Abstract
VITA-1 and VITB-1 are low- and elevated level CRMs, respectively, which were certified for minerals and vitamin contents by National Research Council Canada. The proportions of key ingredients are the same in each formula, and they were prepared in such a way that VITB-1 has twice the amount of minerals and vitamins as VITA-1. The availability of these two matrix-matched CRMs, with different concentrations for all analytes, will not only assist in the validation of procedures and the development of methods for the determination of respective analytes in multivitamins or samples of a similar matrices, but also will be useful for calibration purposes (for the values that are certified). Tablets were individually packaged in trilaminate foil pouches and values presented in the certificate of analysis are expressed on a “as is” basis, with no need for the determination of moisture content on a separate test portion. In addition, certified/reference values are provided per single tablet (mass fraction expressed as both mg/kg and µg/tablet), eliminating the need of a homogenization step prior to analysis. Homogeneity and stability were assessed and included in the final expanded uncertainties of the certified values. Quantity value assignment for both VITA-1 and VITB-1 was based on the use of at least two independent methods, using data generated at NRC and data from external collaborators for confirmation purposes. As a result of this campaign, value assignment was possible for a total of 39 analytes: 24 trace elements and 15 vitamins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of dietary supplements has been showing a rising interest in the last few decades, with these products being used by a large fraction of the population in an effort to increase their daily nutrient intake and/or for overall health promotion as vitamins and minerals play an essential role in metabolic pathways and the functioning of the human body [1,2,3]. Vitamins and minerals are often added to food to address public health issues and maintain the nutritional quality of the food supply. For example, vitamin D is added to milk to combat the childhood deficiency disease of rickets, and folic acid is added to flour to reduce birth defects.
Nearly 45 % of Canadians (aged 1 year and older) used at least one nutritional supplement in 2015, with the highest percentile being females over 50 years old [4]. Dietary supplements come in a variety of forms such as tablets, capsules, and gummies and they often contain a combination of vitamins and minerals. They are usually complex products and in Canada they are controlled under the Natural Health Products Regulations which ensure that Canadians have access to safe, effective, and high quality natural health products. According to this regulation, all natural health products must be licensed by Health Canada before they are allowed to be legally sold in Canada. A quick search in the Licensed Natural Health Products Database from the Health Canada website [5] for multivitamins products shows about 100 different brand names for products that have been licensed by Health Canada. Those products range from multivitamins for toddlers to those for adults over 50 years old. It should be noted that each age group has different recommended daily intakes as set by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) [6]. Also, these recommendations may differ for men and women [6]. For instance, recommended magnesium intake varies from 60 mg/day for 1–3 year old children to 230 mg/day for males over 65 years [6]. Exceeding the recommended daily dosage of some vitamins and mineral supplements could be detrimental and lead not only to some considereably minor health issues such as nausea and diarrhea, but also to heart problems (vitamin D, calcium) [7], liver damage (vitamin A) [8,9,10], or nerve damage (vitamin B6) [11, 12], among other serious health issues. Therefore, levels of vitamins and minerals in multivitamin products must be accurately assessed in order to guarantee that a dose meets the required daily intake. Also, earlier studies [13,14,15,16] have shown that the actual amount of “active ingredient” in some multivitamins and supplements currently available on the market can vary significantly from the amount listed on the label. This may be due to the lack of uniformity of the ingredients added to the formulation. Thus there is a need for chemical metrology, not only from a health and safety perspective but also to support/improve any future clinical studies which might rely on the use of commercially available multivitamins. The ability to provide consumers with assurance of the accurate concentrations of minerals and vitamins presented in those products depends on the implementation of precise and accurate methods of analysis which employ proper quality control (QC) procedures such as the use of Certified Reference Materials (CRMs) of multivitamins and similar matricies.
In recent years CRM producers have developed such reference materials. For example, the National Metrology Institute of Korea (KRISS) released two CRMs, both in powder form: one for nutritional supplements for elemental analysis (108-10-012) [17] which is certified for Se and a multivitamin powder for analysis of organic nutrients (108-10-010) [18] which is certified for seven water-soluble vitamins. The National Institute of Standards and Technology (NIST) released a Standard Reference Material (SRM) for multivitamin/multiement tablets (SRM 3280), with certified and reference mass fraction values for 13 vitamins, 26 elements, and 2 carotenoids. This material is offered as five units, each containing 30 tablets. During the certification campaign of SRM 3280, it was verified that the material used showed a large tablet-to-tablet variability, ranging from approximately 15 % to 25 % for measured element mass fractions presented in the certificate of analysis [19,20,21]. In order to use the SRM, users are instructed to grind at least 15 tablets to obtain a homogeneous sample prior to sampling a test portion for analysis [19,20,21]. For the vitamin analysis, it was also recommended to use a freshly ground portion as some vitamins were observed to be unstable in the ground material [19]. This can significantly increase the cost of the analysis, especially for the vitamins.
National Research Council Canada (NRC) has produced two new CRMs for multivitamins: VITA-1 (low-level), and VITB-1 (elevated level). These two matrix-matched CRMs, with different concentrations for all analytes, will assist not only the validation of procedures and development of methods for the determination of respective analytes in multivitamin or samples of similar matrices, but also will be used for calibration purposes (for certified values). VITA-1 and VITB-1 were prepared in such a way that apart from having the similar matrices, the nutrient’s proportion between both CRMs was kept constant (with VITB-1 having twice the amount of minerals and vitamins as VITA-1). Thus, when both CRMs are used together, they can aid a calibration.
The NRC CRMs aim to achieve a higher degree of homogeneity and provide certified values per single tablet (mass fraction expressed as both mg/kg and µg/tablet), eliminating the need of a homogenization step prior to the analysis. In addition, tablets were individually packaged in trilaminate foil pouches and values presented in the certificate of analysis are expressed on a wet weight basis, eliminating the need for the determination of moisture content on a separate test portion.
The certification campaign for VITA-1 and VITB-1 is described here. Both materials were produced in compliance with the NRC Metrology Quality System, which conforms to the requirements of ISO/IEC 17025 and ISO 17034. Quantity value assignment for both VITA-1 and VITB-1 was based on the use of at least two independent methods, preferably one of them being a single primary method performed at NRC. Confirmation of these results by external collaborators’ results (ISO/IEC 17025 accredited) was achieved.
Experimental section
Reagents and solutions used at NRC
Nitric and hydrochloric acids were purified in-house by sub-boiling distillation of reagent grade feedstock in a quartz still. High-purity deionized water (DIW) was produced by reverse osmosis of tap water followed by deionization (Barnstead/Thermolyne, Dubuque, IA, USA) to yield an 18 MΩ·cm resistance. Methanesulfonic acid (≥ 99.0 %), ammonium acetate (≥ 98 %), and acetic acid (≥ 99.7 %) were purchased from Sigma-Aldrich (Oakville, ON, Canada).
All plastic and glass lab ware were cleaned by immersion in 5 % (volume fraction) HNO3 for at least 24 h and thoroughly rinsed with DIW before use.
Natural isotopic abundance As, Cd, Cr, Co, Cu, Fe, Hg, Pb, Mn, Mo, Ni, V, and Zn stock solutions at 1000–3000 mg/kg were prepared by dissolving NRC-traceable high-purity metals, previously characterized by glow discharge mass spectrometry (GDMS) for purity (> 99.9 %), in high-purity HNO3 or mixture of HNO3 and HCl, and diluted with DIW. Natural isotopic abundance B, Ca, Mg, P, K, and Se stock solutions of SRM 3107, SRM 3109a, SRM 3131a, SRM 3139a, SRM 3141a, and SRM 3149 at 10000 mg/kg were obtained from National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA).
Enriched 10B, 57Fe, 53Cr, 65Cu, 67Zn, 82Se, 100Mo, 111Cd, 201Hg, and 207Pb isotopes, purchased from Oak Ridge National Laboratory (Oak Ridge, TN, USA) or Trace Sciences International (Richmond Hill, ON, Canada), were dissolved in high-purity HNO3 or mixture of HNO3 and HCl and diluted with DIW to prepare spike stock solutions.
NIST SRM 3280 multivitamin/multielement tablets, NRC CRM DORM-4, NIST SRM 8435 whole milk powder, NIST SRM 1566a oyster tissue, and NRC CRM SELM-1 were used for method validation.
Commercial USP cyanocobalamin and L-selenomethionine standards, both from Sigma-Aldrich were used. The purity of both reagents were assigned traceable to NIST SRM 84L (potassium hydrogen phthalate), via proton quantitative nuclear magnetic resonance spectroscopy (1H-qNMR). All other water-soluble standards were of USP grade.
Instrumentation used at NRC
A high-resolution HR-ICP-MS Element XR (Thermo Fisher Scientific, Bremen, Germany) equipped with a combination of cyclonic and Scott-type spray chamber and 50 μL/min MCN50 PFA nebulizer (Elemental Scientific, Omaha, NE, USA). A plug-in quartz torch with a quartz injector and a platinum guard electrode was used. The Element XR is equipped with an additional Faraday cup detector in addition to the secondary electron multiplier (SEM) detector. An Agilent 8800 triple quadrupole ICP-QQQ-MS (Agilent Technologies, Mississauga, ON, Canada) was also used for the analysis of minerals. Both ICP-MS instruments were optimized daily according to the manufacturers’ recommendations. Typically, the extent of oxide or doubly charged ion formation was less than 1.5 %. Typical operating conditions of HR-ICP-MS and triple quadrupole ICP-MS, when used for total mineral analysis, are summarized in Table 1.
A Varian 720 ES Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES, Agilent Technologies, Mississauga, ON, Canada) with a Meinhard concentric nebulizer coupled to a cyclonic spray chamber (Glass Expansion Inc., Pocasset, MA, USA) were also used for the determination of minerals. Optimization of the ICP-AES was performed as recommended by the manufacturer and typical operating conditions are summarized in Table 2. Multiple wavelengths were utilized to assess spectral interferences and instrument software was employed to correct interferences where present.
All sample preparations and dilutions for ICP-MS and ICP-AES methods were conducted in a class 100 clean room or in fume hoods of class 10 air quality.
A Multiwave 3000® microwave sample preparation system (Anton Paar, Graz, Austria), equipped with Conventional PTFE-TFM liner vessels with a maximum pressure of 70 bar and maximum temperature of 240 °C was used for conventional microwave-assisted acid digestion prior to total elemental analysis. Evaporation of excess acid resulting from the initial digestions was achieved with a Digiprep Jr block heater (SCP Science, Baie-D’Urfe, QC, Canada) also located in a class 10 fume hood.
For the determination of cyanocobalamin, selenomethionine, and chromium picolinate, an Agilent 1200 Series HPLC coupled to an Agilent 8800 triple quadrupole ICP-QQQ-MS (Agilent Technologies, Mississauga, ON, Canada) was used. Water-soluble vitamins were analyzed using an Agilent Infinity 1290 HPLC (Agilent Technologies) interfaced with a Quantiva TSQ electrospray ionization (ESI) triple quadrupole mass spectrometer (Thermo Fisher Scientific, Ottawa, ON, Canada). Table 3 presents the instrumental parameters used.
Preparation of VITA-1 and VITB-1 candidate CRMs
Two candidate CRMs for multivitamins, named VITA-1 and VITB-1, were prepared at NRC Canada. VITA-1 is a low-level multivitamin and VITB-1 an elevated level multivitamin CRMs. The proportion of nutrients is the same in each formula and they were prepared in such a way that VITB-1 has twice the amount of each minerals and vitamins as VITA-1. They were both produced by a Canadian commercial nutraceutical manufacturer according to pharmaceutical standards using the product specification presented in the Table 4.
Non-medicinal ingredients such as lactose monohydrate, hydroxypropyl cellulose, silicon dioxide, microcrystalline cellulose, magnesium stearate, and opadryl II white (polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc) were also added to the formulations. Both materials were pressed into tablets, film-coated and individually packed into trilaminate foil pouches. Individual tablets of VITA-1 and VITB-1 weigh approximately 2.57 g and 2.62 g, respectively. Both materials have been stored in a dark + 4 °C temperature environment.
Methods used for the certification
Determination of mass fractions of minerals by double isotope dilution ICP-MS at NRC
Tablets were digested “as it is”, i.e., no subsampling was performed. Individual whole vitamin tablets were accurately weighed into pre-cleaned Teflon microwave digestion vessels. For isotope dilution analysis, the contents were then gravimetrically spiked with known masses of enriched isotopes to achieve an approximately 1:1 ratio in intensities of selected isotope pairs. The following reference/spike isotope pairs were used: 11B/10B, 114Cd/111Cd, 52Cr/53Cr, 63Cu/65Cu, 56Fe/57Fe, 208Pb/207Pb, 202Hg/201Hg, 98Mo/100Mo, 78Se/82Se, and 66Zn/67Zn. Similarly, three procedural blanks were prepared by addition of only 10 % of the mass of enriched isotope spike used for the samples. After addition of 15 mL HNO3, 2 mL HF, and 3 mL H2O2, contents were heated in a hot block at 80 °C for 24 h. Then 3 mL HCl was added prior to the microwave digestion which followed a power cycle of 5 min ramping to 360 W and 5 min hold, another 5 min ramping to 850 W and 20 min hold, then 0 W for the cool down cycle (35 min). The contents were transferred into Teflon tubes and evaporated until 2 mL – 3 mL remained. Another 4 mL of HNO3 and 12 mL of HCl were added to the tubes which were then heated at 60 °C for 60 min. Contents were diluted to 500 g with DIW. Sample solutions were left to stand for at least 5 days prior to analysis to ensure a clear solution, i.e., total dissolution.
The isotope dilution (ID) method is capable of compensating for matrix effects, instrument drift, and any subsequent losses of analyte during sample preparation procedures, provided that isotopic equilibrium has been achieved prior to analysis. In addition, ID provides superior measurement accuracy and precision compared to other calibration strategies. For the certification of VITA-1 and VITB-1, double ID [22] was applied for the determination of mass fractions of B, Cr, Cu, Fe, Pb, Hg, Mo, and Zn in the multivitamin samples using HR-ICP-MS and Cd and Se using the ICP-QQQ-MS.
where: wx is the blank mass fraction of the analyte in the sample (mg/kg); wz is the mass fraction of the analyte in primary standard solution (mg/kg); my is the mass of spike solution used to prepare the mixture of sample and spike (g); mx is the mass of sample used (g); mz is the mass of primary assay standard used (g); my′ is the mass of spike used to prepare the mixture of spike and primary assay standard (g); Ay is the abundance of the reference isotope in the spike; By is the abundance of the spike isotope in the spike; Ax is the abundance of the reference isotope in the sample; Bx is the abundance of the spike isotope in the sample; Az is the abundance of the reference isotope in the primary assay standard; Bz is the abundance of the spike isotope in the primary assay standard; K is the mass bias correction factor; r is the measured reference/spike isotope ratio in the mixture solution of sample and spike; r′ is the measured reference/spike isotope ratio in the mixture solution of spike and primary assay standard; Ar(X) is the atomic weight of the analyte element in the sample; Ar(Z) is the atomic weight of the analyte element in primary assay standard; wb is the mass fraction of the analyte in the procedural blank (mg/kg) calculated using an expression identical to Eq. 1 wherein only the first term on the right-hand side is used and the subscripted variables refer to the relevant blank parameters.
The measurement equation can be simplified to Eq. 2 for all elements whose isotopic abundances are invariant in nature (and therefore Ax = Az = Axz, Bx = Bz = Bxz, Ar(X) = Ar(Z)):
Determination of mass fractions of minerals by standard addition ICP-MS and ICP-AES
The same sample preparation procedure described for the ID-ICP-MS method was applied for standard addition ICP-MS and ICP-AES methods, except isotopically enriched spikes were not added to samples. In this case, a three-point standard addition calibration was used. For that, three 20 g subsamples of digest were weighed into pre-cleaned polyethylene bottles. Appropriate masses of 0.20 and 0.40 g of the mixed standard solution were added to two subsamples to result in approximately a onefold and twofold increase in the analyte mass fractions, respectively.
Equation 3 was used for the calculation of the mass fraction of analytes by ICP-MS (75As, 44Ca, 59Co, 208Pb, 24Mg, 55Mn, 202Hg, 31P, 39K) and ICP-AES (B, Ca, Cr, Cu, Fe, K, Mg, Mn, Mo, P, Se, Zn) using standard addition calibration. [23, 24] In addition, errors-in-variables regression was used to obtain regression coefficients and their uncertainties. [25]
where: wx is the mass fraction of the analyte in the sample (µg/kg); wstd is the mass fraction of the analyte in the primary standard solution (µg/kg); Ii is the measured intensity in the prepared set of samples, i = 0, 1, 2; mstd-i is the mass of natural abundance standard added to the spiked sample (g), i = 1, 2; ms-i is the mass of aliquot of diluted sample used to prepared spiked sample (g), i = 1, 2; msf-i is the final mass of spiked sample (g), i = 1, 2; mdf-i is the final mass of diluted set of samples (g), i = 0, 1, 2; md0-i is the mass of aliquots of spiked samples for dilution (g), i = 0, 1, 2; mx is the mass (g) of the original sample; mxf is the final mass of the original sample after addition of enriched spikes and 1 % HNO3 (g).
It is well known that the determination of mass fractions of trace elements by ICP-MS can be a challenge due to the interferences. For example, the single arsenic isotope suffers from interferences of 40Ar35Cl+ and 36Ar39K+. Those polyatomic interferences needed to be resolved by employing either high mass resolution or a reaction cell.
For the determination of mass fractions of Cr, Co, Cu, Fe, Mn, Ni, V, and Zn using HR-ICP-MS at NRC, medium resolution was used in order to separate possible polyatomic interferences (e.g., 40Ar12C, 40Ar16O and 23Na 40Ar, 36Ar17O, 35Cl17O, 35Cl18O, 40Ar16O,25Mg35Cl, 23Na37Cl, 44Ca16OH, 26Mg35Cl, 23Na38Ar, 45Sc16O, 23Na40Ar, 46Ca16OH, 47Ti16O, 25Mg40Ar, 48Ca16OH, 130Ba++, 49Ti16O, 50Ti16O, 132Ba++, 136Ba++) on isotopes 52Cr, 53Cr, 59Co, 63Cu, 65Cu, 56Fe, 57Fe, 55Mn, 66Zn, and 67Zn. For example, the mass fraction of calcium in VITA-1 is about 28600 mg/kg (double in VITB-1) and will cause interferences in the measurement of 59Co (due to the polyatomic interferences of 43Ca16O + , 42Ca16O1H+), 56Fe (40Ca16O+), and 57Fe (40Ca16O1H+), among others. Also the mass fraction of potassion is also high (12100 mg/kg for VITA-1) which can cause interferences on 55Mn (39K16O+), 98Mo (41K2O+). Arsenic (by hydride generation), Ca, K, and Se were measured at high resolution to separate polyatomic interferences (e.g., 40Ar36Cl and 39Ar1H) on isotopes 75As, 44Ca, 39K, 78Se and 82Se. B, Hg, Pb and Mo were measured at low resolution on 10B, 11B, 202Hg, 201Hg, 207Pb, 208Pb, 100Mo and 98Mo.
When ICP-QQQ-MS was used for the determination of mass fractions of Cd, Co, Hg and Pb by standard addition, helium mode was used (isotopes: 111Cd, 208Pb, 59Co and 202Hg). Oxygen mode was used for As (measurement performed using mass shift 75 > 91 for 75As > 75As16O+), Se (isotope dilution using mass shift (78 > 94)/(82 > 98)), and Cd (isotope dilution at m/z (113 > 113)/(111 > 111)) to avoid possible interferences such as 40Ca162O12H+ on 113Cd+.
Validation of the method at NRC was performed by analysis of established CRMs, NIST-3280, multivitamin/multielement tablets (for all elements/compounds except Hg, selenometionine and chromium picolinate), NRC CRM DORM-4 (for As, Cd, Co, Hg, and Pb), and NRC CRM SELM-1 (for selenomethionine). Sample preparation is the same as described above except that for SRM 3280, a total of 25 tablets were ground and 1 g subsamples were used. For the other CRMs, 0.25 g of sample was used instead. Analysis of the digested samples was performed by ICP-MS or ICP-AES as described above.
Determination of As by hydride generation
The mass fraction of arsenic was also determined by hydride generation using l-cysteine to reduce arsenic species to As(III). Samples were mixed with l-cysteine to result in 2 % l-cysteine and left stand overnight at room temperature in order to reduce all As species into a single form prior to analysis. Samples were mixed online with a solution containing 1 % NaBH4 and 0.1 % KOH and carried by Ar through a PTFE reactor (1.5 m length) and then to a homemade gas–liquid separator (GLS), with 2 mL internal volume. The generated As vapor was directed from the GLS to the gas inlet port of the spray chamber of ICP-MS via a 0.30 m length of Teflon tubing for detection. The method of standard addition was used for calibration.
Determination of cyanocobalamin at NRC
Cyanocobalamin (vitamin B12) was analyzed at NRC by two independent methods: LC-ICP-MS using standard addition and isotope dilution LC–MS/MS, respectively. Information is presented in ref [26]. The ID-LC–MS/MS method is based on high-precision quadruple isotope dilution for quantitation using isotopically enriched 13C15N-B12 as internal standard. Purity assessment of the cyanocobalamin standard was determined by 1H-qNMR against NIST SRM 84L potassium hydrogen phthalate (KHP) [26].
In short, tablets were dissolved in a dilute acetic acid solution, and sonicated at 25 °C for 30 min, followed by centrifugation at 2000 rpm for 10 min. Supernatant was filtered through 0.45-mm nylon filters and concentrated by solid phase extraction (SPE). The SPE tubes (Strata™-X 33 mm Polymeric Reversed Phase, 500 mg/12 mL, Phenomenex) were first conditioned with methanol and DIW. The multivitamin extract was loaded on the SPE followed by a wash with 5 % methanol in water for removing the minerals. Cyanocobalamin was finally eluted with 10 mL methanol which was then evaporated to dryness under nitrogen. The solid residue was reconstituted with 1 mL of water and diluted 1:10 in 1 % acetic acid prior to LC–MS/MS analysis. Sample preparation for the determination of cyanocobalamin by LC-ICP-MS analysis was similar to the one just described, but without the SPE step. There, quantitation was attained by standard addition. A C18 column was used and analytes were eluted in isocratic mode at 25 °C in 15:85 acetonitrile: water mobile phase with 0.1 % formic acid [26], as noted in Table 3.
Determination of water-soluble vitamins (except cyanocobalamin), chromium picolinate and selenomethionine at NRC
For the analysis of thiamine, riboflavin, niacinamide, pantothenic acid, biotin, folic acid, pyridoxine, riboflavin, and thiamine, tablets were dissolved in a 1 % acetic acid solution,and sonicated at 25 °C for 30 min, followed by centrifugation at 2000 rpm for 10 min. The supernatant was filtered through a 0.45 mm nylon filter, and analyzed directly (following appropriate dilution) by LC–MS/MS (see Table 3 for parameters) using external calibration for quantitation (with use of an internal standard for biotin and niacinamide) for quantitation.
For the determination of chromium picolinate and selenomethionine, 20 tablets were crushed and homogenized and aliquots were sub-sampled for extraction and analysis. For chromium picolinate, 0.25 g of power was added to 100 mL of 60 % acetonitrile in deionized water and the mixture was sonicated at 25 °C for 40 min. Following 10 min of centrifugation at 4000 rpm, the supernatant was 0.2 µm syringe filtered and analyzed by LC-ICP-QQQ-MS [27] under the conditions noted in Table 3. For selenomethionine, 0.25 g of powder was refluxed for 16 h in 24 mL of 4.0 M methanesulfonic acid. Samples were cooled, 0.2-µm syringe-filtered, and diluted 1:5 in deionized water prior to LC-ICP-QQQ-MS analysis [16] (Table 3). Quantitation of both chromium picolinate and selenomethione was performed by standard addition.
Determination of mass fractions of Br, I, Cl by ICP-MS (external collaborator)
Br, I, and Cl were analyzed using a microwave-induced combustion method. In short, sample pellets were placed together with the filter paper on the quartz holder and 50 μL of ammonium nitrate solution (6 mol L−1) were added to the paper. The sample holder was introduced into the quartz vessel, previously charged with 6 mL of absorbing solution (100 mmol/L NH4OH). After closing the vessels and capping of the rotor, vessels were pressurized with 20 bar of oxygen. The rotor was placed inside the oven and the selected microwave heating program was started. The microwave irradiation program was 900 W for 5 min and 0 W for 60 min (cooling step). After digestion, the pressure of each vessel was carefully released. The resultant solution was diluted with water up to 25 mL in volumetric vessels [28]. Final digests were analyzed by ICP-MS, measuring isotopes 35Cl, 79Br, and 129I. Stock standard solutions of Cl, Br, and I (1000 mg/L) were prepared by the dissolution of sodium chloride, potassium bromide and potassium iodide salts (Merck), respectively, in water. NIST SRM 3280 multivitamin/multielement tablets, NIST SRM 1566a oyster tissue, and NIST RM 8435 whole milk powder were used as QC samples.
Determination of mass fraction of elements by ICP-MS (external collaborator)
Samples were digested using a closed vessel microwave system using a mixture of mineral acids following EPA Method 3052. Mass fractions of analytes were determined by external calibration ICP-MS. Commercial standards traceable to NIST were used. NIST SRM 3280 multivitamin/multielement tablets and NIST SRM 1566a oyster tissue were used as QC samples.
Determination of water-soluble vitamins by LC–MS/MS (external collaborator)
The determination of water-soluble vitamins by LC–MS/MS was based on extraction using an acidic aqueous-solvent mixture, followed by the addition of a small amount of alkaline solution to precipitate proteins and aid in chromatography. Determination was performed using LC–MS/MS. Stable isotope internal standards of each B-vitamin were used in the quantitation and an external calibration strategy was used.
Microbiological assay (external collaborator)
The microbiological assay on VITA-1 and VITB-1 was performed by an external laboratory according to AOAC 952.20 and 960.46 official methods of analysis for vitamin B12. The test consists in incubating the sample, formerly inoculated with Lactobacillus leichmanii, for 16 h to 24 h and measuring the developed turbidity with a photometer. Commercial USP standard from Sigma-Aldrich was used. A blind sample of NIST 3280 SRM multivitamin tablets was supplied along with VITA-1 and VITB-1 for quality control purposes. This method was used as a confirmatory method.
Results and discussion
Certification campaign
Quantity value assignment for both VITA-1 and VITB-1 was based on the use of at least two independent methods, preferably one of them being a single primary reference method performed at NRC. Confirmation of these results by analysis performed by external collaborators (ISO/IEC 17025 accredited) was also used.
A primary or higher order method is the most preferred to obtain quantity values for a certification and could be applied to obtain certified quantity values on its own or with an additional confirmatory method. Unfortunately, there are a limited number of primary methods available. For this certification campaign, the primary method used for the determination of mass fractions of trace elements was double isotope dilution inductively coupled plasma mass spectrometry (ID-ICP-MS) and it was applied for the determination of the mass fractions of B, Cd, Cr, Cu, Fe, Pb, Hg, Mo, Se, and Zn in both VITA-1 and VITB-1 proposed candidate CRM samples using high-resolution ICP-MS. Mass fractions of As, Co, Mn, and V were determined using a standard addition calibration strategy to account for any possible matrix effects. A second set of data was also produced at NRC by using a standard addition calibration strategy and detection by triple quadrupole (QQQ) ICP-MS or ICP-atomic emission spectrometry (ICP-AES). For the determination of vitamins in both VITA-1 and VITB-1, results from a single method only, liquid chromatography tandem mass spectrometry (LC–MS/MS), were produced at NRC, except for cyanocobalamin which was also determined by LC-ICP-MS and ID-LC–MS/MS.
In addition to NRC’s results, external collaborators provided measurements using their own internal procedures. Each collaborator received blind samples that encompassed a QC sample of a similar matrix and several single tablets from each proposed candidate CRM (VITA-1 and VITB-1). For the determination of the mass fraction of trace elements, collaborators were asked to perform a complete digestion using a microwave-assisted acid digestion procedure.
Gravimetric data, i.e., the amount of each ingredient that was added to the formulations, was also available from the manufacturer of the proposed candidate CRM (VITA-1 and VITB-1) and was used during the certification as an independent data set.
The summary of the analytical methods used in the certification of VITA-1 and VITB-1 is presented in Table 5. For most analytes, three independent methods were used, two of them performed at NRC.
Assessment of homogeneity
It is well known that in the pharmaceutical industry, production of homogenized products in the powder form is challenging [29] as optimum mixing is crucial, especially for low dose products like multivitamins. Homogeneity is a factor of the particle size, shape, and density, among other parameters [30]. During the certification of NIST SRM 3280 multivitamin/multielement tablets, it was verified that the material, which was produced by a manufacturer of multivitamin/multielement tablets using their normal procedure, presented an individual tablet-to-tablet variability ranging from 15 to 25 %; therefore, at least 15 tablets of the material must be ground to obtain a homogenous sample prior to analysis [21]. In light of this, special attention was given during the production of VITA-1 and VITB-1 in order to achieve a better homogeneity in order to permit the use of single tablets for analysis.
An initial homogeneity assessment was carried out during the production by selecting four water-soluble vitamins (pyridoxine HCl, riboflavin, thiamine HCl, and niacinamide) as model analytes and using LC-UV for the respective analyte determination. Eight single tablets of VITA-1 and four single tablets of VITB-1, selected across the entire production, were using for this initial homogeneity assessment. Figure 1 shows the individual results for VITA-1. RSD values of about 5 %, 2.5 %, 7 % and 1 % (VITA-1) and 9.5 %, 2.5 %, 3 %, and 1.6 % (VITB-1) were obtained respectively for pyridoxine HCl, riboflavin, thiamine HCl, and niacinamide.
Initial homogeneity assessment for vitamins during VITA-1 production using eight single tablets selected across the entire production. Analytes were determined by LC-UV. Red dotted line represents the specification for each water-soluble vitamin (as indicated in Table 2), blue solid line represents the average and blue dotted line represents the standard deviation a Pyridoxine HCl, b Riboflavin, c Thiamine HCl, and d Niacinamide (color figure online)
Between-tablet homogeneity for both metals and vitamins in VITA-1 and VITB-1 was assessed at NRC across the entire CRM production series (about 45000 units for each product). For that, individual tablets were randomly selected and analyzed. The between-tablet variation comprises sample heterogeneity (uhom) and variation due to batch characterization (uchar). Results for representative elements are presented in Fig. 2 and refer to the digestion of the whole tablet.
Tablet-to-tablet homogeneity assessment of VITA-1 (blue) and VITB-1 (orange). Solid columns refer to ICP-MS measurement, dashed columns refer to ICP-AES measurements, hollow columns refer to LC–MS–MS (B12). The number on top of each column is the number of tablets that was used for the homogeneity assessment (color figure online)
Both ICP-MS and ICP-AES measurements were used for the between-tablet homogeneity assessment. For most analytes, homogeneity was assessed with the analysis of at least 50 tablets. The relative standard deviation (RSD) values for minerals were less than 5 % for all analytes except for As, Mg and Se in VITA-1 and As and Se in VITB-1. These values were 8.5 % and 9.8 % for As (standard addition ICP-MS) in VITA-1 and VITB-1, 6.2 % for Mg (measured by ICP-OES) in VITA-1, and 6.3 % and 5.4 % for Se (ID-ICP-MS) in VITA-1 and VITB-1, respectively. Cyanocobalamin showed a higher RSD, of about 9 % – 10 % for determination using ID-LC–MS/MS.
Determination of arsenic by ICP-MS suffers from interferences of 40Ar35Cl+ and 36Ar39K+. Those polyatomic interferences needed to be resolved by employing either high mass resolution or a reaction cell. In this work, a reaction cell instrument (triple quadrupole ICP-MS) was used for the determination of As using O2 as reaction gas. Subsequent data using a high mass resolution (as well HG) with smaller RSD was observed (4 %) although the number of tablets analyzed was smaller (10 tablets instead).
The overall mass fraction for magnesium came from contributions of both magnesium oxide (active ingredient) and magnesium stearate, a metallic salt of fatty acids that was added to the formulation to act as a lubricant in order to reduce friction by enhancing the powder flow during the manufacturing process [31]. Another data set for Mg, also performed at NRC, using ICP-MS has a much smaller RSD (1.4 % for the analysis of 15 single tablets) which shows that the higher RSD for Mg for the ICP-OES method is related to the variation due to characterization rather than the sample heterogeneity.
In relation to the mass fraction of selenium, it should be noted that its determination by ICP-MS suffers from several polyatomic interferences (40Ar40Ar+, 40Ca40Ar+, 39K37Cl+, 60Ni16O+, 65Cu17O+, 64Zn18O+). In this certification, a triple quadrupole ICP-MS operating in oxygen mode was used to remove such interferences. A second dataset for Se, performed at NRC (SA, ICP-AES, n = 52) also showed slightly higher RSD but signal intensities were very low in the sample. Similar to Mg, we believe that this variation is also related to the characterization procedure.
The uncertainty component due to possible between-tablet inhomogeneity was evaluated, for both VITA-1 and VITB-1, using the DerSimonian-Laird random effects model [32], an often-used statistical model for evaluating homogeneity study data according to ISO Guide 35:2017 [33]. In the framework of this statistical model, the observed result for any analyte, xi, is modeled as a juxtaposition of the true (unknown) value, the random effect due to homogeneity and random effect due to measurement. Results from the homogeneity assessment were included in the calculation of the certified values.
Stability
For the water-soluble vitamins, a short-term stability study was carried out at NRC using an isochronous experimental design [34] to simulate the effect of the potential elevated temperatures experienced during shipment. For that, multivitamin samples were placed in four different environments (freezer at − 20 °C, fridge at + 4 °C, laboratory at + 20 °C, and oven at + 37 °C) for 14 days. The reference temperature was + 4 °C (which is the storage condition for both VITA-1 and VITB-1). After 14 days, samples were analyzed. No measurable degradation, when compared to the certified uncertainties, was observed after this period at any temperature as exemplified in Fig. 3 for some water-soluble vitamins in VITA-1. Accelerated stability studies were only carried out for thiamine, niacinamide, riboflavin and folic acid and used as proxy for other water-soluble vitamins. No short-term assessment was performed for the minerals in the multivitamins CRMs as similar matrix CRMs have been analyzed and showed no measurable degradation when compared to the certified range so VITA-1 and VITB-1 were assumed to be stable during transport.
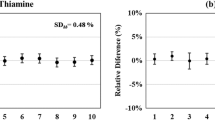
The long-term stability was assessed over a period of 2 years for quantity values for minerals and over a period of 1 year for the water-soluble vitamins (biotin, folic acid, pantothenic acid, pyridoxine HCl, riboflavin, thiamine HCl, niacinamide, and cyanocobalamin). Samples were stored at + 4 °C. No change in measured mass fraction for minerals and vitamins were observed over this period as exemplified for the vitamins in Fig. 4 for VITA-1. Similar behavior was observed for VITB-1. Uncertainty components for long and short-term stability were thus considered negligible and are thus not included in the uncertainty budget. Both materials are subject to ongoing monitoring.
One year long-term stability study under storage conditions (+ 4 °C) for the following water-soluble vitamins in VITA-1: biotin, cyanocobalamin, folic acid, niacinamide, pantothenic acid, pyridoxine HCl, riboflavin and thiamine HCl. Day 1 in blue (left) and Day 365 in orange (right). Error bars represent expanded uncertainty (color figure online)
Each single unit of VITA-1 and VITB-1 is individually packed in a trilaminte foil pouch. It is recommended that both materials be stored in a dark in a + 4 °C or lower temperature environment and the trilaminate foil pouches be opened immediately prior to use. A shelf life of 2 years was assigned for both materials. In case of suspect instabilities, the materials will be re-assessed, values revised and users notified.
Validation of methods for elements
Validation of the methods used for the determination of mass fractions of elements was performed by analysis of QC samples with similar matrices and results obtained were in agreement with the certified values (or reference values were certified values were not available). Several QC samples, such as NIST SRM 3280, NRC CRM DORM-4, and NIST SRM 1566b, were used, and Fig. 5 presents the bias obtained respect to the certified value for various analytes. It can be seen that results for the QC samples were mostly within the 10 % range of the certified values.
Validation of the methods developed for the determination of cyanocobalamin, selenomethionine, and chromium picolinate in multivitamins can be found elsewhere [16, 26, 27].
It should be noted that the observed bias between results obtained for the QC samples and the certified values were compared to the respective measurement uncertainty and, if necessary, incorporated into the uncertainties in the batch characterization.
Value assignment
Tables 6 and 7 present the results obtained for each method as well the consensus values and the category of the value. Quantity values presented in the NRC certificate of analysis fall into three categories: certified, reference, and information values. Certified values are considered to be those for which the National Research Council Canada (NRC) has the highest confidence in accuracy and that all known and suspected sources of bias have been taken into account and are reflected in the stated expanded uncertainties. Certified values are the best estimate of the mean and uncertainty. As a result, certified values were provided for 16 elements (As, B, Ca, Cr, Co, Cu, Fe, Pb, Mg, Mn, Hg, Mo, P, K, Se, Zn) and cyanocobalamin in both VITA-1 and VITB-1.
Reference values are those for which insufficient data are available to provide a comprehensive estimate of uncertainty. Br, Cl, chromium picolinate, iodine, selenomethionine, biotin, folic acid, niacinamide, pantothenic acid, pyridoxine HCl, riboflavin, and thiamine HCl were presented in the VITA-1 and VITB-1 certificates as reference values. Br, Cl and I analysis were not performed at NRC but by an expert laboratory along with several QC samples. From the vitamins listed before, folic acid was not determined by NRC but good agreement was observed between two data sets from external collaborators and the gravimetry data.
At last, information values are those for which insufficient data are available to provide any estimate of uncertainty. They usually have results from either one technique or from two techniques that were not sufficiently independent as required for certification, or if the disagreement among the methods was greater than expected for certified values. Mass fractions of Cd, Na, and Sr were presented in both VITA-1 and VITB-1 certificates as information values, along with quantity values for ascorbic acid, alpha tocopherol, beta carotene, ergocalciferol, lutein, phylloquinone, and retinol acetate.
For the characterization of the proposed candidate CRMs VITA-1 and VITB-1, value assignments were determined by combining measurement results obtained internally with external collaborators’ results and are presented as consensus values and expanded uncertainties (U(k=2)). Individual sets of data are presented as mean and characterization uncertainty (u(k=1)). As many as five data sets were combined to calculate the assigned value. As mentioned before, several blinded samples from each candidate CRM were provided to the external collaborators along with blinded QC samples to support the validity of the analyte measurement data.
Laboratory results were evaluated from their performance of analyzing QC samples. If their results for the QC samples were not in agreement with the certified values, their uncertainties for characterization would be inflated accordingly. The consensus values were obtained by combining the results from individual methods using a random effects statistical model (DerSimonian-Laird). Details can be consulted elsewhere [32]. Note that the uncertainties of the individual method results were adjusted (expanded) based on the QC results.
Uncertainty evaluation
The expanded uncertainty (U) is equal to U = kuc where uc is the combined standard uncertainty calculated according to the Guide to the expression of Uncertainty in Measurement [34] and k is the coverage factor. A coverage factor of two (2) was applied. It is intended that UCRM accounts for every aspect that reasonably contributes to the uncertainty of the measurement.
The standard combined uncertainty, uc, was determined using the following equation:
where: uchar uncertainty from characterization; uhom uncertainty related to possible between-tablet variation; umethod uncertainty related to inconsistency between the various measurement methods and determined as the overdispersion uncertainty using random effects model from the reported results and their uncertainties.
Expressed as standard uncertainties, these components are listed in Table 8 for VITA-1 and Table 9 for VITB-1. NRC data for certified elements in both VITA-1 and VITB-1 are traceable to the SI through gravimetrically-prepared primary standards (As, Cd, Cr, Co, Cu, Fem Pb, Mn, Hg, Mo, and Zn) whose purities were established by glow discharge mass spectrometry (GDMS) at NRC [35] which is validated through international inter-comparisons and inter-laboratory comparisons. Purity of high-purity metals may be assessed using either of two approaches: direct determination of the mass fraction of the main component or by determining each of the elemental impurity mass fractions and subtracting their sum from the ideal value of 1 kg/kg. The GD-MS is ideally suited to the latter approach as it is able to provide, with the exception of H and radioactive elements, full elemental coverage (including C, N and O) with low to sub-ng/g detection limits. For elemental results that cannot be quantitatively determined, a limit of detection (LOD) is reported. More information regarding the use of GDMS for purity assessment can be found in Ref. [36]. For those elements that NRC does not have primary standards assessed by GDMS, NIST SRMs were used (SRM 3107 for B, SRM 3109a for Ca, SRM 3131a for Mg, SRM 3186 for P, SRM 3141a for K, and SRM 3149 for Se).
The purity of cyanocobalamin and selenomethionine (the latter is presented as a reference value) were assigned traceable to NIST SRM 84L (potassium hydrogen phthalate), both via proton quantitative nuclear magnetic resonance spectroscopy (1H-qNMR), validated through international measurement inter-comparisons. NIST traceable calibration standards were used by external collaborators.
Conclusions
Two new multivitamin Certified Reference Materials are now available from National Research Council Canada. VITA-1 [37] and VITB-1 [38] are, respectively, low- and elevated level multivitamin Certified Reference Materials that are certified for minerals and vitamins. These Certified Reference Materials are intended for the calibration of procedures (for certified values) and the development of methods for the determination of mass fraction of trace elements, matrix constituents, and vitamins in multivitamins materials or similar matrices.
Individual tablets could be analyzed without the need of homogenization of the sample. Quantity values for both VITA-1 and VITB-1 are presented as mg/kg as well as µg/tablet. Homogeneity was assessed and included in the calculation of the certified values. Quantity value assignment was based on the use of at least two independent methods, using data generated at NRC and data from external collaborators for confirmation purposes. As a result of this campaign, value assignment was possible for a total of 39 analytes: 24 trace elements and 15 vitamins. Among them, 17 are classified as certified values (16 trace metals and one vitamin), 12 as reference values (5 trace metals and 7 vitamins), and 10 information values (3 for trace metals and 7 for vitamins) for both VITA-1 and VITB-1.
References
Huskisson E, Maggin S, Ruf M (2007) The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res 35:277–289
Somer E (1995) The essential guide to vitamins and minerals. HarperTorch
Marks J (2012) A guide to the vitamins: their role in health and disease. Medical and technical publishing Co Ltd
Statistics Canada (2015). https://www150.statcan.gc.ca/n1/pub/82-625-x/2017001/article/14831-eng.htm. Accessed 9 Jan 2019
Health Canada (2018). https://health-products.canada.ca/lnhpd-bdpsnh/index-eng.jsp. Accessed 9 Jan 2019
Food and Agriculture Organization of the United Nations and World Health Organization WH (2001). http://www.fao.org/3/a-y2809e.pdf. Accessed 9 Jan 2019
Alshahrani F, Aljohan N (2013) Vitamin D: deficiency, sufficiency and toxicity nutrients. Nutrients 5(9):3605–3616
Farrington K, Miller P, Varghese Z, Baillod RA, Moorhead JF (1981) Vitamin A toxicity and hypercalcaemia in chronic renal failure. BMJ 282:1999–2002
Lam HS, Chow CM, Poon WT, Lai CK, Chan KCA, Wai Lan Yeung JH, Chan AYW, Ng PC (2006) Risk of vitamin A toxicity from candy-like chewable vitamin supplements for children. Pediatrics 118(2):820–824
Penniston K, Tanumihardjo SA (2006) The acute ad chronic toxic effects of Vitamin A. Am J Clin Nutr 83(2):191–201
Bender DA (1999) Non-nutritional uses of vitamin B6. Br J Nutr 81:7–20
Morrison B, Chaudhry V (2012) Medication, toxic, and vitamin-related neuropathies. Peripheral Neropathy 18(1):139–160
LeBlanc ES, Perrin N, Jr JDJ, Ballatore A, Hillier T (2013) JAMA INTERN 173 (7):585–586
Niedzielski P, Rudnicka M, Wachelka M, Kozak L, Rzany M, Wozniak M, Kaskow Z (2016) Selenium species in selenium fortified dietary supplements. Food Chem 90:454–549. https://doi.org/10.1016/j.foodchem.2015.05.125
Unceta N, Astorkie M, Abrego Z, Gómez-Caballero A, Goicolea MA, Barrio RJ (2016) A novel strategy for Cr(III) and Cr(VI) analysis in dietary supplements by speciated isotope dilution mass spectrometry. Talanta 154:255–262
LeBlanc KL, Kawamoto MS, Le P-M, Grinberg P, Nadeau K, Yang L, de Araújo Nogueira AR, Mester Z (2019) Quantitation of selenomethionine in multivitamins and selenium supplements by high performance liquid chromatography inductively-coupled plasma mass spectrometry. Food Anal Methods 12:1316–1326
Korea Research Institute of Standards and Science (2011) Certificate of Analysis, CRM # 108-10-012 Nutritional Supplements Powder for Elemental Analysis 108-10-012
Korea Research Institute of Standards and Science (2016) Certificate of Reference Material CRM # 108-10-019 Multivitamin tablets (for the analysis of organic nutrients) 108-10-019
Sander LC, Sharpless KE, Wise SA, Nelson BC, Phinney KW, Porter BJ, Rimmer CA, Thomas JB, Wood LJ, Yen JH, Duewer DL, Atkinson R, Chen P, Goldschmidt R, Wolf WR, Ho I-P, Betz JM (2011) Certification of vitamins and carotenoids in SRM 3280 multivitamin/multielement tablets. Anal Chem 83(1):99–108
Turk GC, Sharpless KE, Cleveland D, Jongsma C, Mackey EA, Marlow AF, Oflaz R, Paul RL, Sieber JR, Thompson RQ, Wood LJ, Yu LL, Zeisler R, Wise SA, Yen JH, Christopher J, Day RD, Long SE, Greene E, Harnly J, Ho I-P, Bet JM (2013) Certification of elements in and use of Standard Reference Material 3280 multivitamin/multielement tablets. J AOAC Int 96(6):1281–1287
Gonzalez CA, Choquette SJ (2016) Certificate of analysis, Standard Reference material 3280, multivitamin/multiemelent tablets
Yang L, Sturgeon RE (2009) High accuracy and precision isotope dilution mass spectrometry: an application to the determination of Mo in seawater. J Anal At Spectrom 24:1327–1335
Gao Y, Sturgeon RE, Mester Z, Hou X, Zheng C, Yang L (2015) Direct determination of trace antimony in natural waters by photochemical vapor generation ICPMS: method optimization and comparison of quantitation strategies. Anal Chem 87:7996–8004
Meija J, Pagliano E, Mester Z (2014) Coordinate swapping in standard addition graphs for analytical chemistry: a simplified path for uncertainty calculation in linear and nonlinear plots. Anal Chem 86:8563–8567
Meija J, Chartrand MMG (2017) Uncertainty evaluation in normalization of isotope delta measurement results against international referencematerials. Anal Bioanal Chem 413:1061–1069
D’Ulivo L, Yang L, Ding J, Pagliano E, Leek DM, Thibeault M-P, Mester Z (2017) Determination of cyanocobalamin by isotope dilution LC–MS/MS. Anal Chim Acta 990:103–109
Mihai O, Kawamoto MS, LeBlanc KL, Grinberg P, Nogueira ARdA, Mester Z (2019) Determination of chromium picolinate and trace hexavalent chromium in multivitamins and supplements by HPLC-ICP-QQQ-MS. J Food Compos Anal 87:103421. https://doi.org/10.1016/j.jfca.2020.103421
Picoloto RS, Doneda M, Flores ELM, Mesko MF, Flores EMM, Mello PA (2015) Simultaneous determination of bromine and iodine in milk powder for adult and infant nutrition by plasma based techniques after digestion using microwave-induced combustion. Spectrochim Acta Part B 107:86–92
Deveswaran R, Bharath S, Basavaraj B, Abraham I, Furtado S, Madhavan V (2009) Concepts and techniques of pharmaceutical powder mixing process: a current update. Res J Pharm Tech 2(2):245–249
Venables HJ, Wells JI (2010) Powder mixing. Drug Dev Ind Pharm 27(7):599–612
Li J, Wu Y (2014) Lubricants in pharmaceutical solid dosage forms. Lubricants 2:21–43
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
ISO Guide 35 (2017), Reference materials—guidance for characterization and assessment of homogeneity and stability. ISO Copyright Office, Switzerland
Evaluation of measurement data—Guide to the expression of uncertainty in measurement. JCGM 100:2008
Bureau International des Poids et Mesures (2017). https://kcdb.bipm.org/AppendixC/QM/CA/QM_CA_1.pdf
Sturgeon RE, Methven B, Willie SN, Grinberg P (2014) Assignment of purity to primary metal calibrants using pin-cell VG 9000 glow discharge mass spectrometry: a primary method with direct traceability to the SI international system of units? Metrologia 51:410–422
Grinberg P, D’ulivo L, Nadeau K, Pihillagawa I, Yang L, LeBlanc K, Mihai O, Kawamoto M, Doneda M, Pereira RM, Bizzi CA, Mello PA, Flores EMM, Mesko MF, Leek DM, Thibeault M-P, Meija J, Mester Z (2018) VITA-1: low-level multivitamin certified reference material for minerals and vitamins. National Research Council Canada, Ottawa
Grinberg P, D’ulivo L, Nadeau K, Pihillagawa I, Yang L, LeBlanc K, Mihai O, Kawamoto M, Doneda M, Pereira RM, Bizzi CA, Mello PA, Flores EMM, Mesko MF, Leek DM, Thibeault M-P, Meija J, Mester Z (2018) VITB-1: elevated-level multivitamin certified reference material for minerals and vitamins. National Research Council Canada, Ottawa
Acknowledgements
The contributions of Enea Pagliano and Phuong-Mai Le (NRC), Mayumi Kawamoto (Universidade de São Paulo, Brazil) Morgana Doneda, Rodrigo M. Pereira, Cezar A. Bizzi, Paola A. Mello, Erico M. M. Flores (Universidade Federal de Santa Maria, Brazil), and Marcia F. Mesko (Universidade Federal de Pelotas, Brazil) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grinberg, P., D’Ulivo, L., Nadeau, K. et al. Development of low and elevated level multivitamin and mineral supplement certified reference materials: VITA-1 and VITB-1. Accred Qual Assur 25, 201–220 (2020). https://doi.org/10.1007/s00769-020-01433-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-020-01433-9