Abstract
Digital X-ray radiogrammetry (DXR) is a computer-assisted diagnosis technique for quantifying cortical hand bone mineral density (BMD) as well as the metacarpal index (MCI) in the metacarpal bones from radiographs. The objective was to compare DXR-BMD and DXR-MCI between healthy individuals and patients with rheumatoid arthritis (RA) and verify the sensitivity and specificity of this technique for the identification of cortical hand bone loss as an additional diagnostic approach in RA. 618 patients were enrolled and divided into two groups: those with RA (n = 309) and a healthy control group (n = 309) as a reference database. DXR-BMD and the DXR-MCI were measured by DXR using hand radiographs. The severity of RA was evaluated by the modified Larsen score. Mean values for DXR-BMD and DXR-MCI in RA patients were significantly lower compared to healthy subjects (−20.7 and −21.1 %, respectively). Depending on the severity of RA-related joint damage, DXR-BMD revealed a significant reduction of –28.1 % and DXR-MCI –28.2 %, comparing score 1 and score 5 of the modified Larsen score. Both DXR-BMD and DXR-MCI had a high sensitivity (DXR-BMD 91 %, DXR-MCI 87 %) and a moderate specificity (DXR-BMD 47 %, DXR-MCI 49 %) to identify RA-related cortical hand bone loss. The DXR technique seems to be able to quantify RA-related periarticular bone loss as a characteristic feature in the course of RA. Consequently, periarticular osteoporosis seems to function as a reliable diagnostic approach comparable to erosions and joint space narrowing in the diagnosis of RA and as a surrogate marker for the progression of bone loss in RA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an inflammatory disease characterized by progressive joint destruction, in particular of the small joints of the hands and feet [1, 2]. Joint destruction includes cartilage dissolution and bone resorption. On radiographs, both features are visualized as joint space narrowing and erosions, the later serving as the radiographic hallmark of RA disease manifestation [1]. The inflammatory origin of RA provokes generalized and also periarticular osteoporosis [3]. Periarticular osteoporosis has been shown to occur only at the early stage of RA [4, 5].
In recent years digital X-ray radiogrammetry (DXR), a feasible quantitative measure of metacarpal cortical bone mass, has been evaluated for the potential to quantify RA-related inflammatory hand bone loss reflecting periarticular osteoporosis [6]. DXR hand bone loss in RA patients has been shown to be associated with markers of disease severity (rheumatoid factor, antibodies to cyclic citrullinated peptide) and with markers of disease activity (disease activity score, C-reactive protein and erythrocyte sedimentation rate) [7, 8]. Furthermore, DXR bone mineral density (BMD) has been shown to be a marker of response to anti-inflammatory treatment [9–11]. Cross-sectional studies have shown a strong association between reduced bone mineral density as measured by DXR and radiographically visible joint destruction [6, 12–15], indicating that DXR estimates function as a surrogate marker of radiographic progression [8]. Longitudinal studies have also confirmed that early hand bone loss may be a predictor of subsequent radiographic joint damage [16–20].
Beside erosions, cortical thinning and cortical hand bone loss are characteristic features of RA [21, 22]. However, there is a persistent lack of data exploring the diagnostic power of quantified inflammatory bone loss as well as the value of the DXR method in identifying RA patients with disease-related periarticular bone loss.
The aim of this study was to evaluate the sensitivity and specificity of the DXR estimates for the quantification of cortical hand bone loss which could appear as the third characteristic sign (besides erosions and joint space narrowing) of RA in the interpretation of hand radiographs as well as in the diagnosis of RA.
Materials and methods
Study cohort
A total of 618 individuals were enrolled in the study, divided into a reference group of healthy subjects and a group of patients suffering from RA (see Table 1). The RA group comprised 309 Caucasian patients with verified RA diagnosed according to the revised criteria of the American College of Rheumatology in 1987 [23]. The median disease duration was 7.6 years. No pre-selection regarding severity of RA or steroid therapy was performed. All patients were treated with disease-modifying antirheumatic drugs. 36 % of the patients received prednisolone (mean dose 4, 5 mg per day). Patients with osteosynthetic material involving the hands and with a Disease Activity Score 28 >3.1 and/or C-reactive protein >7.5 mg/L and/or erythrocyte sedimentation rate in the first hour >15 mm were excluded to preserve stable and established disease conditions.
The control group comprised 309 healthy Caucasian subjects who were part of the German DXR reference cohort [24]. All subjects had been admitted to the university clinic due to trauma and subsequently underwent X-ray imaging of the non-dominant non-injured hand to exclude fractures due to trauma. Based on a questionnaire, all subjects with disease- or drug-related alterations of peripheral and axial bone were excluded. Furthermore, subjects with fractures of the upper extremity were excluded, to eliminate the influence of immobility-induced osteopenia. Further details regarding this study group can be found in Böttcher et al. (2006) [24]. Exclusion criteria were determined by an extensive questionnaire focusing on visible metallic material, any endocrine diseases known to affect bone metabolism, rheumatic disease, renal disease, genetic and oncology diseases, and Kellgren–Lawrence grade greater than 1, as well as incorrect hand positioning.
Methods
Acquisition of hand radiographs
All plain radiographs of the non-dominant left hand were acquired by comparable X-ray devices using standardized conditions. After the radiographs were scanned (Scanner UMAX Power Look 1100, resolution 300 dots per inch) into the DXR system, digitized images were available. DXR digitally performed a continual self-check to maintain the quality of the digital X-ray imaging; the analysis process was halted if the X-ray imaging became inferior during the depiction process (i.e. incorrect contour finding and identification of bone structures).
Measurement of cortical hand bone mass by digital X-ray radiogrammetry
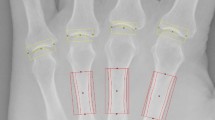
DXR (Pronosco X-Posure System™, Version 2.0; Sectra; Linköping, Sweden) was applied to estimate the BMD (g/cm2) and metacarpal index (MCI; a dimensionless parameter based on the mean cortical thickness normalized with the mean outer bone diameter of the metacarpals), requiring conventional or digital radiographs of the hand in an anterior–posterior projection [25]. After digitalization of the hand radiographs, the computer algorithms automatically defined regions of interest around the narrowest bone parts of metacarpals II, III and IV and subsequently determined the outer and inner cortical edges of the identified cortical bone parts. There is no operator interaction connected to the DXR measurements [26]. The mean of the cortical thickness and overall cortical thickness of the second, third and fourth metacarpals were estimated. The cortical volume per area was subsequently calculated for each bone. Based on the mean cortical volume per area, the DXR-BMD is calculated with a correction for the estimated porosity index [25]. DXR is not affected by variation in exposure level, film brand or sensitivity, nor by film–focus distance during the image capture [27]. Thus, the short-time precision error (0.28 %) [19] and the reproducibility (ranging between 0.05 and 1.50 %) is very low [28]. The DXR method has also been proven to be stable for long-term follow-up based on an in vitro long-term precision ranging between 0.22 and 0.43 % [29]. Focusing on these observations, the estimated bone loss is not based on ‘precision error’ or ‘reproducibility error’ of the osteodensitometric method itself, resulting in a very small detectable difference (0.0012–0.0028 g/cm2) [30]. Additionally, the hand positioning and hand rotation has no influence on DXR-BMD measurements using standardized anterior–posterior radiographs [31].
Scoring of hand radiographs
Each radiograph of the RA cohort was scored by two radiologists in a separated blinded manner using the modified Larsen score which evaluates 32 joints of the feet and hands (total sum of points: 160): score 0 = normal joint; score 1 = periarticular demineralization, soft tissue affected, initial reduction of the joint space width; score 2 = initial erosions and reduction of the joint space width; score 3 = multiple erosions and advanced reduction of the joint space width; score 4 = partial ankylosis; score 5 = ankylosis or mutilation [22]. The sum of the individual scores is then divided by the number of joints evaluated. In cases of ambiguity, a third highly experienced radiologist reviewed the radiographs for a final decision.
Ethical committee
All examinations were performed in accordance with the rules and regulations of the local Human Research and Ethics Committee. As a special note, the authors emphasize that all radiographs (including the reference group of healthy participants) used for the DXR calculations were performed as part of routine clinical care; no additional radiographs were obtained for study purposes.
Statistical analysis
The statistical analysis was performed using SPSS for Windows version 14.0® (SPSS, Chicago, IL, USA). (1) The differences in age and DXR parameters between the reference group and the matched RA group as well as the reduction in DXR parameters between Larsen score 0 and 5 were assessed using the Mann–Whitney U test. (2) Sensitivity and specificity of DXR concerning the quantification of cortical hand bone loss was based on receiver operating characteristic (ROC) curve analysis, including positive predictive values and accuracy. For the evaluation of gender-associated demineralization the gender-specific mean values of DXR-BMD and DXR-MCI in the RA cohort were used as cut-off points. Regarding the RA-associated metacarpal bone loss, the mean values of DXR-BMD and DXR-MCI in the RA group were defined as the cut-off to identify RA patients with disease-related cortical hand bone loss. The area under the curve was calculated with 95 % confidence intervals for the ROC curves. Additionally, the overall significance level was p < 0.05.
Results
Comparison of DXR parameters between healthy subjects and patients with rheumatoid arthritis
As shown in Table 2, DXR-BMD and DXR-MCI was significantly lower in RA patients than in the healthy individuals (−20.7 %, p < 0.01) and −21.1 %, p < 0.01). Compared with healthy individuals, men and women suffering from RA had a significantly lower DXR-BMD (−26.8 vs. −18.2 %, p < 0.01) and DXR-MCI (−26.3 vs. −19.5 %, p < 0.01).
Cortical bone loss estimated by digital X-ray radiogrammetry depending on severity of rheumatoid arthritis
As summarized in Table 3, DXR-BMD and DXR-MCI declined with an increasing Larsen score. DXR-BMD was 28.1 % and DXR-MCI 28.2 % lower in RA patients with Larsen score 5 than in RA patients with Larsen score 1.
When RA patients grouped according to Larsen score were compared with healthy individuals, DXR-BMD declined continuously from −5.4 % in patients with Larsen score 0 to −31.9 % in RA patients with Larsen score 5. Similar results were observed for the DXR-MCI.
Sensitivity and specificity of digital X-ray radiogrammetry in the diagnosis of rheumatoid arthritis (Tables 4, 5)
DXR-BMD provided a sensitivity of 91 %, specificity of 47 % and an accuracy of 83 %. A similar specificity was observed for the DXR-MCI (49 %), with a sensitivity of 87 %. The accuracy for the DXR-MCI was calculated to be 80 %. The positive predictive value for DXR-BMD and DXR-MCI was 84 and 79 %, respectively.
In the context of lower DXR-BMD for men and women with RA, the sensitivity and specificity of the DXR-BMD based on a gender-specific detection of RA was 88 % vs. 47 % for women and 100 % vs. 41 % for men. Similar results were detected for the DXR-MCI, with a sensitivity of 83 % (women) versus 99 % (men) and a specificity of 51 % (women) versus 45 % (men), as shown in Table 5.
Discussion
In recent years, the DXR technique has been introduced as an innovative computer-assisted diagnostic technique and gold standard for the measurement of cortical BMD at the metacarpal bones, based on its high precision and reproducibility [29, 30]. In this context, dual energy X-ray absorptiometry showed a lower reproducibility (1.23–2.48 %) for the quantification of metacarpal BMD [32], which often results in an inadequate detection of therapeutic effects. High-resolution peripheral quantitative computed tomography (HR-pQCT) is now available for the volumetric quantification of BMD and bone structure at the metacarpal heads. Some initial studies present promising results regarding the reduced BMD and detailed bone structure in RA patients [33, 34], based on a more time- and cost-intensive analysis.
Although a strong relationship between reduced BMD as measured by DXR and radiographic joint destruction has been noted, DXR has been explored for its potential diagnostic value for assessment of RA in clinical care.
For healthy women and those with RA, the study presented a significantly lower DXR-BMD compared to men; these findings are comparable to the published reference values [24] and a further German reference cohort of the initial DXR version by Wüster et al. [35].
Our data shows that DXR-BMD (−20.7 %) and DXR-MCI (−21.1 %) are considerably reduced in RA patients compared to healthy subjects. These results indicate that periarticular demineralization of the metacarpal bone, which is specifically an early radiographic sign in RA, can be reliably quantified by DXR. Periarticular demineralization of the metacarpals has been implemented as a diagnostic method for classifying bone involvement in RA, both in the Steinbroker SCORE and the Larsen score [21, 36, 37]. Periarticular loss at the metacarpal bones has traditionally been the first radiological sign of RA considered by the scoring methods, and can be found before erosions or joint space narrowing occur.
The results of this study point to a continuous reduction in periarticular BMD as measured by DXR for the different stages of the modified Larsen score in comparison to healthy subjects, with a decline of −5.4 % (Larsen score 0) to −31.9 % (Larsen score 5), confirming our previously published findings [17, 38]. These results confirm that periarticular demineralization not only occurs in the early stage of RA, but also continues during the later stages of prolonged RA, as indicated by a significant BMD reduction from Larsen score 0 to 5. Additionally, different cross-sectional studies have observed a strong relationship between reduced BMD as measured by DXR and radiographic joint destruction [12, 17, 38]. With regard to the Larsen score, DXR-BMD and DXR-MCI are significantly decreased at –28.1 and –28.2 %, respectively, between Larsen score 0 and 5 in this study. A study of Haugeberg et al. (2004) revealed a comparable reduction of DXR-BMD of –26 % between Larsen score 1 and 5 [12]. In a cohort of 313 RA patients, DXR-BMD showed a significant decline of –27.7 % (Sharp Joint Space Narrowing Score) and –20.4 % (Sharp Erosion Score) [38]. Consequently, periarticular demineralization, which is a characteristic feature of inflammatory bone involvement in RA, may be comparable to the diagnostic impact of joint space narrowing and erosions. As demineralization is difficult to ascertain by simple visualization of radiographs [37], DXR offers the benefit of a reliable quantification of periarticular bone mass in an observer-independent and highly reproducible manner [28].
To our knowledge, this is the first study which evaluates the diagnostic performance of an imaging technique using a comparison of healthy subjects and RA patients. Our data has revealed a high sensitivity of 91 % and accuracy of 83 % for DXR-BMD, enabling a reliable diagnosis of RA without the diagnostic input of erosions and joint space narrowing. These results clearly demonstrate that the diagnosis of periarticular osteoporosis can be significantly improved using DXR; this method is superior to the diagnostic power of the established scoring methods due to their lack of sensitivity and their impaired inter-observer variability.
Other studies have also investigated the association of periarticular bone loss as detected by DXR and the assessment of RA progression. The association between DXR-BMD and functional disability as measured by the Modified Health Assessment Questionnaire score showed a reduced DXR-BMD in association with a poorer functional outcome [19]. The measurement of periarticular bone loss can be considered as a complementary approach to verify RA-related changes in bone involvement, as well as functioning as a potentially important tool in the daily clinical routine supplementing the levels of antibodies to cyclic citrullinated peptide, inflammatory blood markers and radiographs which identify patients with an increased risk of a progressive course of RA [16]. In summary, these investigations indicate that DXR-BMD could be used as a surrogate marker of RA progression, and also correlate to the functional outcome in RA patients [20].
A further potential advantage of DXR consists of the detection of therapy-induced alterations of the hand BMD [39] in RA patients with disease-modifying anti-rheumatic drugs [40] and anti-tumour necrosis factor-α therapy [10, 11].
A limitation of our study is the lack of a gold standard for BMD measurements at the metacarpal bones which would allow a comparison between the DXR technique and a gold standard like DXA. Bejarano et al. showed a limited value of hand DXA measurements in the first year of RA as an additional prognostic tool and concluded for patients with early RA that the DXA technique does not provide any more information than baseline radiographs of hands and feet [41]. In addition, the use of a scanner for the digitalization of hand X-rays bears limitations due to smearing artefacts based on the scanning process. A fully digital version of DXR is now available (Sectra DXR-online) which may compensate for possible influences on image quality during the printing and scanning procedures. A possible limitation could be the analysis of hand radiographs of the non-dominant left hand. The study of Toledo and Jergas presented a highly significant coefficient of correlation (r = 0.953, p < 0.001) of the DXR-BMD between the right and left hand. Additionally, no significant difference was detectable for the DXR-MCI [42]. A further limitation of the study is the absence of information regarding the influence of menstrual status and anthropometric data on the DXR measurement. A possible solution is to introduce a new parameter named the Bone Health Index which includes the size of the metacarpal bones and offers the advantage of an body-size-independent quantification of cortical thickness and periarticular demineralization with a better understanding of the cortical changes (own unpublished data). Another limitation of the study is the absence of information of the postmenopausal status of the women, whereas Desai et al. presented an association of hand bone loss as measured by DXR and BMD of the lumbar spine estimated by dual energy X-ray absorptiometry in postmenopausal women with RA [43].
In conclusion, the development of digital imaging and computer-assisted diagnostic techniques has advanced the precise quantification of hand BMD calculated by DXR. The clinical use of the DXR technology allows the measurement of cortical BMD in patients suffering from RA with high sensitivity and moderate specificity, enabling a reliable diagnosis of RA. Cortical hand bone loss functions as a diagnostic sign during the entire course of RA progression, comparable with other radiographically visible signs such as joint space narrowing and erosions. Consequently, RA-related cortical bone loss of the metacarpals is a characteristic surrogate marker for manifestation of RA which also may improve the planning of appropriate individual therapeutic strategies.
Abbreviations
- BMD:
-
Bone mineral density (g/cm2)
- DXR:
-
Digital X-ray radiogrammetry
- MCI:
-
Metacarpal index
- RA:
-
Rheumatoid arthritis
References
Blair WF (1996) An approach to complex rheumatoid arthritis hand and wrist problems. Hand Clin 12:615–628
Gravallese EM (2002) Bone destruction in arthritis. Ann Rheum Dis 61:84–86
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalised bone loss in patients with early rheumatoid arthritis. Lancet 344:23–27
Haugeberg G, Green MJ, Quinn MA, Marzo-Ortega H, Proudman S, Karim Z, Wakefield RJ, Conaghan PG, Stewart S, Emery P (2006) Hand bone loss in early undifferentiated arthritis: evaluating bone mineral density loss before the development of rheumatoid arthritis. Ann Rheum Dis 65:736–740
Haugeberg G, Morton S, Emery P, Conaghan PG (2011) Effect of intra-articular corticosteroid injections and inflammation on periarticular and generalised bone loss in early rheumatoid arthritis. Ann Rheum Dis 70:184–187
Böttcher J, Pfeil A, Mentzel HJ, Kramer A, Schäfer ML, Lehmann G, Eidner T, Petrovitch A, Malich A, Hein G, Kaiser WA (2006) Peripheral bone status in rheumatoid arthritis evaluated by digital X-ray radiogrammetry (DXR) and compared with multi-site quantitative ultrasound (QUS). Calcif Tissue Int 78:25–34
Bøyesen P, Hoff M, Ødegård S, Haugeberg G, Syversen SW, Gaarder PI, Okkenhaug C, Kvien TK (2009) Antibodies to cyclic citrullinated protein and erythrocyte sedimentation rate predict hand bone loss in patients with rheumatoid arthritis of short duration: a longitudinal study. Arthritis Res Ther 11:R103
Hoff M, Bøyesen P, Haugeberg G, Vis M, Woolf AD, Havaardsholm EA, Dijkmans BA, Kvien TK, Uhlig T, Lems WF (2010) High disease activity is a predictor of cortical hand bone loss in post-menopausal patients with established rheumatoid arthritis: a 5-year multicentre longitudinal study. Rheumatology 49:1676–1682
Haugeberg G, Strand A, Kvien TK, Kirwan JR (2005) Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis: results from a randomized placebo-controlled trial. Arch Intern Med 165:1293–1297
Hoff M, Kvien TK, Kälvesten J, Elden A, Haugeberg G (2009) Adalimumab therapy reduces hand bone loss in early rheumatoid arthritis: explorative analyses from the PREMIER study. Ann Rheum Dis 68:1171–1176
Güler-Yüksel M, Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Groenendael JH, Mallée C, de Bois MH, Breedveld FC, Dijkmans BA, Lems WF (2009) Changes in hand and generalized bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 68:330–336
Haugeberg G, Lodder MC, Lems WF, Uhlig T, Ørstavik RE, Dijkmans BAC, Kvien T, Woolf AD (2004) Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann Rheum Dis 63:1331–1334
Böttcher J, Malich A, Pfeil A, Petrovitch A, Lehmann G, Heyne JP, Hein G, Kaiser WA (2004) Potential clinical relevance of digital radiogrammetry for quantification of periarticular bone demineralization in patients suffering from rheumatoid arthritis depending on severity and compared with DXA. Eur Rad 14:631–637
Böttcher J, Pfeil A, Rosholm A, Schäfer ML, Malich A, Petrovitch A, Mentzel HJ, Hein G, Kaiser WA (2006) Computerized digital imaging techniques provided by digital radiogrammetry as new diagnostic tool in rheumatoid arthritis. J Digit Imaging 19:279–288
Forslind K, Boonen A, Albertsson K, Hafstrom I, Svensson B (2009) Hand bone loss measured by digital X-ray radiogrammetry is a predictor of joint damage in early rheumatoid arthritis. Scand J Rheumatol 38:431–438
Hoff M, Haugeberg G, Ødegård S, Syversen S, Landewé R, van der Heijde D, Kvien TK (2009) Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis 68:324–329
Böttcher J, Pfeil A, Rosholm A, Petrovitch A, Seidl BE, Malich A, Kramer A, Lehmann G, Hein G, Kaiser WA (2005) Digital X-ray Radiogrammetry combined with semi-automated analysis of joint space distances as a new diagnostic approach in rheumatoid arthritis—a cross-sectional and longitudinal study. Arthritis Rheum 52:3850–3859
Böttcher J, Pfeil A (2008) Diagnosis of periarticular osteoporosis in rheumatoid arthritis using digital X-ray radiogrammetry. Arthritis Res Ther 10:103
Hoff M, Haugeberg G, Kvien TK (2007) Hand bone loss as an outcome measure in established rheumatoid arthritis: 2-year observational study comparing cortical and total bone loss. Arthritis Res Ther 9:R81
Pfeil A, Haugeberg G, Hansch A, Renz DM, Lehmann G, Malich A, Wolf G, Böttcher J (2011) The value of digital X-ray radiogrammetry in the assessment of inflammatory bone loss in rheumatoid arthritis. Arthritis Care Res (Hoboken) 63:666–674
Steinbroker O, Traeger CH, Batterman RC (1949) Therapeutic criteria in rheumatoid arthritis. JAMA 140:659–662
Larsen A, Thoen J (1987) Hand radiography of 200 patients with rheumatoid arthritis repeated after an interval of one year. Scand J Rheumatol 16:395–401
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fies FJ, Cooper NS (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Böttcher J, Pfeil A, Schäfer ML, Petrovitch A, Schmidt M, Mentzel HJ, Lehmann G, Malich A, Heyne JP, Hein G, Wolf G, Kaiser WA (2006) Normative data for digital X-ray radiogrammetry from a female and male German cohort. J Clin Densitom 9:342–350
Rosholm A, Hyldstrup L, Baeksgaard L, Grunkin M, Thodberg HH (2001) Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int 12:961–969
Mentzel HJ, John U, Boettcher J, Malich A, Pfeil A, Vollandt R, Misselwitz J, Kaiser WA (2005) Evaluation of bone-mineral density by digital X-ray radiogrammetry (DXR) in pediatric renal transplant recipients. Pediatr Radiol 35:489–494
Malich A, Boettcher J, Pfeil A, Sauner D, Heyne JP, Petrovitch A, Hansch A, Linss W, Kaiser WA (2004) The impact of technical conditions of X-ray imaging on reproducibility and precision of digital computer-assisted X-ray radiogrammetry (DXR). Skeletal Radiol 33:698–703
Böttcher J, Pfeil A, Rosholm A, Malich A, Petrovitch A, Heinrich B, Lehmann G, Mentzel HJ, Hein G, Linß W, Kaiser WA (2005) Influence of image-capturing parameters on digital X-Ray radiogrammetry. J Clin Densitom 8:87–94
Dhainaut A, Hoff M, Kälvesten J, Lydersen S, Forslind K, Haugeberg G (2011) Long-term in vitro precision of direct digital X-ray radiogrammetry. Skeletal Radiol 40:1575–1579
Hoff M, Dhainaut A, Kvien TK, Forslind K, Kälvesten J, Haugeberg G (2009) Short-time in vitro and in vivo precision of direct digital X-ray radiogrammetry. J Clin Densitom 12:17–21
Pfeil A, Renz DM, Fröber R, Hansch A, Lehmann G, Sommerfeld J, Malich A, Wolf G, Böttcher J (2015) Influence of angulation on metacarpal bone mineral density measurements using digital X-ray radiogrammetry. Int J Comput Assist Radiol Surg 10:587–592
Naumann L, Hermann KG, Huscher D, Lenz K, Burmester GR, Backhaus M, Buttgereit F (2012) Quantification of periarticular demineralization and synovialitis of the hand in rheumatoid arthritis patients. Osteoporos Int 23:2671–2679
Fouque-Aubert A, Boutroy S, Marotte H, Vilayphiou N, Bacchetta J, Miossec P, Delmas PD, Chapurlat RD (2010) Assessment of hand bone loss in rheumatoid arthritis by high-resolution peripheral quantitative CT. Ann Rheum Dis 69:1671–1676
Finzel S, Englbrecht M, Engelke K, Stach C, Schett G (2011) A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann Rheum Dis 70:122–127
Wüster C, Wenzler M, Kappes J, Rehm C, Gühring T, Arnbjerg D (2000) Digital X-ray radiogrammetry as a clinical method for estimating bone mineral density – a German reference database. J Bone Miner Res 15 (Suppl 1):S289
Larsen A, Dale K, Eek M (1977) Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn 18:481–491
Jergas M (2003) Conventional radiographs and basic quantitative methods. In: Grampp S (ed) Radiology of osteoporosis. Springer, Berlin, pp 62–64
Böttcher J, Pfeil A, Rosholm A, Sörös P, Petrovitch A, Schäfer ML, Seidl BE, Malich A, Hansch A, Kaiser WA (2006) Computerized quantification of joint space narrowing and periarticular demineralisation in patients with rheumatoid arthritis based on digital X-ray radiogrammetry. Invest Radiol 41:36–44
Forsblad-d’Elia H, Carlsten H (2011) Hormone replacement therapy in postmenopausal women with rheumatoid arthritis stabilises bone mineral density by digital x-ray radiogrammetry in a randomised controlled trial. Ann Rheum Dis 70:1167–1168
Pfeil A, Lippold J, Eidner T, Lehmann G, Oelzner P, Renz DM, Hansch A, Wolf G, Hein G, Böttcher J (2009) Effects of leflunomide and methotrexate in rheumatoid arthritis detected by digital X-ray radiogrammetry and computer-aided joint space analysis. Rheumatol Int 29:287–295
Bejarano V, Hensor E, Green M, Haugeberg G, Brown AK, Buch MH, Emery P, Conaghan PG (2012) The relationship between early bone mineral density changes and long term function and radiographic progression in rheumatoid arthritis. Arthritis Care Res (Hoboken) 64:66–70
Toledo VA, Jergas M (2006) Age-related changes in cortical bone mass: data from a German female cohort. Eur Radiol 16:811–817
Desai SP, Gravallese EM, Shadick NA, Glass R, Cui J, Frits M, Chibnik LB, Maher N, Weinblatt ME, Solomon DH (2010) Hand bone mineral density is associated with both total hip and lumbar spine bone mineral density in post-menopausal women with RA. Rheumatology (Oxford) 49:513–519
Acknowledgments
The authors thank Monika Arens (managing director, Arewus GmbH) and Jacob Algulin (managing director, Sectra, Sweden) for the use of the Digital X-ray Radiogrammetry technique. Further thanks to Thomas Lehmann (PhD) for statistical advice.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pfeil, A., Haugeberg, G., Renz, D.M. et al. Digital X-ray radiogrammetry and its sensitivity and specificity for the identification of rheumatoid arthritis-related cortical hand bone loss. J Bone Miner Metab 35, 192–198 (2017). https://doi.org/10.1007/s00774-016-0741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-016-0741-3