Abstract
The aim of this study was to evaluate the differences between patients with obstructive sleep apnea syndrome (OSAS) and phenotypically similar subjects without OSAS in terms of bone mineral density (BMD) and bone turnover markers. The study was conducted on 30 males diagnosed with OSAS and 20 healthy males. All subjects underwent polysomnographic testing. Calcium, phosphorus parathyroid hormone, thyroid stimulating hormone, bone-specific alkaline phosphatase, 25-hydroxyvitamin D3, osteocalcin, and beta-CrossLaps (β-CTx) were measured. BMD in the lumbar spine (L1–L4) and femoral neck was measured by dual energy X-ray absorptiometry. There was no statistically significant difference between the two groups in terms of demographic data with the exception of bone mass index and waist circumference. (p < 0.05). Analyses showed significantly lower BMD measurements in the femoral neck and T-scores in the femoral neck in patients diagnosed with OSAS. Serum β-CTx levels were found to be statistically significantly higher in the OSAS group (p = 0.017). In multivariate assessments performed for apnea/hypopnea index values, mean saturation O2 levels were found to be significantly associated with osteocalcin levels and neck BMD. OSAS patients might represent a risk group with respect to loss of BMD and bone resorption. It is important to evaluate bone loss in these patients. Further studies should be carried out on larger study populations to evaluate the effects of chronic hypoxia on BMD in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a sleep disorder encompassing recurrent apnea and intermittent hypoxia episodes [1]. Hypoxia associated with OSAS causes an increase in oxidative stress, endothelial dysfunction, and sympathetic activity [1], which in turn negatively affects other organs and systems [3–5], particularly the cardiovascular system [2]. There is still controversy over the effects of OSAS on bone metabolism, and there is also paucity of data on this topic in the literature [6–8].
Patients with OSAS may have multiple morbidities and may have been exposed to systemic responses which might affect bone metabolism. Studies have shown that chronic hypoxia accelerates bone resorption by down-regulating osteogenic differentiation and stimulating osteoclastic activity [10, 11]. Intermittent hypoxia occurring during apnea episodes [9] is considered to have a negative impact on bone metabolism [8]. Studies indicate the presence of increased systemic inflammation in patients with OSAS, and increased interleukin (IL)-6, tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) have been demonstrated [12–14]. Increased inflammation can be a risk factor for bone mineral loss [8]. OSAS is a clinical condition often occurring in obese patients. The obesity affects bone metabolism through weight loading and production of cytokines or hormones [15].
To our knowledge, there are a limited number of studies in the literature indicating a relationship between OSAS and bone mineral density (BMD) [6–8]. Among these studies, no study evaluated both BMD and bone turnover markers and vitamin D levels. The aim of our study was to identify the differences in BMD and bone turnover markers between OSAS patients and patients without OSAS. We also wanted to evaluate the correlation between OSAS parameters (apnea/hypopnea index [AHI], mean O2 saturation) and bone turnover markers and BMD.
Materials and methods
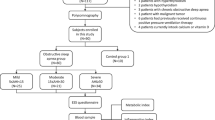
The study was conducted on 30 males diagnosed with OSAS and 20 healthy males between 2012 and 2013. The control group consisted of patients who were referred to the sleep laboratories for polysonographic tests due to various reasons and had normal polysonographic test results, who were living in the same region as the OSAS patient group, and who met the study eligibility criteria. Patients who had a known history of chronic renal, pulmonary, and rheumatic disorders, a previous history of a disease that could result in malabsorption syndrome, diabetes, impaired thyroid function, anorexia nervosa, prolonged immobilization, and those who received therapy for osteoporosis or medications that could affect bone metabolism (steroids, thiazides, drugs for dyslipidemia, warfarin, heparin, HRT, and chemotherapy) and patients who previously received CPAP were excluded from the study. Demographic data of all subjects were recorded, and a detailed medical history was obtained, and all underwent a physical examination. The habits of the patients and duration of the complaints were recorded. Approval was obtained from the ethics committee for the current study.
Anthropometric measurements
Body mass index (BMI) was calculated by dividing weight by the square of the height (kg/m2). Neck circumference was measured at the midpoint of the neck between the mid-cervical spine and the mid-anterior neck 0.5 cm below the laryngeal prominence. Waist circumference was measured midway between the lower rib margin and the iliac crest.
Polysomnographic test
All subjects underwent overnight polysomnographic recording (Embla N 7000). The polysomnography included electroencephalogram, electrooculogram, chin and leg electromyography, electrocardiography, snoring, oronasal thermistor, nasal pressure transducer, finger pulse oximeter, thoracic and abdominal respiratory movements, and body position. The scoring was conducted based on the criteria of American Academy of Sleep Medicine (AASM) published in 2007. Apnea was defined as ≥90 % decrease from baseline in the oronasal thermistor signal amplitude for at least 10 s, and hypopnea was defined as ≥50 % decrease from baseline in the nasal cannula signal amplitude for at least for 10 s, and decrease in oxygen saturation was defined as ≥3 % decline or presence of arousal. The AHI was defined as the number of occurrences of apnea/hypopnea per hour (/h) during sleep. Patients with an AHI ≥5/h were considered to have OSAS. An AHI of 5–15/h was considered mild OSAS, 15–30/h was considered moderate, and ≥30/h was considered severe.
Biochemical measurements
A fasting blood specimen was drawn from each subject. Blood samples were collected between 8:00 and 10:30 a.m., and parathyroid hormone (PTH), thyroid stimulating hormone (TSH), calcium, and phosphorus levels were measured by automated standard methods. Plasma osteocalcin and β-isomerized form C-terminal telopeptideof type I collagen (β-CTx) were measured via the electrochemiluminescence method using the Cobas® kit (Roche, Germany) in the Cobas e601® analyzer (Roche, Germany). The IDS® bone-specific alkaline phosphatase (Immunodiagnostic Systems Ltd., UK) was studied using the enzyme-linked immunosorbent assay (ELISA) kit in the BioTek ® ELISA reader (BioTek Inc., USA). Additionally, 25-hydroxyvitamin D3 (25(OH)D) was chromatographically measured using the D3 Waters® UPLC/MS/MS device (Roche, UK).
BMD measurement
BMD at the lumbar spine and femoral neck were measured by dual energy X-ray absorptiometry (Lunar Dpx-Pro). Lumbar spine BMD was defined as the mean BMD in lumbar vertebrae 1–4. Patients were divided into two groups according to their BMD results for the femoral neck and lumbar spine using World Health Organization criteria—those with normal BMD (T-score > −1.0 SD) and those with osteopenia and osteoporosis (T-score ≤ −1.0 SD).
Statistical analysis
Statistical analysis was performed using NCSS (Number Cruncher Statistical System) 2007 and PASS (Power Analysis and Sample Size) 2008 Statistical Software (NCSS LLC, Kaysville, UT, USA) packages. In addition to the descriptive statistics (mean, standard deviation, median, frequency, and ratio), Student’s t test was used to compare normally distributed variables, and the Mann–Whitney U test was used to compare variables that did not show normal distribution. ANCOVA (analysis of covariance) was used to evaluate the effects of BMI. Spearman’s correlation analysis was used to evaluate the association between the parameters. Yates’ continuity correction test was used to compare qualitative data. The effects of other variables on AHI, mean O2, and minimum O2 levels were assessed by simple linear regression and multiple linear regression analyses. The results were expressed at a 95 % confidence interval, and the level of statistical significance was established at p < 0.05.
Results
The study was conducted on 30 male patients diagnosed with OSAS and 20 healthy males in the control group. The mean age was 52.37 ± 8.58 years in patients with OSAS, and 50.60 ± 10.84 years in the control group. There were significant differences between the two groups in terms of BMI and waist circumference (p < 0.05). There was no significant difference between the two groups in terms of neck circumference and smoking status (p > 0.05) (Table 1).
Sleep test parameters in the two groups are presented in Table 2. Of 30 patients with OSAS, 11 had moderate and 19 had severe OSAS. None of the patients were diagnosed with mild OSAS.
Analyses showed significantly lower BMD measurements in the femoral neck and T- scores in the femoral neck in patients diagnosed with OSAS (p < 0.05). Although BMD in L1–4 and lumbar T-scores were found to be lower in patients with OSAS compared to the control group, the difference did not reach statistical significance (p > 0.05) (Table 3).When the two groups were statistically compared considering the effect of BMI, no statistically significant difference was noted with respect to the levels of vitamin D, whereas the CTX level was noted to be statistically significantly higher in the OSAS group (Table 4).
Univariate analyses of AHI showed a significant positive correlation between AHI and age, and significant negative correlations between AHI and osteocalcin, femoral neck T value, and femoral neck BMD value (p < 0.05). In multivariate analyses, a linear regression analysis was carried out, and the results suggested that age, osteocalcin, and femoral neck BMD values had significant effects on the established model (p < 0.05) (Table 5). Univariate analyses of mean saturation O2 levels showed a significant negative correlation between mean saturation O2 level and BMI, and a significant positive correlation between mean saturation O2 level and osteocalcin, femoral neck T value, and femoral neck BMD value (p < 0.05). In the case of multivariate analyses on mean saturation O2 levels, a linear regression analysis was carried out, and the results suggested that BMI, osteocalcin, and femoral neck BMD values had significant effects on the established model (p < 0.05) (Table 5).
Discussion
Several studies have shown the negative effects of hypoxia occurring in patients with OSAS on bone metabolism. Hypoxia has been shown to increase the number and volume of osteoclasts in human and animal models [16, 17], promote the function of osteoclasts by increasing the expression of IL-6 and vascular endothelial growth factor [16], and decrease osteoclast maturation and differentiation [18]. Hypoxia also stimulates lipogenesis. Osteoblastic cells and adipocytes originate from the same precursor cells, and lipogenesis inhibits differentiation of osteoblasts [20]. Chronic hypoxia decreases collagen synthesis by decreasing the levels of oxygen-dependent enzymes [21]. Intermittent hypoxia could reduce the expressions of Cbfa1, bone ALP, and type I collagen [16]. Patients with OSAS also develop acidosis [24]. Studies showed that bone resorption was increased by one-fold after every 0.1 decrement in pH value [23]. In addition, acidosis negatively affects bone matrix mineralization [24]. Considering all these effects, OSAS may be suggested to have unfavorable effects on the bone metabolism. To our knowledge, there are three studies in the literature that evaluated the relationship between bone metabolism and OSAS. The first two studies were conducted by Uzkeser et al. [7] and Tomiyama et al. [8] on a small study population, and their findings provided evidence for impaired bone metabolism in this patient group. Tomiyama et al. studied 50 patients with OSAS and 15 subjects in the control group, and as markers of bone resorption, they found significantly higher CTX and cytokine levels in patients with severe OSAS compared to subjects in the control group and patients with mild OSAS. They noted a decrease in these markers after 3 weeks of CPAP [8]. In the present study, serum CTX levels were similarly significantly higher in the OSAS group.
In the study by Uzkeser et al., the risk of osteoporosis was found to be higher in 21 patients with OSAS compared to the control group, and they also found significantly lower lumbar T-scores and BMD and femoral neck BMD in the OSAS group [7]. In the present study, femoral neck BMD and femoral neck T-scores were significantly lower in patients with OSAS; however, there was no significant difference in the lumbar region.
In contrast to these two studies, Sforza et al. [6] studied elderly patients and found higher femoral and spinal BMD values in the OSAS group compared to the control group. When compared to the other two studies, this study offers the advantage of a larger patient population (n = 832). However, this study may also have disadvantages in that the study population averaged 65 years of age, bone turnover markers were not evaluated, and ambulatory polygraphy was used.
The results of the present study support the negative effects of OSAS on BMD. At the same time, serum CTX levels were significantly higher in the OSAS group. This suggests that bone metabolism might have been affected towards a catabolic state.
In the present study, vitamin D levels were found to be lower in the OSAS group; however, there was no statistically significant difference after adjustments have been made for BMI. Likewise, Erden et al. found higher PTH and lower 25(OH)D3 levels in the OSAS group compared to the control group [25]. Studies to date have usually found lower vitamin D levels in obese patients [26]. Vitamin D levels are often lower due to a lack of sun exposure in relation to insufficient physical activity, excessive deposition or sequestration of vitamin D in the fat tissue [27].
The limitations of our study include the absence of an assessment of other factors (level of physical activity, nutrition, calcium intake, and sunlight exposure) that might affect BMD, absence of an examination with respect to vertebral osteoarthritis, and the low sample size. In particular, the limited number of patients in our study population might have led us to overlook the potential differences in bone mass density in the lumbar region.
Our study is superior in that the control group comprised subjects without OSAS, the diagnosis was confirmed by polysomnographic recording and not based on history and symptoms. Furthermore, to our knowledge, this is the first study to evaluate BMD and bone turnover markers together with vitamin D levels. Similar studies in a larger number of patients are needed and, in addition to BMD, further studies related to bone quality and microarchitecture in this patient population would be helpful in evaluating the relationship between OSAS and bone changes.
References
Shahar E, Whitney CW, Redline S et al (2001) Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med 163:19–25
Khan F, Walsh C, Lane SJ et al (2014) Sleep apnoea and its relationship with cardiovascular, pulmonary, metabolic and other morbidities. Ir Med J 107:6–8
Barceló A, Piérola J, de la Peña M et al (2011) Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J 37:1418–1423. doi:10.1183/09031936.00050410
Nair D, Dayyat EA, Zhang SX et al (2011) Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS ONE 6:e19847. doi:10.1371/journal.pone.0019847
Destors M, Tamisier R, Baguet JP et al (2014) Cardiovascular morbidity associated with obstructive sleep apnea syndrome. Rev Mal Respir 31:375–385. doi:10.1016/j.rmr.2013.12.003
Sforza E, Thomas T, Barthélémy JC et al (2013) Obstructive sleep apnea is associated with preserved bone mineral density in healthy elderly subjects. Sleep 36:1509–1515. doi:10.5665/sleep.3046
Uzkeser H, Yildirim K, Aktan B et al (2013) Bone mineral density in patients with obstructive sleep apnea syndrome. Sleep Breath 17:339–342
Tomiyama H, Okazaki R, Inoue D et al (2008) Link between obstructive sleep apnea and increased bone resorption in men. Osteoporos Int 19:1185–1192
Fujimoto H, Fujimoto K, Ueda A, Ohata M (1999) Hypoxemia is a risk factor for bone mass loss. J Bone Miner Metab 17:211–216
Arnett TR (2010) Acidosis, hypoxia and bone. Arch Biochem Biophys 503:103–109
Svensson A, Venge P, Janson C, Lındberg E (2012) Relationship between sleepdisordered breathing and markers of systemic inflammation in women from the general population. J Sleep Res 21:147–154
Almendros I, Carreras A, Montserrat JM et al (2012) Potential role of adult stem cells in obstructive sleep apnea. Front Neurol 3:112
Guven SF, Turkkani MH, Ciftci B et al (2012) The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath 16:217–221. doi:10.1007/s11325-011-0492-2
Yokoe T, Minoguchi K, Matsuo H et al (2003) Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107:1129–1134
Mariani S, Fiore D, Varone L et al (2012) Obstructive sleep apnea and bone mineral density in obese patients. Diabetes Metab Syndr Obes 5:395–401. doi:10.2147/DMSO.S37761
Arnett TR, Gibbons DC, Utting JC et al (2003) Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol 196:2–8
Utting JC, Flanagan AM, Brandao-Burch A et al (2010) Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct 28:374–380
Orriss IR, Knight GE, Utting JC et al (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol 220:155–162
Fink T, Abildtrup L, Fogd K et al (2004) Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells 22:1346–1355
Utting JC, Robins SP, Brandao-Burch A et al (2006) Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 312:1693–1702
Song L, Liang X, Zhou Y (2014) Estrogen-mimicking isoflavone genistein prevents bone loss in a rat model of obstructive sleep apnea-hypopnea syndrome. Int J Clin Exp Pathol 7:1687–1694 (eCollection 2014)
Lemann J Jr, Litzow JR, Lennon EJ (1966) The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45:1608–1614
Arnett TR, Spowage M (1996) Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18:277–279
Brandao-Burch A, Utting JC, Orriss IR et al (2005) Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int 77:167–174
Erden ES, Genc S, Motor S et al (2014) Investigation of serum bisphenol A, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine 45:311–318. doi:10.1007/s12020-013-0022-z
Wortsman J, Matsuoka LY, Chen TC, Lu Z et al (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693
Buffington C, Walker B, CowanJr GS et al (1993) Vitamin D deficiency in morbidly obese. Obes Surg 3:421–424
Conflict of interest
The authors declared no conflict interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Terzi, R., Yılmaz, Z. Bone mineral density and changes in bone metabolism in patients with obstructive sleep apnea syndrome. J Bone Miner Metab 34, 475–481 (2016). https://doi.org/10.1007/s00774-015-0691-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0691-1