Abstract
The primary aim of this study was to investigate the impact of treatment with low-temperature plasma (LTP) for varying exposure durations on a multispecies cariogenic biofilm comprising C. albicans, L. casei, and S. mutans, as well as on single-species biofilms of L. casei and C. albicans, cultured on hydroxyapatite discs. Biofilms were treated with LTP-argon at a 10 mm distance for 30 s, 60 s, and 120 s. Chlorhexidine solution (0.12%) and NaCl (0.89%) were used as positive (PC) and negative controls (NC), respectively. Argon flow only was also used as gas flow control (F). Colony-forming units (CFU) recovery and confocal laser scanning microscopy (CLSM) were used to analyze biofilm viability. LTP starting at 30 s of application significantly reduced the viability of multispecies biofilms by more than 2 log10 in all treated samples (p < 0.0001). For single-species biofilms, L. casei showed a significant reduction compared to PC and NC of over 1 log10 at all exposure times (p < 0.0001). In the case of C. albicans biofilms, LTP treatment compared to PC and NC resulted in a significant decrease in bacterial counts when applied for 60 and 120 s (1.55 and 1.90 log10 CFU/mL, respectively) (p < 0.0001). A significant effect (p ≤ 0.05) of LTP in single-species biofilms was observed to start at 60 s of LTP application compared to F, suggesting a time-dependent effect of LTP for the single-species biofilms of C. albicans and L. casei. LTP is a potential mechanism in treating dental caries by being an effective anti-biofilm therapy of both single and multispecies cariogenic biofilms.
Similar content being viewed by others
Introduction
Dental caries of deciduous and permanent teeth is the most prevalent disease and the second-highest incidence of oral conditions in the world1. Caries decay is more prevalent in socioeconomically disadvantaged people2, and even though it is a disease that can be physically and chemically controlled, studies indicate that its prevalence has not decreased in the last three decades3. The caries process depends on forming a cariogenic biofilm on the tooth surface4. In a biofilm, microorganisms grow in a matrix rich in extracellular polysaccharides5, creating a well-organized community6 that protects against penetration of chemical and antimicrobial agents7. However, the resistance of the cariogenic bacteria present in this biofilm to antimicrobials may occur due to the frequent contact of the biofilm with these agents6,8. Microbiologically, Streptococcus mutans is the most well-known pathogen in caries formation and development9,10, frequently used to assess caries risk11 due to its strong acid production capacity and its acid tolerance. Additionally, these microorganisms have vesicles in their membranes containing proteins, extracellular DNA, and other substances capable of activating cell-to-cell communication, assisting biofilm formation and disease progression12.
Studies have shown a low amount or absence of S. mutans in patients with dental caries decay13,14 and also in the caries-free group, indicating that other species of bacteria that are associated in the cariogenic biofilm together with S. mutans may also be responsible for the formation and development of caries15,16. S. mutans was correlated with Lactobacillus spp.15,16 and Candida albicans 17 in dental caries. Gregoire et al. demonstrated through an in vitro study that the glycosyltransferase B (GtfB) released by S. mutans might be able to bind to cell surfaces of C. albicans through enzymatic reactions with sucrose present in the biofilm18. A study has reported the mechanism of adhesion of C. albicans to hydroxyapatite surfaces, dentin, and even cementum19. Besides, C. albicans has been reported in the oral mycobiome of early childhood caries and dental cavities established in adults20. For more than six decades, Lactobacillus species have been associated with tooth decay21. They are recognized as significant contributors to dental caries, particularly in developing advanced caries lesions in adults and children22. Lactobacilli are frequently isolated from sites of active and advanced caries lesions in adult and pediatric patients14,21 but are absent in caries-free children. As they metabolize dietary sugar, they can significantly contribute to the progression of caries23. Both S. mutans and Lactobacillus species can survive in low-pH environments commonly found in carious lesions21. However, Lactobacillus species exhibit greater acid tolerance than S. mutans, allowing them to survive at even lower pH levels and favoring their persistence in deeper carious lesions21.
Improving the current protocols for treating dental caries is urgent24, especially after the COVID-19 pandemic caused by the new coronavirus SARS-CoV-2, as conventional restorative treatments can generate aerosols and spread bacteria and viruses25. In this scenario, minimally invasive dentistry (MID) is a safer alternative, using manual instruments instead of rotating equipment26. MID preserves healthy dental tissue and prevents damage to the pulpal dentin complex26. Low-temperature plasma (LTP) therapy has been presented as an innovative therapeutic tool in treating dental caries27,28,29. Plasma is the fourth state of matter, consisting of ionized gas formed by atoms and/or molecules and ions at different densities and temperatures. It is a conductive medium and responds to electric and magnetic fields30. LTP can be applied in a laboratory setting using a specialized plasma machine that provides an appropriate electrical stimulus, such as high voltage, microwave, or radiofrequency, and utilizes noble or molecular gases like argon, helium, oxygen, and nitrogen. Ensuring that the laboratory is equipped with adequate safety measures for this process is crucial30,31,32. For plasma to be considered a biologically viable energy source, it must reach a temperature of 40 °C or lower30,32. A partially ionized gas produces LTP after an electrical discharge33. LTP generated at 40 °C or less triggers the production of ROS in the gas phase from reactive oxygen species (ROS) and reactive nitrogen species (RNS), including hydroxyl radicals (OH), superoxide radicals (O2−), ozone (O3), atomic oxygen and δ singlet oxygen and RNS, including peroxynitrite34.
This study aimed to assess the impact of LTP-argon treatment on multispecies cariogenic biofilms composed of C. albicans, L. casei, and S. mutans, as well as on single-species biofilms of C. albicans and L. casei. Notably, the effects of LTP-argon treatment on single-species biofilms of S. mutans were not included in this study, as they were previously investigated under identical parameters in the study by Figueira et al.28. Here, we explore the novel application of LTP-argon treatment on a model of cariogenic biofilms that includes not only S. mutans but also two other species closely related to caries—C. albicans and L. casei, aiming to expand evidence of its efficacy in MID for dental caries management in the future. Given the importance of a site-specific treatment to control dental caries despite conventional treatments, exploring alternative therapies like LTP-argon is crucial. Our findings could impact future dental research involving more intricate in vivo and clinical models, potentially modernizing clinical practices by refining MID protocols, including LTP application to manage cariogenic biofilms, as a safe and minimally invasive approach. LTP-argon therapy offers promise in biofilm management, which is critical for controlling dental caries progression. We hypothesize that LTP-argon treatment will effectively reduce cariogenic biofilms' viability and structural integrity, demonstrating its potential as a viable therapeutic option for cariogenic biofilm management. By addressing these aspects, this study aims to advance knowledge in dental microbiology and therapeutic interventions, potentially paving the way for improved treatment modalities in dental caries management.
Materials and methods
Plasma device and parameters
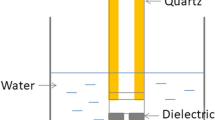
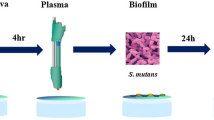
LTP jet was generated with argon gas through the KINPen09™ device (Leibniz Institute for Plasma Science and Technology, INP, Germany). The apparatus comprises a hand-held unit (170 mm in length, 20 mm in diameter, and 170 g) that generates the plasma jet, a DC power supply (system power: 8 W at 220 V, 50/60 Hz), and a gas supply unit. The LTP jet was produced from the top of the central electrode and expanded to the surrounding air outside the nozzle28. Biofilms formed on the top of hydroxyapatite discs were treated at a distance of 10 mm from the biofilm surface to the tip of the plasma device for 30 s, 60 s, and 120 s of exposure in continuous working mode28. Support developed by the laboratory collaborators and a calibrated operator trained to experiment with a consistent approach was used to guarantee the standardization of the distance between the plasma tip and the sample. The specimens were moved horizontally using sterile tweezers during LTP application to scan the whole surface. Argon gas flow was set to 5 slm, and the flow rate was controlled using a flow controller (MKS Instruments, Germany).
Microbial growth on hydroxyapatite discs
The following strains were used for the formation of the biofilm: Streptococcus mutans UA159 (ATCC 700610), L. casei (ATCC 10801), and C. albicans (SC 5314). The effects of LTP-argon treatment on single-species biofilms of S. mutans were not assessed in this study, as they had been previously investigated under identical parameters in the study by Figueira et al.28. Consequently, our investigation focused exclusively on single-species biofilms of C. albicans and L. casei and multispecies biofilms formed by S. mutans, C. albicans, and L. casei. Growth curves were performed to standardize the inoculum for the biofilm, and the equivalent colony-forming units (CFU) were determined. Stock cultures were maintained at − 80 °C. S. mutans was reactivated in BD BBL™ CDC Anaerobe 5% Sheep Blood agar and incubated at 37 °C for 48 h, L. casei was reactivated in BHI agar (Brain Heart Infusion, Difco, Detroit, USA), and C. albicans was reactivated in Sabouraud dextrose chloramphenicol Agar (SDA) 0.5 g/L. The plates were incubated at 37 °C for 72 and 24 h, respectively. S. mutans and L. casei were submitted to a captophilic (5% CO2) environment, requiring a carbon dioxide concentration between 5 to 10% and approximately 15% oxygen. After that, for the formation of the pre-inoculum, ten isolated colonies were collected with the aid of a sterile loop and subsequently transferred to a falcon centrifuge tube containing 10 mL of Tryptic soy broth (TSB) supplemented with 1% glucose at 37 °C for 18 h. Then, 1 mL of the pre-inoculum was transferred to a falcon centrifuge tube containing 9 mL of TSB supplemented with 1% glucose. The same procedures were done for all microorganisms separately. The growth of microorganisms was monitored aseptically every hour (0–24 h) through subsequent absorbance readings (optical density) in a spectrophotometer using the 600 nm wavelength to ensure the reproducibility of the biofilm model.
Multispecies cariogenic biofilm
S. mutans, L. casei, and C. albicans were incubated at 37 °C for 48, 72, and 24 h, respectively35. To prepare the pre-inoculum, the microorganisms were transferred from the agar plate to a centrifuge tube containing 10 mL of TSB broth supplemented with 1% glucose. Tubes were incubated at 37 °C, 5% CO2 for 18 h. Then, 1 mL of the pre-inoculum was transferred to another tube containing 9 mL of TSB supplemented with 1% sucrose, and the tubes were incubated as described in 2.2. According to the determination of the growth curve performed for each microorganism under analysis, the inoculum corresponded to 108 CFU/mL after incubation. The tubes were centrifuged, the supernatant was discarded, and 10 mL of saline was added. This same process was performed for two more times. The inoculum's microorganism concentration (108 CFU/mL) was confirmed with a spectrophotometer. Then, the L. casei suspensions were concentrated to 109 CFU/mL, C. albicans diluted to 105 CFU/mL, and de S. mutans to 107 CFU/mL. To form multispecies biofilms, an equal part of each standardized suspension was mixed up using a vortex. For single and multispecies biofilms, 200 µL of the inoculum was added to the wells of 24-well plates containing sterile hydroxyapatite discs, plus 800 µL of TSB broth supplemented with 0.2% sucrose was added28. Biofilms were incubated for 48 h (37 °C, 5% CO2). The fresh culture medium was replaced after 24 h.
Biofilm treatments and processing
Single and multispecies biofilms were treated with LTP for 30, 60, and 120 s, as described in 2.1. As a control for LTP efficacy, we applied only argon gas flow (F) for the same periods as LTP. Chlorhexidine (0.12%) was used as a positive control (PC), and 0.89% NaCl was used as a negative control. Both Chlorhexidine and NaCl were in contact with the samples for 1 min. The number of samples per group was six (n = 6). After that, the biofilms were scratched from the top of the hydroxyapatite discs using a sterile spatula and immediately diluted in 5 mL of sterile 0.89% NaCl solution. Biofilm suspension was sonicated for 10 s pulses at an output of 9 W (Fisher Scientific, Sonic Dismembrator model 100, USA) three times28. The same procedures were performed for each experimental group and control. The obtained suspensions were serially diluted and plated on selective agar. SDA with chloramphenicol (0.1 mg/mL) for C. albicans, Mitis Salivarius-Bacitracin (MSB) (0.1 unit/mL) sucrose (1%) for S. mutans, and Rogosa agar 1.32 mL/L glacial acetic acid were used to recover L. casei. After incubation at 37 °C for 24 h for C. albicans and 5% CO2 at 37 °C for 48 and 72 h for S. mutans and L. casei, respectively, the number of CFU/mL of recovered C. albicans, L. casei, S. mutans from the biofilm was recorded.
Confocal laser scanning microscopy (CLSM) of single- and multispecies biofilm
Multispecies biofilms composed of C. albicans, L. casei, and S. mutans (formed as described at 2.3) were stained with LIVE/DEAD® BacLight™ Bacterial Viability Kit (L13152; Molecular Probes, Inc.) and incubated in the dark at room temperature for 15 min to allow penetration of the fluorophores inside the bacterial cells. Specimens were then washed twice with 0.89% NaCl and examined under a Leica SP8 Resonant-scanning confocal/multiphoton microscope using a Leica Fluotar VISIR 25×/0.95 water objective, with a free working distance of 2.3 mm. Serial sessions on the XYZ plane were observed.
Statistical analysis
Normal data distribution was verified by the Shapiro–Wilk test and the Levene test checked the homogeneity of variance (α = 0.05). Analyzes were performed with the IBM SPSS statistical software package (version 25) for Windows (IBM Corp., New York, NY, USA). Results were presented in mean values (± standard deviation), being analyzed by ANOVA and Tukey's post-hoc test, considering α = 0.05. For this analysis, the GraphPad Prism 5.0 program was used.
Results
Single species biofilm of C. albicans and L. casei
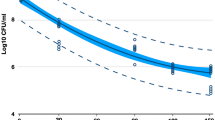
Figure 1 shows the results for single-species biofilms formed by C. albicans. A significant reduction in all exposure times (p < 0.0001) was observed for the LTP-treated groups compared to NC. There was no statistically significant difference between the exposure to LTP or F for 30 s (p > 0.05). However, LTP demonstrated better results than F for 60 and 120-s applications (p ≤ 0.05). LTP application for 60 s or 120 s showed similar results to 0.12% chlorhexidine (PC) (p > 0.05). Both LTP treatments for 60 and 120 s showed a reduction of more than 1 log10 CFU/mL (1.55 and 1.90 log10 CFU/mL, respectively) compared to both PC and NC. Figure 2 shows the results obtained for single-species biofilms formed by L. casei. Significant reduction in all exposure times (p < 0.0001) was observed in all LTP-treated groups compared to negative and positive controls. The results for L. casei single biofilms were similar to those observed for C. albicans with respect to the comparison between LTP and F. There was no statistically significant difference between the exposure to LTP or F for 30 s (p > 0.05). Yet, LTP demonstrated better results than F for 60 and 120-s applications (p ≤ 0.05). This suggests that the effects of LTP on single-species biofilms depend on the exposure time, with at least 60 s of exposure needed to observe a significant reduction.
Mean values and standard deviations log10 (CFU/mL) of single-species biofilms composed by C. albicans treated with LTP-argon 0.12% chlorhexidine digluconate (positive control) or 0.89% saline solution (negative control). *Values equal to zero. Different letters indicate significant statistical differences (n = 9; P ≤ 0.05; ANOVA, Tukey's Test). F30, F60, and F120 (argon gas–flow control treatment for 30, 60, and 120 s); P30, P60, and P120 (LTP-argon treatment for 30, 60, and 120 s), PC (positive control) and NC (negative control).
Mean values and standard deviations log10 (CFU/mL) of single-species biofilms composed by L. casei treated with LTP-argon, 0.12% chlorhexidine digluconate (positive control) or 0.89% saline solution (negative control). *Values equal to zero. Different letters indicate significant statistical differences (n = 9; P ≤ 0.05; ANOVA, Tukey's Test). F30, F60, and F120 (argon gas–flow control treatment for 30, 60, and 120 s); P30, P60, and P120 (LTP-argon treatment for 30,60 and 120 s), PC (positive control) and NC (negative control).
Multispecies biofilms: C. albicans, L. casei, and S. mutans
Figure 3 shows the log10 CFU/mL results for the multispecies biofilms formed by C. albicans, L. casei, and S. mutans. In the counts of these three microorganisms recovered from the multispecies biofilms, a significant log10 CFU/mL reduction was observed in LTP-treated samples in all exposure times (30, 60, and 120 s) in comparison to PC, NC, and F (p < 0.0001). Both C. albicans and L. casei LTP-treated for 120 s groups showed a more than 2 log10 CFU/mL reduction. Finally, for S. mutans, the LTP-argon treatments for 60 and 120 s, in addition to having similar reductions, were greater than 2 log10 CFU/mL. Furthermore, 0.12% chlorhexidine treatment per 1 min for all microorganisms recovered from the multispecies biofilms showed no statistically significant log10 CFU/mL reduction (p < 0.05) compared to the negative control.
Mean values and standard deviations log10 (CFU/mL) of polymicrobial biofilms composed by Candida albicans, Lactobacillus casei, and Streptococcus mutans treated with LTP-argon, 0.12% chlorhexidine digluconate (positive control) or 0.89% saline solution (negative control). *Values equal to zero. Different letters indicate significant statistical differences (n = 9; P ≤ 0.05; ANOVA, Tukey's Test). F30, F60, and F120 (argon gas–flow control treatment for 30. 60, and 120 s); P30, P60, and P120 (LTP-argon treatment for 30, 60, and 120 s); PC (positive control) and NC (negative control).
Confocal scanning laser microscopy (CSLM)
Figure 4 presents a CSLM representative image of the morphology and structural organization of multispecies biofilm after the treatment with LTP for 120 s compared to the negative control. The photos show live cells stained green (Syto 9), while dead cells are stained red (Propidium Iodine). As the picture shows, samples treated with LTP for 120 s present a visually larger area covered in dead cells when compared to the negative control, which can be visualized in the overlaid images (P120 live/dead), as well as when images are separated in live or dead channels.
Confocal laser scanning microscopy of multispecies biofilm formed by Streptococcus mutans, Streptococcus gordonii, and Streptococcus sanguinis in the negative control group and LTP-argon (120 s) group; 25 × zoom. Biofilm stained with Live/Dead BacLight Viability kit. Live cells are in fluorescent green, and dead cells are in red. Live and Dead (live/dead) cells mixed in fluorescent green and red.
Discussion
In this study, we worked with three different species of microorganisms commonly related to the composition of cariogenic biofilm on the tooth surface. Here, we could show that the cariogenic biofilm formed by the association of these microorganisms was significantly reduced after treatment in all plasma-treated samples compared to both negative and positive controls.
Our results indicate that LTP treatment reduced the viability of C. albicans by 1.95 log10 CFU/mL on the multispecies biofilm after 120 s of treatment compared to negative control. This is corroborated by a previous study, where the authors used LTP-helium at a 1.5 cm distance from the plasma tip to the sample. They observed significant viable C. albicans cell reduction. Approximately 2-log reduction was observed after 7.5 min of exposure36. Another study that also used an LTP-helium analyzed the capacity of the LTP-helium to disrupt the biofilm matrix, the cellular structure, and the C. albicans viability after exposure by applying 60 s of treatment and 10 mm of distance from the plasma tip to the biofilm surface. The results indicated that after treatment, significant log reductions were observed by changing the microorganism morphology compared to controls37.
Ebrahimi-Shaghaghi et al. evaluated the effects of LTP-helium/O2 (2%) on the growth of C. albicans, submitted to 90, 120, 150, 180, and 210 s of treatment. The percentage of biofilm inhibition was 31.43% after 90 s of exposure and reached 41.15% after 120 s of treatment38. Similar trends were observed in the present work both for monospecies and multispecies biofilms; as the dosage of LTP increased, a more significant biofilm reduction was observed.
This study also significantly reduced L. casei in multispecies cariogenic biofilms. Another study analyzed the action of the LTP-argon brush on monospecies biofilms formed by Lactobacillus and Streptococcus on hydroxyapatite discs. Three, 9, 13, 15 and 18 s of treatment were applied. Initially, the biofilm was created by a lower concentration of inoculum (between 2.1 × 108 and 2.4 × 108 CFU/mL). After treatments, the authors observed that applying the plasma brush for just 13 s was necessary to reduce the viability of the biofilms to the point that they could not be recovered. However, when biofilms were formed at a higher concentration (between 1.7 × 1010 and 3.5 × 1010 CFU/mL), the biofilm showed greater resistance to LTP-argon and the reductions were between 1.5 and 2. 5 logs for both biofilms, respectively39. This study may help to understand better how LTP and its generated reactive oxygen species can act to reduce the viability of biofilms of Lactobacillus and S. mutans, two sugar metabolizers that are important in the process of caries evolution, and also to understand better how single jet that can only treat a specific point can act in comparison to LTP-brush that produces more quantity reactive species.
In a previous study carried out by our team, we analyzed the action of LTP-argon using the same plasma device commercially obtained, as well as the same parameters28; the argon gas flow was also used, and the effect of LTP-argon jet was analyzed on single and multispecies biofilms formed by S. mutans, Streptococcus sanguinis, and Streptococcus gordonii on top the same hydroxyapatite disk. After treatment with LTP-argon per 30 s, 60 s, and 120 s, we observed a significant reduction in viability (log10 CFU/mL) for S. mutans on single and muti-species biofilm28. This study obtained similar results for S. mutans on multi-species biofilm formed by C.albicans, L. casei, and S. mutans. We observed a significant reduction in viability (log10 CFU/mL) for all plasma-treated samples. This allowed us to understand better the action of LTP-argon on different associations of cariogenic microorganisms present in the biofilm, especially the activity of LTP-argon on S. mutans.
Qing et al. investigated the effect of LTP-argon brush treatment on the biofilm of S. mutans nomo-species40 by applying a 6 mm distance from the plasma tip to the sample surface. Biofilms were treated for 1, 2, and 5 min. After just 1 min of treatment, results show 90% biofilm reduction. Our results were significant for better understanding the LTP jet since the plasma brush can treat a larger surface, producing more oxygen species, nitrogen, and other agents. The plasma jet can only treat a specific point, which leads to less production of these agents that cause the effect41 Nima et al. evaluated the action of LTP against S. mutans biofilms formed on sterile resin discs under anaerobic conditions. The plasma jet was applied for 30, 90, 120, and 150 s. After CFU analysis, a significant reduction was proven at all times of treatment, using jets of LTP formed by a device similar to a brush42. Yang et al. observed the effectiveness of the LTP device in reducing the viability of biofilms formed by S. mutans and Lactobacillus acidophilus. The authors conclude in this study that the complete elimination of S. mutans took only 15 s and 5 min to eradicate L. acidophilus43.
Using 0.12% chlorhexidine as a positive control was based on knowing that chlorhexidine is a gold-standard chemical substance with an antibacterial action against gram-positive and gram-negative bacteria44. In this study, treatment with 0.12% chlorhexidine reduced C. albicans, L. casei, and S. mutans log10 CFU/mL amount according to the exposure. However, there is an urgency to develop alternative methods to contain the exponential growth of the microbial population comprised in biofilm without increasing the burden of bacterial and fungal resistance. In this sense, LTP is an optimal alternative for the microbial resistance response because it is safe, effective, and non-toxic, and, to this date, no bacterial or fungal resistance to LTP has been reported45.
Plasmas are produced at various pressures, generally close to atmospheric pressure by high electric field intensities, which have electromagnetic radiation, ultraviolet radiation, and light in the visible spectrum, free radicals, free electrons, neutral reactive oxygen species (ROS–O, O2·−, O3, OH), and nitrogen (RNS–N, N2*, NO, NO2) that play a synergistic role in antimicrobial action46. LTPs are produced at the temperature of heavy species (neutral ions), which is much lower than the electron temperature and can provide energetic fluxes of ions to the substrates. This gaseous reaction at low temperatures is the main reason for using LTPs for biological interests46, and studies have demonstrated the safety of LTP in various oral tissues, such as keratinocytes, gingival fibroblasts, and reconstituted tissue, showing that LTP has lower cytotoxicity and high viability of cells after the treatment, proved by histological images37,47. Due to these fundamental characteristics, scientists began to innovate to update existing dentistry protocols.
It is essential to acknowledge the inherent limitations of an in vitro study. In our research, hydroxyapatite was used as a substrate, which does not fully replicate natural teeth' complex structure and composition. Consequently, the interactions between dental plaque, saliva, and the tooth surface in a living oral environment cannot be fully replicated. In addition, in vitro models lack dynamic oral conditions such as saliva flow, pH fluctuations, and bacterial colonization patterns. These constraints underscore the necessity for complementary in situ research to validate our findings and obtain a more comprehensive understanding of low-temperature plasma (LTP) for caries management. Nevertheless, our study has laid the groundwork by establishing initial trends and hypotheses to guide future experiments. By meticulously controlling variables and conditions in a laboratory setting, we systematically investigated the effects of LTP on individual microorganisms and as part of multispecies biofilms, aiming to assess its potential as a targeted treatment to reduce cariogenic biofilms. Subsequent studies will be crucial for evaluating the action of LTP on biofilms formed in situ.
To conclude, LTP-argon demonstrated a significant antibiofilm effect against both single biofilms of C. albicans and L. casei and a multispecies biofilm comprising C. albicans, L. casei, and S. mutans. LTP-argon effectively reduced cariogenic biofilms in short exposure times, as brief as 30 s for multispecies biofilms and 60 s for single-species biofilms; therefore, we recommended an application time of 60 s. These findings suggest potential advancements in LTP technology, paving the way for developing equipment with customized parameters for effective dental caries treatment. Low-temperature plasma is considered a low-cost technology48 that could be implemented in clinical settings for caries management, following the principles of MID. Our study has yielded promising results, particularly in managing cariogenic biofilms in vitro through targeted, brief applications of LTP-argon.
Data availability
All data are included in the manuscript, and the corresponding author can provide additional information and answer questions about sharing data.
References
Zhou, N. et al. Dental caries and associated factors in 3 to 5-year-old children in Zhejiang Province, China: An epidemiological survey. BMC Oral Health 19, 9 (2019).
Schwendicke, F. et al. Socioeconomic inequality and caries: A systematic review and meta-analysis. J. Dent. Res. 94, 10–18 (2015).
Pitts, N. et al. Understanding dental caries as a non-communicable disease. Br. Dent. J. 231, 749–753 (2021).
- Rusu, L., Roi, A., Roi, C., Tigmeanu, C.V. & Ardelean, L.C. The Influence of Salivary pH on the Prevalence of Dental Caries (ed. Intech open) 108 (2022).
Jamal, M. et al. Bacterial biofilm and associated infections. J Chin. Med. Assoc. 81, 7–11 (2018).
Kim, D. & Koo, H. Spatial design of polymicrobial oral biofilm in its native disease state. J. Dent. Res. 99, 597–603 (2020).
Sims, K. R. Jr. et al. Dual antibacterial drug-loaded nanoparticles synergistically improve treatment of Streptococcus mutans biofilms. Acta Biomater. 115, 418–431 (2020).
Perez-Diaz, M. A. et al. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C Mater. Biol. Appl. 55, 360–366 (2015).
Palmer, R. J. J. R. Composition and development of oral bacterial communities. Periodontology 2000(64), 20–39 (2014).
Grier, A. et al. Oral microbiota composition predicts early childhood caries onset. J. Dent. Res. 100, 599–607 (2021).
Edelstein, B. L., Ureles, S. D. & Smaldone, A. Very high salivary Streptococcus mutans predicts caries progression in young children. Pediatr. Dent. 38, 325–330 (2016).
Cao, Y. & Lin, H. Characterization and function of membrane vesicles in gram-positive bacteria. Appl. Microbiol. Biotechnol. 105, 1795–1801 (2021).
Eriksson, L. et al. Microbial complexes and caries in 17-Year-Olds with and without streptococcus mutans. J. Dent. Res. 97, 275–282 (2018).
Gross, E. L. et al. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 7, e47722 (2012).
Belstrøm, D. et al. Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. J. Oral Microbiol. 8, 30170 (2016).
Richards, V. P. et al. Microbiomes of site-specific dental plaques from children with different caries status. Infect. Immun. 85, 8 (2017).
Wu, R., Cui, G., Cao, Y., Zhao, W. & Lin, H. Streptococcus Mutans membrane vesicles enhance Candida albicans pathogenicity and carbohydrate metabolism. Front. Cell Infect. Microbiol. 12, 940602 (2022).
Gregoire, S. et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 77, 6357–6367 (2011).
Thanh, N. H. et al. Candida albicans Bgl2p, Ecm33p, and Als1p proteins are involved in adhesion to saliva-coated hydroxyapatite. J. Oral Microbiol. 13, 1879497m (2021).
Alkhars, N. et al. Oral candida predicts Streptococcus Mutans emergence in underserved US infants. J. Dent. Res. 101, 54–62 (2022).
Badet, C. & Thebaud, N. B. Ecology of lactobacilli in the oral cavity: A review of literature. Open Microbiol. J. 2, 38–48 (2008).
Wen, Z.T., Huang, X., Ellepola, K., Liao, S., Li, Y. Lactobacilli and human dental caries: more than mechanical retention. Microbiology. 168, (2022).
Caufield, P. W., Schön, C. N., Saraithong, P., Li, Y. & Argimón, S. Oral Lactobacilli, and dental caries: A model for niche adaptation in humans. J Dent Res. 94(Suppl), 110S-S118 (2015).
Gao, S. S., Du, M. & Maharani, D. A. Editorial: Minimally invasive dentistry for caries management. Front. Oral Health. 7, 940177 (2022).
Eden, E., Frencken, J., Gao, S., Horst, J. A. & Innes, N. Managing dental caries against the backdrop of COVID-19: Approaches to reduce aerosol generation. Br. Dent. J. 229, 411–416 (2022).
BaniHani, A., Hamid, A., Van Eeckhoven, J., Gizani, S. & Albadri, S. Minimal Intervention Dentistry (MID) mainstream or unconventional option? Study exploring the impact of COVID-19 on paediatric dentists’ views and practices of MID for managing carious primary teeth in children across the United Kingdom and European Union. Eur. Arch. Paediatr. Dent. 23, 835–844 (2022).
Jungbauer, G. et al. The antimicrobial effect of cold atmospheric plasma against dental pathogens—A systematic review of in-vitro studies. Antibiotics (Basel). 10, 211 (2021).
Figueira, L. W., Panariello, B. H. D., Koga-Ito, C. Y. & Duarte, S. Low-temperature plasma as an approach for inhibiting a multispecies cariogenic biofilm. Appl. Sci. 11, 570 (2021).
Nima, G. et al. Antibacterial efficacy of non-thermal atmospheric plasma against Streptococcus mutans biofilm grown on the surfaces of restorative resin composites. Sci. Rep. 11, 23800 (2021).
Laroussi, M. Plasma medicine: A brief introduction. Plasma. 1, 47–60 (2018).
Bourke, P., Ziuzina, D., Han, L., Cullen, P. & Gilmore, B. F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 123, 308–324 (2017).
Neyts, E. C. & Brault, P. Molecular dynamics simulations for plasma-surface interactions. Plasma Process. Polym. 14, 1–2 (2017).
Tanaka, H. et al. Plasma-treated solutions (PTS) in cancer therapy. Cancers. 13, 1737 (2021).
Privat-Maldonado, A., Bengtson, C., Razzokov, J., Smits, E. & Bogaerts, A. Modifying the tumour tmicroenvironment: Challenges and future perspectives for anticancer plasma treatments. Cancers. 11, 1920 (2019).
Arthur, R. A., Waeiss, R. A., Hara, A. T., Lippert, F., Eckert, G. J., Zero, D. T. Caries Res. 47, 318–324 (2013).
Borges, A. C. et al. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 7, 9–15 (2017).
Delben, J. A. et al. Low-temperature plasma: An effective approach against Candida albicans biofilm. Plasma Med. 4, 231–244 (2014).
Ebrahimi-Shaghaghi, F., Noormohammadi, Z., Atyabi, S. M. & Razzaghi-Abyaneh, M. Inhibitory effects of cold atmospheric plasma on the growth, virulence factors and HSP90 gene expression in Candida albicans. Arch Biochem. Biophys. 30, 108772 (2021).
Blumhagen, A. et al. Plasma deactivation of oral bacteria seeded on hydroxyapatite disks as tooth enamel analogue. Am. J. Dent. 27, 84–90 (2014).
Qing, H. et al. Disinfection of Streptococcus mutans biofilm by a non-thermal atmospheric plasma brush. Appl. Phys. 55, 07LG02 (2016).
Duan, Y., Rani, S., Newberg, J. T. & Teplyakov, A. V. Investigation of the influence of oxygen plasma on supported silver nanoparticles. J. Vac. Sci. Technol. A. 1, 01B101 (2018).
Nima, G. et al. Antibacterial efficacy of non-thermal atmospheric plasma against Streptococcus mutans biofilm grown on the surfaces of restorative resin composites. Sci. Rep. 11, 23800 (2021).
Yang, B. et al. Oral bacterial deactivation using a low-temperature atmospheric argon plasma brush. J. Dent. 39, 48–56 (2011).
Kamath, D. G., Nadimpalli, H., Nayak, S. U., Rajendran, V. & Natarajan, S. Comparison of antiplaque and anti-gingivitis effects of aloe vera mouthwash with chlorhexidine in fixed orthodontic patients—A randomized controlled trial. Int. J. Dent. Hyg. 21, 211–218 (2023).
Nicol, M. J. et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 10, 3066 (2020).
Adamovich, I. et al. The 2022 plasma roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 55, 373001 (2022).
Bekeschus, S., Miebach, L., Pommerening, J., Clemen, R. & Witzke, K. Biological risk assessment of three dental composite materials following gas plasma exposure. Molecules. 27, 4519 (2022).
Machala, Z. & Graves, D. B. Frugal biotech applications of low-temperature plasma. Trends Biotechnol. 36, 579–581 (2017).
Acknowledgements
This work was supported by The São Paulo Research Foundation/FAPESP (Process# 2018/17707-3, 2019/01676-4 and 2019/05856-7). This work was conducted while the corresponding author was an Associate Professor at Indiana University School of Dentistry.
Author information
Authors and Affiliations
Contributions
C.Y.K.I., S.D., L.W.F.: the conception and design of the study L.W.F., C.Y.K.I., S.D., B.P.: acquisition of data, analysis and interpretation of data. L.W.F., B.P.: Drafted the article and revised it critically for important intellectual content. L.W.F., C.Y.K.I., S.D., B.P.: final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Figueira, L.W., Panariello, B., Koga-Ito, C.Y. et al. Exploring the efficacy of in-vitro low-temperature plasma treatment on single and multispecies dental cariogenic biofilms. Sci Rep 14, 20678 (2024). https://doi.org/10.1038/s41598-024-70943-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70943-0
- Springer Nature Limited