Abstract
Introduction
To describe the real-world use of romosozumab in Japan, we conducted a chart review of > 1000 Japanese patients with osteoporosis (OP) at high risk of fracture, across multiple medical institutions.
Materials and methods
Treatment-naïve and prior OP-treatment patients who received romosozumab for 12 months followed by ≥ 6 months of sequential OP treatment were included. The primary objective described the baseline demographics and clinical characteristics; secondary objectives evaluated changes in bone mineral density (BMD) and bone turnover markers in all patients and effectiveness of romosozumab in a sub-group of treatment-naïve patients using the fracture risk assessment tool (FRAX®).
Results
Of the 1027 patients (92.4% female), 45.0% were treatment-naïve. The mean ± SD age of treatment-naïve versus prior OP-treatment patients was 76.8 ± 8.5 and 77.1 ± 8.5 years. The most frequent prior OP treatment was bisphosphonates (45.0%). Romosozumab treatment for 12 months increased BMD at the lumbar spine in all groups; the median percent change from baseline in lumbar spine BMD was higher in the treatment-naïve (13.4%) versus prior OP-treatment group (bisphosphonates [9.2%], teriparatide [11.3%], denosumab [DMAb, 4.5%]). DMAb, bisphosphonates, or teriparatide after romosozumab maintained the BMD gains at all skeletal sites at month 18 in treatment-naïve patients. Most treatment-naïve patients were at high risk of fracture, BMD increased consistently with romosozumab regardless of the baseline fracture risk assessed by FRAX.
Conclusion
This large-scale, multicenter chart review provides clinically relevant insights into the profiles of patients initiating romosozumab, effectiveness of real-world romosozumab use, and sequential therapy in Japanese patients at high risk of fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis (OP) is a major public health burden and is one of the leading causes of morbidity and mortality among elderly women and men worldwide [1]. Using the World Health Organization (WHO) bone mineral density (BMD)-based definition of OP (BMD T-score ≤ − 2.5 as assessed by dual-energy X-ray absorptiometry [DXA]), Japan had the highest prevalence of OP (38%) among women in industrialized countries [2]. Management of OP includes the use of antiresorptive treatments (such as bisphosphonates, selective estrogen receptor modulators [SERMs], and the RANKL inhibitor denosumab [DMAb]) and osteoanabolic or bone-forming agents (such as teriparatide, abaloparatide, and romosozumab) with various mechanisms of action [3].
Romosozumab, a sclerostin antibody, has a dual effect of promoting bone formation and reducing bone resorption [4, 5]. In global and regional clinical trials, treatment with romosozumab was shown to significantly increase BMD and reduce fracture risk when compared with placebo (FRAME [6], Japan phase 2 trial [7]), alendronate (ARCH [8]), or teriparatide (STRUCTURE [9]) in postmenopausal women with OP; in men with OP romosozumab significantly increased spine and hip BMD (BRIDGE [10]). In a subgroup analysis of FRAME, Japanese women at high risk of fracture showed significant BMD gains and a reduced trend in fracture risk with 12 months of romosozumab versus placebo, followed by DMAb for 24 months [11].
For the prevention and treatment of OP, Japanese guidelines have incorporated the Fracture Risk Assessment Tool (FRAX®), which estimates the 10-year probability of major osteoporotic (defined as clinical vertebral, hip, forearm, or proximal humerus fracture) and hip fractures [12], into the criteria for initiating pharmacological treatment [13]. The interaction between treatment efficacy and baseline fracture risk characterized using FRAX has been explored in several analyses performed using placebo-controlled studies [14,15,16,17,18,19,20,21,22]. Efficacy of romosozumab on clinical, osteoporotic, and major osteoporotic fractures was significantly greater in patients with high baseline FRAX fracture probability compared with that of placebo in the FRAME study [22].
Romosozumab was approved in Japan in January 2019 for the treatment of OP in patients at a high risk of fracture [23]. Since its approval, many retrospective and prospective studies describing the real-world effectiveness of romosozumab have been published [24,25,26,27,28,29,30,31]. However, most of the current real-world evidence from Japan comes from single-center studies with a sample size of < 250 patients. Therefore, to gain more in-depth real-world clinical insights into patient profiles and the effectiveness of romosozumab in larger patient populations in Japan, we conducted a large-scale, multicenter, retrospective chart review in Japanese patients with OP at a high risk of fracture. To evaluate the effectiveness of romosozumab and sequential OP treatment, we examined patients who were treated with romosozumab for 12 months and then transitioned to a sequential OP treatment for at least 6 months. In addition, we explored the relationship between baseline fracture probability (as measured by FRAX) and the effectiveness of romosozumab in treatment-naïve patients in the real-world.
Materials and methods
Patients and study design
This observational, multicenter, retrospective review of medical charts of Japanese patients included data from March 4, 2018, to June 30, 2021. For this review, the number of study sites was determined based on the distribution of healthcare organizations with > 50 patients with a prescription for romosozumab in five geographic regions; the percent of patients with diagnosed OP at a high risk of fracture in each region was 12.6% in Hokkaido/Tohoku, 33.3% in Kanto, 15.6% in Chubu, 18.6% in Kansai, and 20% in Chugoku/Shikoku/Kyushu (Supplemental Fig. 1) [32].
This analysis included patients with OP at a high risk of fracture as defined by the latest (year 2012 revision) Japanese diagnostic criteria for primary OP [33], and who received romosozumab for 12 months starting March 4, 2019 (ie., romosozumab Japan launch date) or after, and then transitioned to another OP therapy for ≥ 6 months. All patients met at least one of the following criteria: (1) BMD T-score < − 2.5 with one or more prevalent fragility fracture at any skeletal site according to WHO’s severe osteoporosis definition [33]; (2) lumbar spine BMD T-score < − 3.3; (3) presence of two or more prevalent vertebral fractures; (4) any prevalent vertebral fracture with semi-quantitative grade 3 [34]; or (5) prevalent hip fractures (considered as high risk of fracture).
Patients initiating treatment on March 4, 2019, or thereafter, with conditions specified as contraindications in the Japan EVENITY® (romosozumab) label, (ie., a history of hypersensitivity to any of the ingredients or the presence of hypocalcemia) were excluded [23]. In September 2019, after the study enrollment began, the Japan romosozumab label was updated to include a warning regarding the imbalance of major adverse cardiac events (MACE), a composite endpoint of cardiovascular (CV) death, nonfatal myocardial infarction, and nonfatal stroke observed in patients receiving romosozumab in a clinical study conducted outside of Japan and in Japanese patients in a postmarketing surveillance. The update also included advisement to assess the benefit of fracture reduction and risk of a CV event when prescribing romosozumab as well as monitoring of any CV events [23].
Patients were categorized as either treatment-naïve (if they had not received any previous OP treatment) or as patients with prior OP treatment (if they had received any OP treatment within 6 months before romosozumab initiation), as reported in the case report form. Data were collected during the baseline period (12 months before romosozumab initiation [ie., index date]), the romosozumab treatment period (12 months), and a follow-up of ≥ 18 months after the index date (Fig. 1).
Study design. The baseline period was defined as the 12 months before romosozumab initiation. The period including 12 months of romosozumab treatment and at least 6 months of the first sequential therapy was defined as the follow-up period. BMD, bone mineral density; BTM, bone turnover marker; FRAX, Fracture Risk Assessment Tool; OP, osteoporosis; QM, once monthly; SC, subcutaneous
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. Though this was a retrospective chart review without any patient intervention, the study protocol was approved by the institutional ethical review board/ethics committee at each study site. Based on the recommendation of the ethics committee, an “opt out” approach was implemented at all study sites.
Outcome measures
The primary objective of this study was to describe the baseline demographic and clinical characteristics of Japanese patients with OP at a high risk of fracture who completed 12 months of romosozumab treatment and transitioned to other OP therapies for at least 6 months.
The secondary objectives were to describe changes in BMD and bone turnover markers (BTMs) from baseline to 12 months and to 18 months of treatment. BMD at 12-month was defined as BMD observed post completion of romosozumab therapy and prior to initiation of sequential therapy within a window of 12 ± 2 months from the initiation of romosozumab. Similarly, BMD at 18-month was defined as the last observed BMD at least 6 months after the first sequential therapy post romosozumab therapy within a window of 18 ± 2 months. The following variables were captured based on the availability in the patient’s medical charts: demographics and medical history including prevalent fractures, history of CV disease, most recent prior OP therapy, and most recent BMD T-score from the initiation date of romosozumab in the baseline period, healthcare utilization, and substance use (smoking status and alcohol consumption at romosozumab initiation). For the first sequential therapy chosen after romosozumab treatment, the name and dose of the drug, the start and end date of treatment, and the reason for choice of treatment were collected for each patient in all treatment groups. For BMD, absolute values and percent changes from baseline over 18 months at the lumbar spine, total hip, and femoral neck were described. The machine type used for the DXA measurement at each visit was also recorded.
Although serum type I collagen cross-linked C-terminal telopeptide (CTX), is a globally recommended marker for bone resorption [35], tartrate-resistant acid phosphatase 5b (TRACP-5b) is the most commonly evaluated bone resorption marker in Japan; thus, instead of CTX we obtained TRACP-5b data for all patients, consistent with practice patterns in Japan. Absolute values and percent changes from baseline in serum concentrations over 18 months were described for the following BTMs: procollagen type 1 N-propeptide (P1NP), bone-specific alkaline phosphatase (BSAP), TRACP-5b, and type I collagen cross-linked N-terminal telopeptide (NTX). For NTX, urine concentrations were also obtained. Safety data were not collected in this study.
In treatment-naïve patients with a baseline FRAX score, we analyzed the relationship between baseline fracture probability, and the effectiveness of romosozumab based on absolute and percent change in BMD from baseline. In addition to the variables captured for all the patients, 10-year probability of a major osteoporotic fracture and of a hip fracture was included for treatment-naïve patients. Ten-year probabilities of major osteoporotic and hip fractures, as assessed by FRAX, were calculated with actual femoral neck BMD (in g/cm2) or T-score at baseline.
Statistical analyses
Statistical analyses were descriptive in nature and no formal hypotheses were tested in this study. The following summary statistics were reported for continuous variables: number of patients, mean, median, standard deviation (SD), first quartile (Q1), and third quartile (Q3). For categorical variables, the number and percents were reported.
As the BMD data for all skeletal sites were positively skewed, percent change from baseline in BMD values were summarized using median and quartile range values instead of mean and SD values. For comparison between groups, only patients with BMD or BTM data available at both baseline and month 12 (at the respective skeletal site) were included.
Unless specified otherwise, the analyses were performed for full analysis set (FAS), which was defined as all patients who had a romosozumab initiation date. No statistical imputation was employed for addressing missing data. All statistical analyses were conducted using SAS software, version 9.4.
Results
Baseline characteristics of overall population grouped by prior therapy
This retrospective, observational study was conducted at 19 sites across five regions in Japan (Supplemental Fig. 1). All 1027 patients were included in the FAS; of whom, 1002 (97.6%) patients received 12 doses of romosozumab, while 25 (2.4%) patients received 11 doses of romosozumab. Due to the retrospective nature of the study, some data were not available for all the patients. Supplemental Table 1 shows the number of patients for whom the data were available for each baseline demographic and clinical characteristic. The number of recorded scans by each type of DXA machine are shown in Supplemental Table 2. Hologic Discovery was the most commonly used DXA machine (eight study sites), followed by Hitachi Aloka Medical DCS-900 (four study sites), GE Prodigy (three study sites), GE Lunar iDXA (two study sites), and Hitachi Aloka ALPHYSLF (two study sites). Hologic Horizon machines were used at one study site, while the DXA machine type used was unknown at three study sites.
Of the 1027 patients enrolled in this study, 462 (45.0%) patients were treatment-naïve and 565 (55.0%) received prior OP treatment; as only 2 patients received calcitonin as a prior OP treatment and data were missing for 3 patients, no further data analysis was performed for these 5 patients (Table 1). Overall, 949 (92.4%) were women, 77 (7.5%) were men, and the sex of 1 patient was unknown. The mean (SD) age was 76.8 (8.5) years for treatment-naïve patients and 77.1 (8.5) years for patients with prior OP treatment. Most patients had no prevalent vertebral fracture at baseline. Among patients with prior OP therapy, patients treated with DMAb before romosozumab had the highest mean (SD) FRAX score (10-year probability) of 33.02% (16.10) for major osteoporotic fracture. The duration of the most recent prior OP therapy showed a skewed distribution for each treatment group, with a median duration that was different between groups. The most used prior treatment was bisphosphonates (254/565, 45.0%). Supplemental Table 3 provides further details on the prior OP treatments received.
Other patient characteristics such as the number of patients with a history of major osteoporotic fracture, cerebrovascular disease, and ischemic heart disease were similar between treatment-naïve patients and patients with prior OP treatment. No patients with a history of cerebrovascular disease and/or ischemic heart disease within 1 year before study enrollment were initiated on romosozumab following the label update; however, one patient with a history of stroke was included, but the timing of stroke in relation to initiation of romosozumab was not collected. Per the label update, for patients with a high risk of ischemic heart disease or cerebrovascular disease, the decision to prescribe romosozumab should be made after considering the benefit of fracture reduction versus the risk of a CV event [23].
Baseline BMD and BTMs values in patients with month 12 data
Due to the retrospective nature of this chart review, BMD and BTM data were not available for all patients at month 12. Therefore, for a better understanding and comparison of the clinical characteristics of the patients at baseline and at month 12, we summarized the baseline BMD and BTM values in patients for whom respective data were available at both time points (Supplemental Table 4). Notable differences in baseline absolute BMD values and T-scores between the treatment-naïve group and other prior OP treatment groups were observed at all skeletal sites.
In general, patients previously treated with teriparatide had a higher baseline level of P1NP, while patients previously treated with DMAb had a lower baseline level of TRACP-5b than that seen among patients from other groups. These trends were consistent with the accepted mechanisms of action/pharmacodynamics of these prior treatments. The median BSAP value was higher in patients treated with teriparatide compared with patients from other treatment groups for whom data were available. However, BSAP data were available for only a small number of patients in each treatment group. NTX data were limited with less than 10% of patients having both pre- and post-baseline NTX data collected and hence are not included in this report.
Changes in BMD in patients with 12-month data
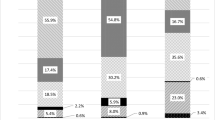
The median percent change from baseline in BMD values after administration of romosozumab over 12 months was numerically higher for the treatment-naïve group; however, the interquartile ranges (IQR) for these values overlapped between the treatment-naïve group and prior OP treatment groups at the lumbar spine, total hip, and femoral neck (Fig. 2a). The median percent change from baseline in lumbar spine BMD was numerically higher in the treatment-naïve group (13.4%) compared with that in prior OP treatment groups such as bisphosphonates (9.2%), teriparatide (11.3%), or DMAb (4.5%). In the DMAb group, the percent increase in lumbar spine and femoral neck BMD from baseline to month 12 was numerically smaller compared with the increases observed in other groups; moreover, a small decrease in BMD was observed at the total hip at month 12.
Percent change in BMD and BTM from baseline to month 12 in patients treated with romosozumab grouped by prior OP therapy. a. Median (Q1, Q3) percent change in BMD from baseline at the lumbar spine, total hip, and femoral neck in patients receiving romosozumab treatment for 12 months and for whom the baseline and month 12 data were available at the respective skeletal site. Patients were grouped based on prior OP treatment received. The table shows the number of patients for whom data were available for at each time point. b. Median (Q1, Q3) percent change from baseline in P1NP, TRACP-5b, and BSAP in patients receiving romosozumab treatment for 12 months and for whom baseline and month 12 data for the respective BTM were available. Patients were grouped based on the prior OP treatment received. The table shows the number of patients for whom data were available at each time point. *No data were available for month 3 for patients receiving TPTD as prior OP therapy. BIS, bisphosphonate; BMD, bone mineral density; BSAP, bone-specific alkaline phosphatase; BTM, bone turnover marker; DMAb, denosumab; OP, osteoporosis; P1NP, procollagen type 1 N-propeptide; Q1, first quartile, Q3, third quartile; TPTD, teriparatide; TRACP-5b, tartrate-resistant acid phosphatase-5b
To better understand the effectiveness of sequential therapy after romosozumab treatment, we analyzed the percent change in BMD from baseline in treatment-naïve patients who received 12 months of romosozumab followed by at least 6 months of sequential therapy. At month 18, sequential treatment with DMAb, bisphosphonates, and teriparatide improved or maintained the BMD gains at all skeletal sites in treatment-naïve patients (Supplemental Fig. 2). The median percent change in total hip and femoral neck BMD from baseline was numerically higher for the active vitamin D3 group than in other groups; however, these data were available for < 10 patients and the IQR overlapped with the IQR of the other treatment groups.
Changes in BTMs in patients with 12-month data
We analyzed percent change from baseline in the bone formation markers P1NP and BSAP and the bone resorption marker TRACP-5b during the 12 months of romosozumab treatment for each treatment group (Fig. 2b). The median percent change from baseline for P1NP was lower at all time points in the teriparatide group compared with other treatment groups. The median percent change from baseline for TRACP-5b was higher at all time points in the DMAb group compared with other treatment groups. At month 12, an increase in the median percent change from baseline in BSAP was observed in the DMAb group, while BSAP levels in the other three groups were near or lower than baseline.
In patients with prior DMAb treatment, marked increases in P1NP and TRACP-5b from a low absolute baseline were seen during the 12 months of treatment with romosozumab. In patients with prior teriparatide treatment, levels of both P1NP and TRACP-5b decreased from a high absolute baseline following treatment with romosozumab. In treatment-naïve patients, P1NP levels remained close to baseline while TRACP-5b levels decreased from baseline. In patients treated with bisphosphonates before romosozumab, P1NP levels increased slightly while TRACP-5b levels remained near baseline through month 12.
Choice of OP treatment after romosozumab and reasons for choice
In the overall population, sequential OP treatments after romosozumab included DMAb (575, 56.0%), bisphosphonates (266, 25.9%), active vitamin D3 (102, 9.9%), teriparatide (40, 3.9%), vitamin K2 (29, 2.8%), and SERMs (15, 1.5%) (Supplemental Table 5). No patients received calcitonin as a subsequent therapy.
Of the 462 treatment-naïve patients, most patients chose DMAb as the first sequential therapy (n = 297, 64.3%; ad hoc analysis). In this group, the most frequent reason for selecting sequential therapy after 12 months of romosozumab was to further increase BMD (ranging from 91.7 to 100% across treatments; Table 2). The second most frequent reasons for choosing the sequential therapy were “To simplify the administration method” (active vitamin D3, 60.4%; DMAb, 3.7%) and “To simplify the dosing schedule” (DMAb, 3.4%). Additional details on the sequential OP treatments for treatment-naïve patients and the overall patient population are presented in Supplemental Table 5.
Baseline fracture risk and BMD response to romosozumab in treatment-naïve patients
Of the 462 treatment-naïve patients, 217 patients had a baseline FRAX score. The baseline characteristics of treatment-naïve patients with a baseline 10-year FRAX probabilities are summarized in Supplemental Table 6. At baseline, the mean (SD) 10-year FRAX probabilities of major osteoporotic fractures and hip fracture were 28.38% (13.37) and 13.52% (10.59), respectively (Table 3). The majority of patients had a FRAX score of ≥ 15% based on 10-year probability of major osteoporotic fractures (82.0%) and a FRAX score of ≥ 3% based on 10-year probability of hip fractures (94.9%).
In 217 treatment-naïve patients, treatment with romosozumab for 12 months resulted in an increase in BMD from baseline at all skeletal sites (Fig. 3a). The median (Q1, Q3) percent increase from baseline in BMD at month 12 was 13.58% (8.66, 19.62) at the lumbar spine, 4.95% (2.28, 8.77) at the total hip, and 5.10% (1.32, 9.03) at the femoral neck, (Fig. 3b). At month 12, 69% (49/71) of patients with a baseline lumbar spine BMD T-score of < − 3.3, threshold for “high-risk” in Japan for lumbar spine BMD [13] showed an improved T‑score of ≥ − 3.3 to < − 2.5 or a T-score within the osteopenia range (≥ − 2.5). In addition, 25.3% (25/99) and 13.8% (24/174) of patients with baseline total hip and femoral neck BMD T-scores of < − 2.5, respectively, showed an improved T-score within the osteopenia range (≥ − 2.5) at month 12 (Fig. 3c).
Change in BMD and T-score at 12 months from baseline with romosozumab in treatment-naïve patients with baseline 10-year FRAX probabilities. a. Median (Q1, Q3) absolute change in BMD at 12 months from baseline with romosozumab at the lumbar spine, total hip, and femoral neck in treatment-naïve patients with baseline 10-year FRAX probabilities. b. Median (Q1, Q3) percent change in BMD at 12 months from baseline with romosozumab at the lumbar spine, total hip, and femoral neck in treatment-naïve patients with baseline 10-year FRAX probabilities. c. Shifts in T-score from baseline at month 12 with romosozumab at the lumbar spine, total hip, and femoral neck in treatment-naïve patients with baseline FRAX score. Patient percent was calculated from the total patients in each row (T-score). BMD, bone mineral density; FRAX, Fracture Risk Assessment Tool; Q1, first quartile, Q3, third quartile
In a scatter-plot analysis, BMD gains were observed at all skeletal sites regardless of the baseline FRAX 10-year probability for major osteoporotic fracture (Fig. 4a) and hip fracture (Fig. 4b).
Scatter plot of baseline fracture risk and BMD response to 12-month romosozumab in treatment-naïve patients with baseline FRAX 10-year probabilities. a. Scatter plot of percent change in BMD at month 12 from baseline and FRAX 10-year probability for major osteoporotic fracture. b. Scatter plot of percent change in BMD at month 12 from baseline and FRAX 10-year probability for hip fracture. BMD, bone mineral density; FRAX, Fracture Risk Assessment Tool
Discussion
In this retrospective, observational, real-world study conducted at 19 sites distributed across five geographic regions in Japan, we describe the baseline demographics and clinical characteristics of 1027 Japanese patients with OP at a high risk of fracture who were treated with romosozumab after its launch in Japan. We also describe the change from baseline in BMD and BTMs in patients who received romosozumab for 12 months followed by sequential therapy for at least 6 months, where data were available. Unlike the previous real-world studies, where the number of patients enrolled were < 300 and were conducted for < 12 months, we provide a more detailed and in-depth understating of romosozumab treatment followed by sequential therapy in Japanese patients with OP.
In our study, the baseline demographic characteristics were largely similar between the groups based on prior OP treatment. However, as expected, the absolute baseline values of BMD and BTMs differed between the various treatment groups.
The percent increases in BMD after 12 months of romosozumab treatment at all skeletal sites measured in the treatment-naïve group were similar to those observed in previous clinical trials such as FRAME [6] and its subgroup analysis in Japanese women with postmenopausal OP [36] as well as Japanese women with OP at a high risk of fracture [11]. The observed increase in BMD after 12 months of romosozumab treatment was higher in the treatment-naïve group compared with that in other prior OP treatment groups. These findings are in line with those observed in phase 2 and phase 3 studies with romosozumab [9, 37]. In addition, the percent changes in BMD from baseline at the lumbar spine, femoral neck, and total hip observed in patients previously treated with DMAb are similar to findings reported in the post hoc analysis of clinical trials evaluating the effect of treatment sequence on romosozumab response [38] as well as some real-world studies in Japanese patients with OP [25, 27, 28]. The observed difference in the apparent effect of romosozumab on percent change from baseline in BMD between the treatment-naïve group and the groups with various prior OP therapies could be attributed to the difference in baseline values, such as BMD or BTMs, as a result of previous treatment. Similar to our study, other real-world studies have shown that the rate of increase in BMD with 12 months of romosozumab treatment was lower in patients with prior OP treatment than in treatment-naïve patients [25, 28]. However, previous studies did not describe the differences at baseline in the absolute BMD values between various treatment groups which, in particular, may explain the apparent differences in the percent change in BMD.
In treatment-naïve patients, we found that the IQR of the median percent change in BMD at month 18 (following transition) overlapped at all three skeletal sites for different sequential therapies, suggesting that all sequential therapies (including DMAb, bisphosphonates, teriparatide, and active vitamin D3) were frequently able to maintain BMD gains after 12 months of romosozumab treatment. The most common reason for transitioning to sequential therapy after romosozumab treatment was to further increase BMD, and DMAb was the most frequently selected sequential treatment. In the DMAb group, there was an increasing trend of BMD gains up to 18 months. This choice of sequential therapy is supported by clinical trial data which show that in treatment-naïve patients with OP at a high risk of fracture, treatment with romosozumab followed by DMAb is associated with additional BMD increases [6, 11, 36]. However, BMD data were difficult to interpret for the active vitamin D3 treatment group due to the high variability in response at all skeletal sites and the low number of patients (< 10) with available data.
The observed difference in median percent change from baseline values in P1NP between the teriparatide group and the other treatment groups may be due to the markedly higher baseline value for P1NP observed in this group. For TRACP-5b, the difference in the pattern of change over time between the DMAb group and other treatment groups could be largely attributed to the median baseline level of TRACP-5b in the DMAb group, which was less than half of the median baseline value observed in the treatment-naïve and teriparatide groups. At month 12, the pattern of median percent change in BSAP was similar to that of P1NP among the different groups with prior OP treatment. However, as BSAP data were available for very few patients, the results were difficult to interpret.
Due to the high variability observed in the duration of the most recent prior OP therapy across and within the different treatment groups, any residual effect of prior treatment could not be accounted for correctly. However, such data provide a better representation of real-world conditions and play an important role in bridging the gap between clinical trials and the real world. Given the effect of prior therapy on baseline BTMs values, it is important to consider both absolute differences and median percent changes from baseline in BTMs values following transition to romosozumab, to fully appreciate its treatment effect.
Our FRAX results showed that treatment-naïve patients included in this analysis were at a high risk of fracture based on the 10‑year probability for either a major osteoporotic fracture or a hip fracture at baseline. More than 80% of the patients exceeded the Japanese pharmacological intervention threshold score for major osteoporotic fracture (15%) [13], and 95% of the patients exceeded the Bone Health and Osteoporosis Foundation (BHOF) guideline threshold score for hip fracture (3%) [39]. At month 12, 69% of treatment-naïve patients who had a baseline T-score of < − 3.3 (ie, Japanese high-risk criteria for lumbar spine BMD) [33] and 25.3% and 13.8% of patients with a baseline total hip and femoral neck BMD T-score of < − 2.5, respectively, showed an improvement in T-score. Of note, our study found that there was consistent increase in BMD with romosozumab regardless of the baseline FRAX estimated 10‑year probability in treatment-naïve patients.
Strengths of this study include the large number of patients and the study design, which sought to recruit a representative sample across Japan. As a real-world study, this patient population is more diverse and is thus closer to those observed in real-world practice than in clinical trials, and may provide more insights into the effectiveness of OP therapeutic interventions in the clinical setting. Medical chart reviews provide an opportunity to collect and analyze information on clinical characteristics, treatment patterns, and outcomes that might not otherwise be available in alternative administrative data, such as insurance claims or electronic health records [40]. Results presented here reflect real-world treatment patterns and outcomes of romosozumab treatment in patients with OP at a high risk of fracture in Japan. In addition, the assessment of the effectiveness of romosozumab in this study was not solely based on the percent change in BMD and BTMs from baseline; to account for the skewed data distribution of BMD and BTM values at baseline, we used median percent change with IQR. Finally, this is the first real-world study of Japanese patients with OP at high risk of fracture treated with romosozumab analyzing the relationship between change in BMD and baseline fracture risk by FRAX.
An inherent limitation to this study is the use of real-world data which includes factors that may have affected the data analysis, such as smaller sample size in some of the prior OP treatment groups, missing data, absence of DXA equipment standardization with phantom calibration between study sites and standardized interpretation, and variance in BTM data collection and analysis, eg., different laboratories and assays. Because, we only evaluated patients with baseline and 12-month data, additional studies are required to analyze change in BMD in patients who did not complete 11 or 12 doses. The primary objective of this study was to conduct a descriptive review; therefore, this study was not designed to generate between-group comparisons by any statistical method. In particular, the observed heterogeneity in baseline BMD and BTM values between the prior OP treatment groups did not allow for the comparison between these groups following romosozumab administration, and the interpretation of results may be inferred based on other observed and/or unobserved covariates.
In conclusion, this large-scale, multicenter chart review describes clinical characteristics of > 1000 Japanese patients with OP at a high risk of fracture initiating romosozumab. In this real-world clinical setting, treatment with romosozumab for 12 months resulted in increased BMD gains in both treatment-naïve patients and patients with prior OP treatment. We also found that most treatment-naïve patients met the criteria for high risk of fracture, and their BMD increased consistently with romosozumab treatment regardless of their baseline fracture risk as assessed by FRAX; further, these BMD gains were maintained or improved after 6 months of sequential therapy. These findings from a large sample size across geographical regions in Japan will help healthcare providers in making informed decisions on the use of romosozumab and sequential therapy in patients with OP at a high risk of fracture.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/
References
Bliuc D, Nguyen ND, Alarkawi D, Nguyen TV, Eisman JA, Center JR (2015) Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporos Int 26:1331–1339
Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD (2014) Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos 9:182
Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, Van K, Hyun D (2018) Osteoporosis: a review of treatment options. Pharm Ther 43:92–104
Padhi D, Jang G, Stouch B, Fang L, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26
Ominsky MS, Boyce RW, Li X, Ke HZ (2017) Effects of sclerostin antibodies in animal models of osteoporosis. Bone 96:63–75
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Ishibashi H, Crittenden DB, Miyauchi A, Libanati C, Maddox J, Fan M, Chen L, Grauer A (2017) Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone 103:209–215
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377:1417–1427
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP et al (2017) Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390:1585–1594
Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, Miyauchi A, Maddox J, Chen L, Horlait S (2018) A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 103:3183–3193
Miyauchi A, Hamaya E, Yang W, Nishi K, Libanati C, Tolman C, Shimauchi J (2021) Romosozumab followed by denosumab in Japanese women with high fracture risk in the FRAME trial. J Bone Miner Metab 39:278–288
Kanis JA on behalf of the World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary healthcare level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Arch Osteoporos 7:3–20
Donaldson MG, Palermo L, Ensrud KE, Hochberg MC, Schousboe JT, Cummings SR (2012) Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: the fracture intervention trial. J Bone Miner Res 27:1804–1810
Harvey NC, Kanis JA, Oden A, Burge RT, Mitlak BH, Johansson H, McCloskey EV (2015) FRAX and the effect of teriparatide on vertebral and non-vertebral fracture. Osteoporos Int 26:2677–2684
Kanis JA, Johansson H, Oden A, McCloskey EV (2009) Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 44:1049–1054
Kanis JA, Johansson H, Oden A, McCloskey EV (2010) A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone 47:729–735
Kanis JA, Johansson H, Oden A, McCloskey EV (2011) A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX(®). Osteoporos Int 22:2347–2355
McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, Lewiecki EM, Lorenc R, Libanati C, Kanis JA (2012) Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 27:1480–1486
McCloskey EV, Johansson H, Oden A, Harvey NC, Jiang H, Modin S, Fitzpatrick L, Kanis JA (2017) The effect of abaloparatide-sc on fracture risk is independent of baseline FRAX fracture probability: a post hoc analysis of the ACTIVE study. J Bone Miner Res 32:1625–1631
McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, Jalava T, Kanis JA (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy–additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20:811–817
McCloskey EV, Johansson H, Harvey NC, Lorentzon M, Shi Y, Kanis JA (2021) Romosozumab efficacy on fracture outcomes is greater in patients at high baseline fracture risk: a post hoc analysis of the first year of the frame study. Osteoporos Int 32:1601–1608
EVENITY Japanese package insert (online). Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/112292_3999449G1025_2_01. Accessed 23 June 2023
Kobayakawa T, Miyazaki A, Saito M, Suzuki T, Takahashi J, Nakamura Y (2021) Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci Rep 11:11801
Kobayakawa T, Suzuki T, Nakano M, Saito M, Miyazaki A, Takahashi J, Nakamura Y (2021) Real-world effects and adverse events of romosozumab in Japanese osteoporotic patients: a prospective cohort study. Bone Rep 14:101068
Tominaga A, Wada K, Okazaki K, Nishi H, Terayama Y, Kato Y (2021) Early clinical effects, safety, and predictors of the effects of romosozumab treatment in osteoporosis patients: one-year study. Osteoporos Int 32:1999–2009
Ebina K, Hirao M, Tsuboi H, Nagayama Y, Kashii M, Kaneshiro S, Miyama A, Nakaya H, Kunugiza Y, Okamura G, Etani Y, Takami K, Goshima A, Nakata K (2020) Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis. Bone 140:115574
Ebina K, Tsuboi H, Nagayama Y, Kashii M, Kaneshiro S, Miyama A, Nakaya H, Kunugiza Y, Hirao M, Okamura G, Etani Y, Takami K, Goshima A, Miura T, Nakata K, Okada S (2021) Effects of prior osteoporosis treatment on 12-month treatment response of romosozumab in patients with postmenopausal osteoporosis. Joint Bone Spine 88:105219
Shimizu T, Arita K, Murota E, Hiratsuka S, Fujita R, Ishizu H, Asano T, Takahashi D, Takahata M, Iwasaki N (2021) Effects after starting or switching from bisphosphonate to romosozumab or denosumab in Japanese postmenopausal patients. J Bone Miner Metab 39:868–875
Kobayakawa T, Miyazaki A, Takahashi J, Nakamura Y (2022) Verification of efficacy and safety of ibandronate or denosumab for postmenopausal osteoporosis after 12-month treatment with romosozumab as sequential therapy: the prospective VICTOR study. Bone 162:116480
Ebina K, Etani Y, Tsuboi H, Nagayama Y, Kashii M, Miyama A, Kunugiza Y, Hirao M, Okamura G, Noguchi T, Takami K, Goshima A, Miura T, Fukuda Y, Kurihara T, Okada S, Nakata K (2022) Effects of prior osteoporosis treatment on the treatment response of romosozumab followed by denosumab in patients with postmenopausal osteoporosis. Osteoporos Int 33:1807–1813
Japan National Receipt Database Open Data (online). Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00003.html. Accessed on 20 June 2023
Soen S, Fukunaga M, Sugimoto T, Sone T, Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, Ohta H, Yoneda T, Tomomitsu T, Japanese Society for Bone Mineral Research and Japan Osteoporosis Society Joint Review Committee for the Revision of the Diagnostic Criteria for Primary Osteoporosis (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257
Lenchik L, Rogers LF, Delmas PD, Genant HK (2004) Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. AJR Am J Roentgenol 183:949–958
Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA, Group I-IBMSW (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22:391–420
Miyauchi A, Dinavahi RV, Crittenden DB, Yang W, Maddox JC, Hamaya E, Nakamura Y, Libanati C, Grauer A, Shimauchi J (2019) Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension. Arch Osteoporos 14:59
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
Cosman F, Kendler DL, Langdahl BL, Leder BZ, Lewiecki EM, Miyauchi A, Rojeski M, McDermott M, Oates MK, Milmont CE, Libanati C, Ferrari S (2022) Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int 33:1243–1256
LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 33:2049–2102
Fujiwara S, Miyauchi A, Hamaya E, Nicholls RJ, Weston A, Baidya S, Pinto L, Barron R, Takada J (2018) Treatment patterns in patients with osteoporosis at high risk of fracture in Japan: retrospective chart review. Arch Osteoporos 13:34
Acknowledgements
The authors would like to thank the 19 site investigators (Osteoporosis Specialist) for conducting this study: Dr. Akimitsu Miyauchi (Miyauchi Internal Medicine Clinic, Osaka), Dr. Akira Yoshida (Yoshida Orthopedic Clinic, Hokkaido), Dr. Shinya Yoshioka (Yoshioka Hospital, Yamagata), Dr. Satoshi Kamiishi (Kamiishi Spine, Osteoporosis Clinic, Tochigi), Dr. Masafumi Maruyama (Maruyama Memorial General Hospital, Saitama), Dr. Naoya Takada (Higashi Osawa Department of Orthopaedic Surgery, Internal Medicine & Rehabilitation Clinic, Saitama), Dr. Shinkou Rin (Gyoda Central General Hospital, Saitama), Dr. Hirotoki Soma (Kobari General Hospital, Chiba), Dr. Seiji Kaneko (Ushitsu General Hospital, Ishikawa), Dr. Koji Ishikawa (Yamanashi Red Cross Hospital, Yamanashi), Dr. Tomonori Kobayakawa (Kobayakawa Orthopedic and Rheumatologic Clinic, Shizuoka), Dr. Hitoshi Tanigawa (Japan Community Healthcare Organization Shiga Hospital, Shiga), Dr. Masayuki Ishihara (Kansai Medical University Hospital, Osaka), Dr. Yasuhiro Terashima (Konan Kakogawa Hospital, Hyogo), Dr. Yoshiyuki Ito (Yamamoto Orthopedic Hospital, Hiroshima), Dr. Nobuyuki Akimoto (Nozomi Orthopaedic Clinic Saijo, Hiroshima), Dr. Hitoshi Morita (Seiyo Municipal Nomura Hospital, Ehime), Dr. Kohei Kamito (Shinnakama Hospital, Fukuoka), and Dr. Satoru Nakagawa (Nakagawa Orthopedic Clinic, Kagoshima). Lisa Humphries, PhD (Amgen) and Archana Patkar, PhD (Cactus Life Sciences on behalf of Amgen), provided medical writing support.
Funding
Amgen Inc., Astellas Pharma, and UCB Pharma sponsored this study.
Author information
Authors and Affiliations
Contributions
AM, EH, and JS were involved in the study conceptualization and methodology. All authors were involved in writing the original draft and reviewing and editing of subsequent drafts of the manuscript. JS led the formal data analysis. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
Akimitsu Miyauchi has received consulting fees from Amgen and Teijin Pharma. Etsuro Hamaya and Kiyoshi Nishi are employees and stockholders of Amgen. Junichiro Shimauchi is an employee of Amgen. Yoko Yoshinaga is a former employee of Amgen.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Miyauchi, A., Hamaya, E., Shimauchi, J. et al. Effectiveness of romosozumab in patients with osteoporosis at high fracture risk: a Japanese real-world study. J Bone Miner Metab 42, 77–89 (2024). https://doi.org/10.1007/s00774-023-01477-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01477-0