar plasma treatment was not significantly different from the control mean of 8.03 log10 CFU/ml (RF = 0.15). Significant differences compared to the control were found after treatment with CHX + Ar plasma (mean = 6.77) and NaOCl + Ar plasma (mean = 6.36 log10 CFU/ml, p < 0.01) as well as after treatment using Ar gas (p < 0.05) and CHX + Ar gas (p < 0.01). The mean value of 7.73 log10 CFU/ml after 30 min CHX treatment was not significantly different from the negative control (RF = 0.30). The mean value of NaOCl treatment alone (6.41 log10 CFU/ml) was significantly lower than not only the control but also than values after treatment using Ar plasma and CHX (p < 0.01). The means after Ar gas treatment decreased significantly with additional CHX pre-treatment from a value of 7.52 to 6.61 log10 CFU/ml (p < 0.01).
For 16-day-old biofilms, the lowest means were shown after treatment with Ar plasma in combination with NaOCl pre-treatment (6.42 log10 CFU/ml) as well as after NaOCl treatment alone (5.85 log10 CFU/ml) (RF = 1.30 and 1.88), which were significantly different compared to the control (7.72 log10 CFU/ml) (p < 0.005). Treatment with Ar plasma alone (7.98 log10 CFU/ml) and Ar plasma after CHX pre-treatment (7.41 log10 CFU/ml) showed no significant effect against biofilms after 16 days of cultivation compared to the control. CHX treatment alone showed no significant difference on 16-day-old biofilms (RF −0.03), but in combination with Ar gas, the mean value decreased significantly (RF = 0.47) compared to the control.
Discussion
The main reservoir of C. albicans is the surface of the upper and lower dentures covering the mucosa, which can cause stomatitis [19]. Brushing is insufficient for plaque control on dentures [20, 21]. The routine use of chemical denture cleansers may lead to color changes of the denture base and denture teeth or increase the roughness [10, 20, 22, 23], which increases the ability for microorganisms to adhere and form biofilms. New and highly effective treatment regimens for inactivating C. albicans embedded in biofilms on dentures are warranted. Cold plasma at atmospheric pressure is highly effective against C. albicans biofilms [15] and could be an alternative denture-cleaning approach that minimizes the risk to the denture wearer of contracting candidiasis.
We investigated the anticandidal effect of a VDBD plasma device on C. albicans biofilms using argon as the working gas with and without 1 % oxygen admixed and examined the interaction with the antiseptics CHX and NaOCl. CHX and NaOCl served as positive controls. CHX solution is a standard antiseptic to inactivate or prevent dental plaque formation [24] and is used as a disinfectant in denture-cleaning solutions [25]. In commercial denture cleansers, a CHX concentration of 0.1 % is used [26], and NaOCl is used in concentrations between 0.05 and 2 %. These concentrations are also employed in cleanser research [1, 27–30]. To distinguish statistically significant differences from clinically meaningful reduction rates, we regarded an RF of 4 log10 as the threshold in accordance with EN 1275 [31], which requires an RF of 4 log10 in the quantitative suspension test within 15 min for fungicidal or basic yeasticidal activity of chemical disinfectants.
Since all intra-oral surfaces become coated with salivary proteins within minutes, it is important to generate a pellicle for biologically inert surfaces such as PMMA to simulate in vivo conditions. A salivary coating increases the adhesion, influences the early colonization of C. albicans, and serves as a source of nutrients for the microorganisms [2]. Because saliva is a highly complex fluid, we used sterile filtered saliva collected and pooled from different healthy individuals for our experiments instead of artificial saliva.
In trial 1 with 2-day-old biofilms, we focused on the effect of both an additional admixture of 1 % oxygen to argon for plasma generation and different exposure times. Oxygen was added, because it can increase the concentration of free oxygen radicals in the plasma-gas compound [32, 33] and thus promote biofilm removal [34]. Contrary to our expectations, the oxygen admixture in Ar plasma did not lead to a reduction of C. albicans superior to that after Ar plasma treatment and did not reach our predefined threshold of clinical relevance. The use of Ar plasma alone led to significant decreases of CFU depending on exposure time, and achieved an RF of 4 log10 after 10 min of exposure (Tables 1 and 2). Therefore, only 10 min of plasma treatment was further used in trail 2 and 3.
In trial 2 with 2-day-old biofilms, we focused on the adjunctive effect of CHX, the gold standard of dental plaque control by antiseptics. A synergistic effect for Ar plasma or Ar + 1 % O2 plasma by pre-treatment with CHX solution was not shown. These results are in agreement with Koban et al. [35], where no adjunctive effects of CHX combined with Ar plasma on different oral biofilms were demonstrated. Ar plasma alone and CHX + Ar plasma were significantly more effective than CHX alone or Ar + 1 % O2 plasma and reached our threshold of an RF at 4 log10 (Table 2). Based on these results, Ar + 1 % O2 plasma was not investigated further. The repeatedly unexpectedly low anticandidal efficacy of oxygen admixture could be explained by quenching of argon-excited species [36], with a loss of reactive oxygen species generated in contact with O2 [37], because the excitation energy required to ionize the O2 in the gas stream was presumably too low.
For trial 3, NaOCl solution was additionally used as sole treatment or as an adjunctive pre-treatment with Ar plasma to cause rapid formation of active oxygen species on 7- or 16-day-old biofilms. NaOCl is used worldwide to inactivate microorganisms in root canals [38] and on dentures [1], and shows high antimicrobial efficacy [39]. An anticandidal effect on 7-day-old biofilms was found after Ar plasma treatment in combination with CHX or NaOCl or after NaOCl treatment alone. For 16-day-old biofilms, only treatment with Ar plasma with NaOCl as well as NaOCl alone showed anticandidal effects. Because the difference between the efficacy of plasma alone and in combination with NaOCl is very small, and on 16-day-old-biofilms the efficacy of the combination was even smaller, it may be assumed that NaOCl was inactivated by plasma. All RFs were under the threshold of 4 log10 and are too low to eradicate strong biofilms on dentures. Interestingly, the anticandidal effect of Ar plasma decreased dramatically for older biofilm. Only NaOCl treatment alone showed a consistent reduction of CFU on 7- and 16-day-old biofilms after 30 min incubation time (RF = 1.62 and 1.88, respectively) (n) as well as on 2-day-old biofilms, presented in a previous study, with an RF of 1.5 [15]. The reduced efficacy of CHX may be caused by impaired penetration into the mature biofilm matrix [40]. The reduced efficacy of the Ar plasma may be caused by a dense layer of damaged microorganisms and degraded matrix compounds produced after reactions of the reactive plasma species with the superficial organic biofilm compounds covering the biofilm surface [41].
Against 2-day-old biofilms, VDBD-ignited Ar plasma was highly effective and superior to 1 % CHX and, in view of the results of Koban et al. [15], presumably also superior to 0.6 % NaOCl treatment. However, the effects on 7- and 16-day-old biofilms were clinically irrelevant. In everyday life, a daily Ar plasma treatment would result in better anticandidal effects than rinsing in 1 % CHX solution. Dentures with thick, mature biofilms should be additionally pre-treated with mechanical methods like a brush or ultrasound to reduce the organic burden and to enhance disinfection processes.
A device incorporating both a plasma unit and a denture cleaner working with ultrasound technology is conceivable. To simplify its construction, air could replace argon as the working gas, because air plasma also exhibits high antimicrobial [41] and anticandidal effects [42] on biofilms. Even a strong plasma effect will act on topic surface areas; the generated air plasma atmosphere in the cleaner chamber will show slight antimicrobial effects too caused by ozone for example [43, 44]. An interesting additional benefit of plasma treatment of dentures might be a decreased initial adhesion of C. albicans on denture surfaces, as was described by Zamperini et al. [45, 46]. Those results and the present study’s findings both indicate that plasma could not only improve daily denture cleaning but may also prevent colonization of dentures by Candida and thus offer two mechanisms to prevent candidiasis.
Conclusion
The VDBD argon plasma treatment showed a high anticandidal effect on biofilms grown for 2 days that was superior to CHX treatment and which increased with increasing treatment time. No clinically relevant effect was shown against C. albicans biofilms cultivated for 7 or 16 days. These trials showed no increased antimicrobial effects after the combined treatment with CHX or NaOCl solutions and plasma. A daily treatment regimen of dentures with plasma is a promising alternative disinfection method. For treatment of dentures with older biofilms, a mechanical pre-treatment is recommended to enhance disinfection effects.
References
Skupien JA, Valentini F, Boscato N, Pereira-Cenci T (2013) Prevention and treatment of Candida colonization on denture liners: a systematic review. J Prosthet Dent 110(5):356–362. doi:10.1016/j.prosdent.2013.07.003
Radford DR, Challacombe S, Walter JD (1999) Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med 10(1):99–116. doi:10.1177/10454411990100010501
Perezous LF, Stevenson GC, Flaitz CM, Goldschmidt ME, Engelmeier RL, Nichols CM (2006) The effect of complete dentures with a metal palate on candida species growth in HIV-infected patients. J Prosthodont 15(5):306–315. doi:10.1111/j.1532-849X.2006.00127.x
Davenport JC (1970) The oral distribution of candida in denture stomatitis. Br Dent J 129(4):151–156
Jainkittivong A, Aneksuk V, Langlais RP (2010) Oral mucosal lesions in denture wearers. Gerodontology 27(1):26–32. doi:10.1111/j.1741-2358.2009.00289.x
Hawser SP, Baillie GS, Douglas LJ (1998) Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 47(3):253–256
Hawser SP, Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39(9):2128–2131. doi:10.1128/AAC.39.9.2128
Pietrokovski J, Azuelos J, Tau S, Mostavoy R (1995) Oral findings in elderly nursing home residents in selected countries: oral hygiene conditions and plaque accumulation on denture surfaces. J Prosthet Dent 73(2):136–141
Hong G, Murata H, Li Y, Sadamori S, Hamada T (2009) Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J Prosthet Dent 101(3):205–213. doi:10.1016/S0022-3913(09)60032-9
Durkan R, Ayaz EA, Bagis B, Gurbuz A, Ozturk N, Korkmaz FM (2013) Comparative effects of denture cleansers on physical properties of polyamide and polymethyl methacrylate base polymers. Dent Mater J 32(3):367–375. doi:10.4012/dmj. 2012-110
Glass RT, Goodson LB, Bullard JW, Conrad RS (2001) Comparison of the effectiveness of several denture sanitizing systems: a clinical study. Compend Contin Educ Dent 22(12):1093–1096, 1098, 1100-2 passim; quiz 1108
Lucena-Ferreira, de Silvia Carneiro, Cavalcanti, Indira Moraes Gomes, Cury, Altair Antoninha Del Bel (2013) Efficacy of denture cleansers in reducing microbial counts from removable partial dentures: a short-term clinical evaluation. Braz Dent J 24(4):353–356. doi:10.1590/0103-6440201302183
Kramer A, Lademann J, Bender C, Sckell A, Hartmann B, Münch S, Hinz P, Ekkernkamp A, Matthes R, Koban I, Partecke L, Heidecke C, Masur K, Reuter S, Weltmann K, Koch S, Assadian O (2013) Suitability of tissue tolerable plasmas (TTP) for the management of chronic wounds. Clin Plasma Med 1(1):11–18. doi:10.1016/j.cpme.2013.03.002
von Woedtke T, Reuter S, Masur K, Weltmann K (2013) Plasmas for medicine. Phys Rep 530(4):291–320. doi:10.1016/j.physrep.2013.05.005
Koban I, Matthes R, Hübner N, Welk A, Meisel P, Holtfreter B, Sietmann R, Kindel E, Weltmann K, Kramer A, Kocher T (2010) Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J Phys 12(7):073039. doi:10.1088/1367-2630/12/7/073039
He M, Du M, Fan M, Bian Z (2007) In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia 163(3):137–143. doi:10.1007/s11046-007-0097-2
Sánchez MC, Llama-Palacios A, Blanc V, León R, Herrera D, Sanz M (2011) Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J Periodont Res 46(2):252–260. doi:10.1111/j.1600-0765.2010.01341.x
European Committee for Standardisation (CEN) (2006) German version EN 1040:2005: chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of basic bactericidal activity of chemical disinfectants and antiseptics—test method and requirements (phase 1). Beuth Verlag GmbH, Berlin
Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW (2004) Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98(1):53–59. doi:10.1016/j.tripleo.2003.04.002
Izumida FE, Ribeiro RC, Giampaolo ET, Machado AL, Pavarina AC, Vergani CE (2011) Effect of microwave disinfection on the surface roughness of three denture base resins after tooth brushing. Gerodontology 28(4):277–282. doi:10.1111/j.1741-2358.2010.00393.x
Paranhos, de Freitas Oliveira H, Salles, Sparca AE, Macedo, de Dorigan L, Silva-Lovato, da Claudia Helena, Pagnano VO, Watanabe E (2013) Complete denture biofilm after brushing with specific denture paste, neutral soap and artificial saliva. Braz Dent J 24(1):47–52. doi:10.1590/0103-6440201301946
Pisani MX, Bruhn JP, Paranhos, Oliveira HF, Silva-Lovato CH, de Souza, Freitas R, Panzeri H (2010) Evaluation of the abrasiveness of dentifrices for complete dentures. J Prosthodont 19(5):369–373. doi:10.1111/j.1532-849X.2010.00592.x
Paranhos, de Freitas Oliveira H, Peracini A, Pisani MX, Oliveira, de Cássia V, de Souza, Freitas R, Silva-Lovato CH (2013) Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Braz Dent J 24(2):152–156. doi:10.1590/0103-6440201302151
Matthews D (2011) No difference between 0.12 and 0.2% chlorhexidine mouthrinse on reduction of gingivitis. Evid Based Dent 12(1):8–9. doi:10.1038/sj.ebd.6400771
de Andrade, Machado I, Cruz PC, Silva-Lovato CH, de Souza, Raphael F, Souza-Gugelmin, Monteiro MC, Paranhos, de Freitas Oliveira H (2012) Effect of chlorhexidine on denture biofilm accumulation. J Prosthodont 21(1):2–6. doi:10.1111/j.1532-849X.2011.00774.x
Felipucci, Borges DN, Davi LR, Paranhos, Oliveira HF, Bezzon OL, Silva RF, Pagnano VO (2011) Effect of different cleansers on the surface of removable partial denture. Braz Dent J 22(5):392–7
Barnabe W, de Mendonca Neto T, Pimenta FC, Pegoraro LF, Scolaro JM (2004) Efficacy of sodium hypochlorite and coconut soap used as disinfecting agents in the reduction of denture stomatitis, Streptococcus mutans and Candida albicans. J Oral Rehabil 31(5):453–459. doi:10.1111/j.1365-2842.2004.01254.x
Brożek R, Koczorowski R, Rogalewicz R, Voelkel A, Czarnecka B, Nicholson JW (2011) Effect of denture cleansers on chemical and mechanical behavior of selected soft lining materials. Dent Mater 27(3):281–290. doi:10.1016/j.dental.2010.11.003
de Sousa P, Rodrigues S, de Lucena-Ferreira, Carneiro S, da Silva, Wander J, Del Bel Cury, Altair Antoninha (2013) Evaluation of sodium hypochlorite as a denture cleanser: a clinical study. Gerodontology. doi:10.1111/ger.12104
Piskin B, Sipahi C, Akin H (2014) Effect of different chemical disinfectants on color stability of acrylic denture teeth. J Prosthodont 23(6):476–483. doi:10.1111/jopr.12131
European Committee for Standardisation (CEN) (2005) European standard EN 1275: Chemical disinfectants and antiseptics - Quantitative suspension test for the evaluation of basic fungicidal or basic yeasticidal activity of chemical disinfectants and antiseptics - Test method and requirements (phase 1). Brussels, Belgium
Lim JP, Uhm HS, Li SZ (2007) Influence of oxygen in atmospheric-pressure argon plasma jet on sterilization of Bacillus atrophaeous spores. Phys Plasma 14(9):093504. doi:10.1063/1.2773705
McKay K, Liu DX, Rong MZ, Iza F, Kong MG (2012) Generation and loss of reactive oxygen species in low-temperature atmospheric-pressure RF He + O 2 + H 2 O plasmas. J Phys D Appl Phys 45(17):172001. doi:10.1088/0022-3727/45/17/172001
Fricke K, Koban I, Tresp H, Jablonowski L, Schroder K, Kramer A, Weltmann K, von Woedtke T, Kocher T (2012) Atmospheric pressure plasma: a high-performance tool for the efficient removal of biofilms. PLoS ONE 7(8):e42539. doi:10.1371/journal.pone.0042539
Koban I, Geisel MH, Holtfreter B, Jablonowski L, Hübner N, Matthes R, Masur K, Weltmann K, Kramer A, Kocher T (2013) Synergistic effects of nonthermal plasma and disinfecting agents against dental biofilms in vitro. ISRN Dent 2013(2):1–10. doi:10.1155/2013/573262
Wagatsuma K, Hirokawa K (1995) Effect of oxygen addition to an argon glow-discharge plasma source in atomic-emission spectrometry. Anal Chim Acta 306(2–3):193–200. doi:10.1016/0003-2670(95)00007-M
Moravej M, Yang X, Hicks RF, Penelon J, Babayan SE (2006) A radio-frequency nonequilibrium atmospheric pressure plasma operating with argon and oxygen. J Appl Phys 99(9):093305
Spencer HR, Ike V, Brennan PA (2007) Review: the use of sodium hypochlorite in endodontics—potential complications and their management. Br Dent J 202(9):555–559. doi:10.1038/bdj.2007.374
Bürgers R, Witecy C, Hahnel S, Gosau M (2012) The effect of various topical peri-implantitis antiseptics on Staphylococcus epidermidis, Candida albicans, and Streptococcus sanguinis. Arch Oral Biol 57(7):940–947. doi:10.1016/j.archoralbio.2012.01.015
Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55(Pt 8):999–1008. doi:10.1099/jmm. 0.46569-0
Matthes R, Bender C, Schlüter R, Koban I, Bussiahn R, Reuter S, Lademann J, Weltmann K, Kramer A (2013) Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS ONE 8(7):e70462. doi:10.1371/journal.pone.0070462
Maisch T, Shimizu T, Isbary G, Heinlin J, Karrer S, Klampfl TG, Li Y, Morfill G, Zimmermann JL (2012) Contact-free inactivation of Candida albicans biofilms by cold atmospheric air plasma. Appl Environ Microbiol 78(12):4242–4247. doi:10.1128/Aem. 07235-11
Estrela C, Estrela CRA, Decurcio DA, Silva JA, Bammann LL (2006) Antimicrobial potential of ozone in an ultrasonic cleaning system against Staphylococcus aureus. Braz Dent J 17(2):134–138. doi:10.1590/S0103-64402006000200010
Pavlovich MJ, Chang HW, Sakiyama Y, Clark DS, Graves DB (2013) Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J Phys D Appl Phys 46(14):145202. doi:10.1088/0022-3727/46/14/145202
Zamperini CA, Machado AL, Vergani CE, Pavarina AC, Giampaolo ET, da Cruz, Cristino N (2010) Adherence in vitro of Candida albicans to plasma treated acrylic resin. Effect of plasma parameters, surface roughness and salivary pellicle. Arch Oral Biol 55(10):763–770. doi:10.1016/j.archoralbio.2010.06.015
Zamperini CA, Carneiro, de Lima H, Rangel EC, Cruz NC, Vergani CE, Machado AL (2013) In vitro adhesion of Candida glabrata to denture base acrylic resin modified by glow-discharge plasma treatment. Mycoses 56(2):134–144. doi:10.1111/j.1439-0507.2012.02223.x
Acknowledgments
This work was realized within the framework of the multidisciplinary research cooperation “Campus PlasmaMed,” particularly within the project “PlasmaDent.” This work was supported by a grant from the German Ministry of Education and Research (BMBF, Grant no. 13N9779), and by the Ministry of Science and Culture of the State of Mecklenburg-Western Pomerania, and the European Union, European Social Fund within the project “Plasmamedical Research – New pharmaceutical and medical fields of application” (Grant no. AU 11 038; ESF/IV-BM-B35-0010/13). The authors thank Claudia Lehnert for her excellent technical assistance and Rüdiger Titze for his skillful support in operating the plasma equipment.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matthes, R., Jablonowski, L., Koban, I. et al. In vitro treatment of Candida albicans biofilms on denture base material with volume dielectric barrier discharge plasma (VDBD) compared with common chemical antiseptics. Clin Oral Invest 19, 2319–2326 (2015). https://doi.org/10.1007/s00784-015-1463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1463-y