Abstract
VITA-1 and VITB-1 multivitamin and mineral supplement candidate reference materials from the National Research Council Canada were analyzed for their total selenium and selenomethionine contents. Following a methanesulfonic acid reflux to extract selenomethionine from the selenized yeast in the multivitamins, analysis by high-performance liquid chromatography-inductively coupled plasma triple quadrupole mass spectrometry resulted in concentrations of 7.4 ± 3.0 μg SeMet g–1 and 16.4 ± 6.5 μg SeMet g−1 in VITA-1 and VITB-1, respectively. Twelve commercially available multivitamins and selenium supplements were analyzed following the same protocol. Seven of these were noted to contain 85–115% of the selenium stated on the label; the others ranged from 5 to 147% of the claimed amount. Only one multivitamin contained selenomethionine at a concentration above the detection limit, but the amount found in the selenium supplements matched the label claims within a reasonable level of uncertainty. For comparison, two certified reference materials—wheat gluten and egg powder, both certified for total selenium only—were also examined and it was determined that selenomethionine accounted for 58% and 25% of the selenium in these food products, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From a human health viewpoint, selenium (Se) is an interesting element in that, while first recognized as a toxic substance, it is now known for its essential role as an antioxidant as well as in a variety of other functions performed by the 25 selenoproteins in the human selenoproteome (Kryukov et al. 2003; Rayman 2012). Although Se is present naturally in soils, its concentration varies significantly worldwide, which leads to a broad range of Se concentrations in different foods. For example, the concentration of Se in cereals and grains is highly dependent on the soil conditions in which they are grown, resulting in a large variation. Fruits and vegetables generally contain relatively little Se, while seafood contains much more (Rayman 2012). Brazil nuts, in particular, are known for being very high in Se—concentrations above 100 μg/g have been measured (Lopes et al. 2015; Palmer et al. 1982). Extreme cases of Se deficiency are fairly rare, the most prominent being that of Keshan disease, an often fatal cardiomyopathy (Chinese Medical Association 1979; Whanger 2001). Se toxicity is generally discussed in an environmental context where it is a reproductive toxin in oviparous vertebrates, but has also been noted in humans living in regions with seleniferous soils—symptoms include skin and nervous system disorders, nail and hair loss, and paralysis (Johnson et al. 2010; Rayman 2012).
Moving from the extreme ends of the spectrum to more typical scenarios, the narrow range of optimal physiological Se concentration means that we typically discuss its effects on human health in a more focused and therefore much more complex manner. Recently, there has been increasing evidence supporting a “U”-shaped trend between Se status (typically measured as serum Se concentration) and all-cause mortality (Rayman 2012). A 12-year study following 13,887 American adults demonstrated a non-linear correlation between serum Se concentrations and both cancer-related and all-cause mortality. Decreased mortality rates were noted for increasing serum Se (to about 130 ng/mL), but the study concluded that higher concentrations may be linked to increased risks of mortality (Bleys et al. 2008).
Globally, the recommended daily intake of selenium averages at 53 and 60 μg/day for women and men, respectively (Rayman 2004; Rayman 2012). Health Canada (2016), for example, recommends a daily intake of 55 μg Se for adults, which is based on the values put forth by the Institute of Medicine (2000). Actual Se intake has been estimated at 98–224 μg/day in adult Canadians (Combs Jr 2001; Gissel-Nielsen 1998), which is similar to the estimated American intake of 93–134 μg/day and somewhat higher than the European average of 40 μg/day (Rayman 2012). Despite Se intake being above recommended values, the average serum Se measured in adult Americans was 125.6 ng/mL (Bleys et al. 2008)—close to the 122 ng/mL suggested to be the concentration that separates decreased from increased risk of cancer-related mortality (Rayman 2012) (130 ng/L for all-cause mortality (Bleys et al. 2008)). Therefore, many North Americans and a good portion of Europeans (Rayman 2012) could benefit from Se supplementation, and in fact, the Council for Responsible Nutrition (2016) estimates that 68% of Americans regularly take dietary supplements and 40% take multivitamins regularly (Gahche et al. 2011).
Unfortunately, there appears to be limited consistency in the preparation and labeling of many commercially available Se-containing multivitamins and supplements, and it is not uncommon for the true Se content of these products to differ significantly from their label claims. For example, Kubachka et al. (2017) observed discrepancies between labeled and measured total Se content in their study of 13 Se supplements on the American market, with one containing 162% of the declared Se and another only 70%. Similarly, of the 86 commercially available Se-containing supplements purchased in Poland and examined by Niedzielski et al. (2016), only 12 (14%) were shown to contain the amount declared on the label (± 10%). Twenty-two (26%) of the supplements contained 70% or less of the declared content (less than 30% in 10 of these samples), and eight contained 151 to 280% of the label’s declared Se. In an extreme case in 2008, a misformulated supplement containing 200 times the declared amount of Se reached the US market resulting in several cases of acute Se poisoning (MacFarquhar et al. 2010; National Institutes of Health 2018).
Clearly, there is a need for stricter monitoring during the preparation of commercial multivitamins and nutritional supplements, at least in regard to Se content. Likely, the discrepancies between labeled and actual Se content are not intentional, meaning the difficulty may lie in the validation of analytical methodologies employed by the manufacturers of these products—the availability of a certified reference material (CRM) of a similar nature could help to overcome this issue. There are a few multivitamin CRMs currently available for purchase; among these are standard reference material (SRM) 3280 (“multivitamin/multielement tablets”) from the National Institute of Standards and Technology (NIST) which is certified for total Se and MX008 (“trace elements in nutritional supplement”) from the National Metrology Institute of Australia (NMIA) which is not certified for Se but lists the total Se content as an information value. The National Research Council of Canada (NRC) is now preparing two new multivitamin, mineral supplement CRMs, VITA-1, and VITB-1, which in addition to the total Se, the selenomethionine (SeMet) content will be certified in the tablets. Due to the difference in bioavailability between Se species, supplements often contain SeMet and/or other organic Se species rather than inorganic selenite (SeO32−, Se(IV)) or selenate (SeO42−, Se(VI)), sometimes in the form of a selenized yeast, typical samples of which commonly contain SeMet as 60–84% of their total Se (Rayman 2004). The source of Se in the NRC candidate CRMs VITA-1 and VITB-1 is selenized yeast.
Here, we discuss the process involved in the extraction and measurement of SeMet in the candidate CRMs, using NRC’s SELM-1 (Se-enriched yeast CRM) for quality control. Additionally, we examine the SeMet content in 12 commercially available multivitamins and Se supplements, labeled to contain Se from a variety of sources, as well as in two food CRMs that are certified for total Se.
Materials and Methods
Health and Safety Considerations
Hydrofluoric (HF) acid should be handled with caution, inside a working fume hood, and only by individuals wearing appropriate personal protective equipment and having calcium gluconate cream readily available in case of accidental exposure. Samples digested with HF should be evaporated to near dryness inside a properly functioning fume hood.
Vitamin/Supplement/Food Samples
The candidate reference materials VITA-1 and VITB-1 and the NRC CRMs SELM-1 (certified for total Se at 2031 ± 70 μg Se g−1 and SeMet at 3190 ± 260 μg SeMet g−1 or 1284 ± 105 μg Se g−1 (Mester et al. 2006)), GLUT-1 (wheat gluten; total Se 2.58 ± 0.19 μg Se g−1), and EGGS-1 (egg powder; total Se 1.39 ± 0.17 μg Se g−1) were analyzed, as was NIST SRM-3280 (17.42 ± 0.45 μg Se g−1, or approximately 26.13 μg Se/tablet based on the average tablet mass of 1.5 g stated in the certificate).
Twelve packages of multivitamins or selenium supplements were purchased locally (Ottawa, Canada) or online. Selections were made based on the type of Se listed on the package; those listing “sodium selenite,” “sodium selenate,” or a similar inorganic form were intentionally excluded.
Standards and Reagents
l(+)-Selenomethionine (+99%, Acros Organics) was purchased from Fisher Scientific (Ottawa, Ontario) and its purity was determined in-house by quantitative nuclear magnetic resonance spectrometry. Briefly, this determination was based on NIST SRM 84L potassium hydrogen phthalate (KHP) as an internal standard (IS), which was co-dissolved with SeMet in a known mass ratio. The integrated signal area per proton per mole for the IS at 7.65 ppm (4H), including both 13C satellites, and for analyte SeMet at 2.04 ppm (5H) and 2.55 ppm (2H) were used to calculate the purity. The material was gravimetrically prepared (three replicates) in D2O (Cambridge Isotope Laboratories, Andover, MA), transferred to precision 5 mm NMR tubes (Wilmad LabGlass, Buena, NJ). 1H-qNMR spectra were acquired at 399.94 MHz (Varian Innova, Santa Clara, CA) at 23 °C. A standard 1H pulse sequence (s2pul) was employed. Following a 90° pulse (determined as 360°/4), followed by a delay of 7× T1, where T1 is the spin-lattice relaxation time. Free induction decays were employed (collecting 32 scans into 44915 points with a spectral width of 20 ppm), apodized with a decreasing exponential (0.1 Hz), Fourier transformed, then manually phased and integrated using NMR data processing software (Delta, JEOL USA, Inc.). This method is an adaptation of that which was described by Le et al. (2016).
Sodium selenite (≥ 99%) and sodium selenate decahydrate (99.999%) were purchased from Sigma Aldrich (Oakville, Ontario). High-purity deionized water (DIW) was obtained from a mixed bed ion exchange system (Millipore Corporation, Etobicoke, Ontario) fed with reverse osmosis domestic feed water. 1000 mg Se L−1 stock solutions were prepared in DIW, stored at 4 °C, and gravimetrically diluted to working standards. Hydrochloric and nitric acids were obtained by sub-boiling distillation (Milestone Inc., Shelton, Connecticut) of the reagent grade acids. Methanesulfonic acid (≥ 99.0%), ammonium acetate (≥ 98%), and acetic acid (≥ 99.7%) were purchased from Sigma Aldrich (Oakville, Ontario).
Sample Preparation
For VITA-1, VITB-1, and the commercial multivitamin and supplement samples, 20 tablets were weighed, crushed by hand using a glass mortar and pestle, and thoroughly homogenized. Samples M1, M4, S3, S4, and S5 contained powder within a water-soluble capsule; here, the capsules were opened and the powder extracted and homogenized. Due to the small size and density of sample S8 tablets, these were not crushed; instead, whole tablets were used for subsequent sample preparation. For NIST SRM-3280, thirty (30) tablets were crushed and homogenized as this was the quantity contained in one bottle of the material. Homogenized material was stored in glass vials, in the dark at room temperature.
The protocol for the extraction of SeMet from the selenized yeast in the samples was optimized from that originally described by Simpson et al. (1976), as described by Yang and coworkers (Yang et al. 2004). Approximately 250 mg of powdered sample was accurately weighed and transferred to a flask containing 24 mL of 4.0 M methanesulfonic acid (MSA). Aliquots of each material were spiked with an appropriate volume of SeMet standard solution. Samples were refluxed for 16 h, allowed to cool, filtered through 0.2-μm syringe filters, and stored in glass vials at 4 °C until analysis. Fifteen subsamples were analyzed from the crushed and homogenized batches of both VITA-1 and VITB-1; all other samples were analyzed in (at least) triplicate. Another extraction efficiency (by spike recovery) test was conducted for VITA-1 and VITB-1. Refluxed samples were split into two aliquots before filtration, one of which was spiked with SeMet (at approximately 1.5× the native concentration), allowed to equilibrate for about 10 min, and then filtered. Based on this, it could be determined whether any SeMet released from Se-containing proteins during reflux adsorbed to the solid material which remained in the samples after they were cooled and was filtered out prior to analysis.
To examine the easily leachable Se in the crushed material, two additional aliquots of each vitamin or CRM sample (approximately 250 mg) were weighed into separate glass vials and 20 mL of DIW or 0.1 M HCl was added. These vials were inverted several times to mix the solid and liquid phases and were allowed to sit at room temperature (~ 22 °C) for 48 h before 0.2-μm filtration. The concentrations of Se(IV) and Se(VI) were quantified in these filtrates.
HPLC-ICP-QQQ-MS Analysis
Sample dilutions and standard additions were prepared using a SeMet standard solution, which was traceable to the SI. Quantification was performed by standard addition: sample concentration was estimated by a rough external calibration and aliquots were spiked at concentrations of SeMet approximately matching and double the native analyte concentration. For VITA-1 and VITB-1 samples, an extra standard addition calibration point was added at four times the native SeMet concentration. Aqueous extracts were quantified by external calibration using SeMet, Se(IV), and Se(VI) standards. These samples were then analyzed by high-performance liquid chromatography with inductively coupled plasma triple quadrupole mass spectrometry (HPLC-ICP-QQQ-MS) using an Agilent Technologies 1200 Series HPLC (Santa Clara, California; see Table 1 for parameters) coupled to an Agilent Technologies 8800 ICP-QQQ-MS using H2 as a cell gas to eliminate polyatomic interferences on the masses of Se. Transitions corresponding to 77Se, 78Se, 80Se, and 82Se (Q1 > Q3: 77 > 77, 78 > 78, 80 > 80, and 82 > 82) were monitored to confirm the presence of Se; 80Se was used for quantification. The chromatographic methods used for these analyses are modified from those described by McSheehy et al. (2005).
Total Se Analysis
The digestion method used for VITA-1 and VITB-1 was optimized and validated by Grinberg et al. (2019). Briefly, whole vitamin pills (of approximate mass 2.6 g) were accurately weighed into pre-cleaned Teflon microwave digestion vessels. The contents were then gravimetrically spiked with a known amount of enriched 82Se isotope solution to achieve an approximately 1:1 ratio for 78Se/82Se. Similarly, three procedural blanks were prepared by addition of 10% of the mass of enriched isotope spike used for the samples. After the addition of 15 mL HNO3, 2 mL H, and 3 mL H2O2, contents were heated on a hot plate at 80 °C for 24 h. Then, 3 mL of HCl was added to each vessel prior to the microwave digestion, which involved a 5-min ramp to 360 W, which was held for 5 min, then another 5 min ramp to 850 W, which was held for 20 min, ending with a 35-min cooling cycle at 0 W. The contents of each microwave vessel were transferred into Teflon tubes and heated to evaporate until 2–3 mL remained. Another 4 mL of HNO3 and 12 mL of HCl were added to the tubes, which were then heated at 60 °C for 60 min. Contents were diluted with DIW to 500 g. Sample solutions were left to stand for at least 5 days prior to analysis by ICP-QQQ-MS using O2 as cell gas (monitoring the Se+ > SeO+ transition; Q1 > Q3: 77 > 93, 78 > 94, 80 > 96, and 82 > 98). For quality control, sample preparation was the same as described above except that for SRM 3280, a total of 25 tablets were crushed and homogenized (as prescribed by the CRM producer) and 1 g of subsamples was analyzed, and for DORM-4 CRM (NRC), 0.25 g was used.
For the commercial multivitamin or Se supplement samples, approximately 250 mg of crushed material was placed into a pre-cleaned microwave digestion vessel to which the following was added: 6 mL HNO3, 0.5 mL H2O2, 0.5 mL HF, and 0.5 mL HCl. Vessels were sealed and allowed to pre-digest at room temperature for 3 days, then placed into the Anton Paar Multiwave 3000 Microwave (Graz, Austria) run on the following program: a 5-min ramp to 800 W (hold 10 min), a 5-min ramp to 1400 W (hold for 10 min), followed by 35 min of cooling at 0 W. Samples were transferred to Teflon tubes, evaporated until about 1 mL remained, then gravimetrically diluted to approximately 60 mL in DIW. Subsequent dilutions were prepared with 1% HNO3 for analysis by ICP-QQQ-MS. Here, H2 and O2 were both used, separately, as cell gasses in two separate total Se analyses to ensure all possible interferences were eliminated. O2 cell gas results were used for final quantitation. Again, transitions corresponding to 77Se, 78Se, 80Se, and 82Se were monitored.
Results and Discussion
Analytical Figures of Merit
Using the qNMR protocol described above, the purity of the SeMet standard was determined to be 0.9819 ± 0.0031 (where a value of 1 represents a pure material). SeMet mass fractions for the VITA-1 and VITB-1 samples, which were determined using this primary standard, are traceable to the International System of Units (SI).
The instrumental detection limit (IDL) was defined as the concentration at which the SeMet peak is present and displaying a signal-to-noise ratio of 3.0. To determine this, a series of SeMet standard solutions of varying concentrations were analyzed and the peak height (“signal”) was measured from the mid-point of the noise. The full range of the noise was measured directly surrounding the chromatographic peak. By this definition, an IDL of 0.00018 μg Se kg−1 (0.00045 μg SeMet g−1) was determined for the method using anion exchange chromatography (AEC) with ICP-QQQ-MS. Based on a fivefold dilution of the refluxed sample (as was used for VITA-1 and VITB-1 samples), an average sample mass of 0.25 g, and average total reflux mass of 26 g, the method detection limit (MDL) for SeMet was calculated to be 0.093 μg Se g−1 (0.23 μg SeMet g−1). The instrumental response to SeMet remained linear from this detection limit to a concentration of approximately 10 μg Se g−1 (24.8 μg SeMet g−1) in the analyzed sample. Slightly higher detection limits were obtained for the reversed phase (RP)-HPLC method where the IDL was determined to be 0.00063 μg Se g−1 (0.0016 μg SeMet g−1) and the MDL 0.33 μg Se g−1 (0.82 μg SeMet g−1), but the response remained linear to 10 μg Se g−1. Chromatograms of SeMet standard solutions of concentrations just above and below this IDL are shown in Fig. 1.
For quality control, SELM-1 was quantified by both chromatographic ICP-QQQ-MS methods. The analysis of refluxed SELM-1 samples by both anion exchange (AEC) and RP-HPLC showed consistent results: SeMet concentrations of 1260 ± 61 μg Se g−1 (3120 ± 150 μg SeMet g−1) and 1200 ± 80 μg Se g−1 (2990 ± 200 μg SeMet g−1) were calculated, respectively. The certified value is 1280 ± 110 μg Se (as SeMet) g−1 (3190 ± 260 μg SeMet g−1).
The observation of coelution between a chemical species in a sample and a standard following two separate chromatographic methods is an excellent means by which the identity of said species can be confirmed in the absence of molecular mass spectrometric detection. Here, AEC was useful for the analysis of SeMet in samples which also contain Se(IV) and Se(VI) as all three are well resolved, with SeMet eluting early and Se(VI) being retained strongly. Conversely, Se(IV) and Se(VI) cannot be examined directly by RP-HPLC as they (co)elute in or near the void volume, though SeMet can be efficiently separated from other low molecular weight organic Se species.
Total Se and SeMet in VITA-1 and VITB-1
Microwave-assisted acid digests of crushed vitamin tablets, followed by ICP-MS analysis revealed that the total Se concentrations in VITA-1 and VITB-1 were 4.2 ± 1.4 μg Se g−1 and 8.2 ± 0.8 μg Se g−1, respectively. The quality control samples also examined during this analysis (NIST SRM 3280, certified at 17.42 ± 0.45 μg Se g−1) showed good agreement with certified values; a concentration of 17.2 ± 1.3 μg Se g−1 was measured.
The reflux extraction protocol using 4 M MSA was previously shown to be efficient for the extraction of SeMet from the selenized yeast CRM SELM-1; when compared to 13 other commonly used methods, MSA was determined to be the most efficient for the extraction of both SeMet and methionine from selenized yeast (Yang et al. 2004). The different sample matrices of the multivitamins did not appear to have a significant effect on the extraction efficiency of the reflux when a similar sample mass (~ 250 mg) was used. Here, the extraction efficiency test showed slightly greater than 100% recovery; therefore, the calculated SeMet concentrations in VITA-1 and VITB-1 did not require correction factors to account for extraction efficiency.
The results of the standard addition quantification are displayed in Table 2. The reported uncertainties are based on the combination of batch characterization (uchar), uncertainties related to possible variations between tablets (uhom), and the uncertainty related to the inconsistencies between various measurement methods (umethod). Since SeMet concentrations in VITA-1 and VITB-1 were fairly close to the method detection limit, uchar accounts for a considerable portion of the uncertainty estimate. Conversely, the characterization of SeMet involved only one measurement method, so umethod does not contribute to the combined uncertainty.
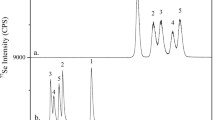
In VITA-1 and VITB-1, the fraction of Se in the form of SeMet is consistent with the use of high-Se yeast in the multivitamin formulation. A sample chromatogram can be found in Fig. 2a, which shows coelution between the sample and an analytical spike. An examination of the easily leachable Se was conducted using both DIW and 0.1 M HCl, but analysis of these extracts did not demonstrate the presence of any Se(IV) or Se(VI) at concentrations above the detection limit.
SeMet in Food Certified Reference Materials
The SeMet contents of two food CRMs, both of which are certified for total Se, were evaluated. The egg powder CRM, EGGS-1, contained 0.88 ± 0.19 μg SeMet g−1, which corresponds to about 25% of its total Se content. The wheat gluten material, GLUT-1, contained both a higher overall amount and proportion of SeMet at 3.70 ± 0.60 μg SeMet g−1, or 58% of the total Se—while the SeMet content of GLUT-1 is not certified, past analysis of this material provided similar results (Wolf et al. 2001; Wolf and Zainal 2002).
Neither of the two food CRMs are Se-enriched, meaning their Se speciation corresponds to the element’s natural distribution rather than being indicative of a stress response. It is not surprising that SeMet is the major species present in GLUT-1 as this material is 82.7% protein (as noted on the certificate) and high-protein foods commonly contain relatively high levels of selenoamino acids compared to other inorganic and non-amino organic Se species (Ullah et al. 2018). Conversely, grain of a wheat plant cultivated in seleniferous soil contained no observable SeMet, but instead 32% of the Se content was from selenocysteine (SeCys) while the remaining 68% was methylselenocysteine (MeSeCys) (Eiche et al. 2015). The production of SeCys by plants is a genetically encoded contribution to selenoproteins, but the production of MeSeCys has been shown to be a detoxification mechanism as large pools of this compound can be accumulated in plants without observable toxic effects, and further metabolism of MeSeCys to volatile dimethyl diselenide (DMDSe) allows it to be excreted (Pilon-Smits and Quinn 2010). Additionally, changes in the chemistry of the soil in which the plant is grown can alter the speciation of the Se formed by the plant’s metabolism (Duncan et al. 2017).
EGGS-1 has a lower protein content at 39.2%, which may account for the lower SeMet fraction (25%) in this material than in GLUT-1. Analysis of fresh (non Se-enriched) eggs has shown that 53–71% of the Se in the white is present in the form of SeMet, while that value is 33–35% in the yolk, giving an overall SeMet content of 22–37% of the total Se in the fresh eggs. Unlike the results observed for wheat, Se-enriched eggs were noted to contain a higher percentage of SeMet (compared to natural eggs), which was balanced by a lower SeCys proportion (Lipiec et al. 2010).
Total Se and SeMet in Commercial Multivitamins and Se Supplements
Spike recovery experiments and the analysis of CRMs (SELM-1 and NIST SRM 3280) demonstrated that the digestion protocol followed for the vitamin and supplement samples provided quantitative recovery of the Se within the samples. Analysis showed that seven of the 12 multivitamins and supplements examined contained a total Se amount within 85–115% of the label claim, as is shown in Table 3. Two more fell within 115–120% of the claimed amount. Two were slightly outside of these ranges at 68% and 147%, while one multivitamin (sample M4) contained less than 5% of the amount of Se that was stated on the label. As was discussed above, these large discrepancies are not unique to this investigation and similar results have been observed in studies of the American (Kubachka et al. 2017) and Polish (Niedzielski et al. 2016) markets, demonstrating that inaccurate label claims are a widespread problem. In one case, the total Se measured was approximately 9 times higher than the declared value (Kozak et al. 2012), and there have been reports of 200 times the claimed amount being present in a formulation (MacFarquhar et al. 2010; National Institutes of Health 2018), meaning that the results seen here do not display the most extreme cases of deviation from label claims.
The gentle extractions performed with water and 0.1 M HCl serve to examine the easily leachable Se in the vitamin pills. The water extraction, in particular, minimizes the potential for Se within yeast degrading to inorganic Se (Kubachka et al. 2017). The intention of this experiment was not to calculate a complete mass balance and determine the inorganic Se speciation in the commercial vitamins and supplements, but rather to determine whether inorganic Se had been added to any of these products. It has been noted that some products claiming to be Se yeasts contain high proportions of Se(IV) not bound to the yeast, and some appear to be a simple mixture of yeast and Se(IV) (i.e., the Se is not actually incorporated in, or bound to, the yeast) (Rayman 2004). Here, inorganic Se was found in the aqueous extracts of two products claiming to contain selenized yeast. In sample M2, 18% of the measured total Se was observed as Se(IV) and Se(VI) in the water extract, while in sample S3, less than 0.1% of the supplement’s total Se content was extracted as Se(VI) into 0.1 M HCl. In comparison, Ayouni et al. (2007) performed sequential extractions on supplements containing selenized yeast and noted that in the first step (“water” extract: 10 mM tris-HCl, pH 8), 12.5% of the total Se was measured, part of which accounted for 1% of the total recovered SeMet—this was based on the subsequent steps of SDS, driselase, and pronase extractions, which overall achieved an 85% recovery of the total Se.
Table 3 also shows that supplements labeled to contain Se as an “amino acid chelate” or “hydrolyzed vegetable protein chelate” do not contain SeMet, but rather inorganic Se, at various degrees of aqueous solubility. Such label claims are potentially misleading and their ambiguity may cause consumers to believe that the products contain selenamino acids when this is not the case, as has been discussed by Amoako et al. (2009) who also observed Se(IV) and Se(VI) in these types of samples. Similarly, in their analysis of a supplement containing Se as an “amino acid chelate,” Kubachka et al. (2017) measured a total Se content slightly lower than the label claim, but observed no SeMet in extracts using water, 4 M MSA, or 0.1 M NaOH. They did note a small amount of inorganic Se (~ 4.5% of measured total Se) in the NaOH extract, but the remainder was not recovered. Conversely, the sample labeled “amino acid complex” yielded 82% of the total Se as inorganic Se during the NaOH extraction (note that this inorganic Se is actually higher than the label’s claim value). Here, our results were similar, with no observed SeMet extracted from sample S1 (Se as “amino acid chelate”) and only 35% of the total Se appearing in the water extract as Se(IV) (25%) and Se(VI) (10%). Samples M1 and S2, which both contained Se labeled as “hydrolyzed vegetable protein chelate,” yielded no detectable SeMet but demonstrated quantitative recovery of the total measured Se in the water extracts—in both cases, the Se(IV) content was higher than the Se(VI), as shown in Fig. 3 for sample S2.
In the two selenized yeast-containing supplements where SeMet was measured in the refluxed products, this species accounted for 61% and 72% of the observed total Se (samples S3 and S6, respectively). This is similar to the fraction of SeMet measured in SELM-1 (Mester et al. 2006) and SelenoExcell® yeast (Uden et al. 2004), as well as in VITA-1 and VITB-1. The difference between measured total Se and SeMet in the refluxed yeast-containing samples may be due to the presence of Se0 or inorganic Se incorporated into the biomass, which may not be extracted with MSA (Barrientos et al. 2016). Additionally, while the majority of the Se in most selenized yeasts is typically in the form of SeMet, various other Se-containing species are also known to be produced during metabolism (Bierla et al. 2012; Rao et al. 2010; Uden et al. 2004). For the samples noted to contain Se as SeMet itself (rather than SeMet in yeast), this amount matched the total Se fairly well—an example of a chromatogram corresponding to sample S8 is displayed in Fig. 2b.
Sample M3 presents an interesting case—while the total Se concentration measured is only slightly lower than stated on the multivitamin’s label, the species identified did not match the label. Se(VI) was recovered quantitatively in the 0.1 M HCl extract. The water extract solubilized a smaller amount of Se(VI), corresponding to 37 μg Se/tablet, but neither Se(IV) nor SeMet was observed following either aqueous extraction method or the MSA reflux. While inaccurate labeling of this degree does not appear to be particularly widespread, other researchers have occasionally observed similar results; for example, Güler and coworkers (2010) noted that in one of the three Se-supplemented vitamin tablets they examined, while the amount stated on the label was correct, the species identified was Se(VI) rather than the SeMet that was claimed to be in the supplement. Conversely, in one supplement analyzed, Gosetti et al. (2007) found no detectable SeMet (despite the label claim) and only recovered 37% of the Se stated to be in the product.
Selenium Speciation in Nutritional Supplements
As discussed briefly in the introduction, the speciation of ingested Se appears to play an important role in the overall Se status of individuals, though the details and the mechanisms involved are still being investigated. An early study conducted by Clark et al. (1996) demonstrated that daily supplementation with 200 μg Se (as selenized yeast) significantly reduced total cancer mortality, total cancer incidence, and incidences of prostate, lung, and colorectal cancers when compared to a placebo group. However, as has been noted here (and elsewhere), not all Se present in yeast is in the form of SeMet, making it difficult to determine if the observed effect(s) were due to SeMet, a different species present in a smaller concentration, or some combination. Based on this, some later studies chose to supplement participants with SeMet, rather than yeast—a noteworthy example is the Selenium and Vitamin E Cancer Prevention Trial (SELECT) which included 35,533 North American men who were given 200 μg Se as SeMet and/or vitamin E or a placebo (Lippman et al. 2009). The results of this study showed that the administration SeMet, alone or combined with vitamin E, did not have a significant effect on the incidence of prostate cancer; however, as Rayman (2012) points out, the trial included very few participants with plasma Se less than 106 μg L−1, which is the baseline concentration above which Se supplementation had previously been shown to be effective in decreasing prostate cancer risk
An examination of Se status without further supplementation has resulted in the U-shaped relationship mentioned previously, where a serum Se concentration of 130 ng L−1 was noted to separate decreased from increased risk of all-cause mortality (Bleys et al. 2008). For individuals looking to raise, their serum Se to optimal levels supplementation is an ideal method but, as already discussed, there are several different forms of Se commercially available for this purpose. In fact, Health Canada’s Multi-Vitamin/Mineral Supplements Monograph (Health Canada 2016) lists 16 different source materials which could be used as a source of Se in these types of products. Inorganic Se—Se(IV) and Se(VI)—is typically the most inexpensive (Rayman 2004), but is also generally less desirable in a nutritional supplement. Selenized yeast has been shown to be highly bioavailable; for example, Sloth et al. (2003) demonstrated that the apparent absorption of Se was 90% after the administration of a single dose of 77Se-labeled selenized yeast, and the overall retention after 72 h was 75%. SeMet appears to be more bioavailable than selenized yeast (McGuire et al. 1993), demonstrating that yeast needs to be metabolized to yield the selenoamino acid. Additionally, SeMet is retained in the human body approximately 2.5× longer than Se(IV), allowing for longer maintenance of selenoenzymes during periods of Se depletion (Rayman et al. 2008). Clearly, SeMet and selenized yeast-containing supplements can be beneficial to achieve the goal of raising the Se status of various individuals.
Conclusions
Herein, two selenized yeast-containing multivitamin and mineral supplement candidate reference materials, VITA-1 and VITB-1, were analyzed for their total Se and SeMet contents. These materials will serve as an important quality control for the analysis of not only Se and SeMet, but also trace elements, with values available for 23 additional elements, and vitamins, with values for 15 water- and fat-soluble vitamins (Grinberg et al. 2019). The examination of 12 commercially available multivitamins and Se supplements demonstrated that the total content as well as the Se species stated on the label is not always what is actually present within the product. Overall, SeMet was determined to be present in most of the supplements claiming to contain either SeMet or selenized yeast, and the fraction of SeMet in the yeast-based products was consistent with typical selenized yeasts. Since these yeasts are known to contain a wide variety of other Se species, it could be beneficial to conduct future studies examining Se nutritional supplements to quantify other species which have shown potential chemopreventative properties.
References
Amoako P, Uden PC, Tyson JF (2009) Speciation of selenium dietary supplements: formation of S-(methylseleno)cysteine and other selenium compounds. Anal Chim Acta 652:315–323. https://doi.org/10.1016/j.aca.2009.08.013
Ayouni L, Barbier F, Imbert J-L, Lantéri P, Grenier-Loustalot M-F (2007) Speciation of selenium in a commercial dietary supplement by liquid chromatography coupled with inductively coupled plasma–mass spectrometry (ICP-MS). J Toxicol Environ Health, A 70:735–741. https://doi.org/10.1080/15287390701236314
Barrientos EY, Wrobel K, Guzman JCT, Escobosa ARC, Wrobel K (2016) Determination of SeMet and Se(IV) in biofortified yeast by ion-pair reversed phase liquid chromatography-hydride generation-microwave induced nitrogen plasma atomic emission spectrometry (HPLC-HG-MP-AES). J Anal At Spectrom 31:203–211. https://doi.org/10.1039/c5ja00276a
Bierla K, Szpunar J, Yiannikouris A, Lobinski R (2012) Comprehensive speciation of selenium in selenium-rich yeast. Trends Anal Chem 41:122–132. https://doi.org/10.1016/j.trac.2012.08.006
Bleys J, Navas-Acien A, Guallar E (2008) Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med 168:404–410. https://doi.org/10.1001/archinternmed.2007.74
Health Canada (2016) Multi-vitamin/mineral supplements monograph. http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=multi_vitmin_suppl. Accessed 20 March 2018
Chinese Medical Association (1979) Observations on effect of sodium selenite in prevention of Keshan disease. Chin Med J 92:471–476
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Park HK, Sanders BB Jr, Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. a randomized controlled trial J Am Med Assoc 276:1957–1963. https://doi.org/10.1001/jama.1996.03540240035027
Combs GF Jr (2001) Selenium in global food systems. Br J Nutr 85:517–547. https://doi.org/10.1079/BJN2000280
Council for Responsible Nutrition (2016) 2015 CRN consumer survey on dietary supplements. http://www.crnusa.org/CRN-consumersurvey-archives/2015/. Accessed 5 April 2018
Duncan EG, Maher WA, Jagtap R, Krikowa F, Roper MM, O’Sullivan CA (2017) Selenium speciation in wheat grain varies in the presence of nitrogen and sulphur fertilisers. Environ Geochem Health 39:955–966. https://doi.org/10.1007/s10653-016-9857-6
Eiche E, Bardelli F, Nothsteina AK, Charlet L, Göttlicher J, Steininger R, Dhillond KS, Sadana US (2015) Selenium distribution and speciation in plant parts of wheat (Triticum aestivum) and Indian mustard (Brassica juncea) from a seleniferous area of Punjab, India. Sci Total Environ 505:952–961. https://doi.org/10.1016/j.scitotenv.2014.10.080
Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C (2011) Dietary supplement use among U.S. adults has increased since NHANES III. Centres for Disease Control and Prevention, Hyattsville, Maryland, pp 1988–1994
Gissel-Nielsen G (1998) Effects of selenium supplementation of field crops. In: Frankenberger WT Jr, Engberg RA (eds) Environmental Chemistry of Selenium. Marcek Dekker, New York, pp 99–112
Gosetti F, Frascarolo P, Polati S, Medana C, Gianotti V, Palma P, Aigotti R, Baiocchi C, Gennaro MC (2007) Speciation of selenium in diet supplements by HPLC–MS/MS methods. Food Chem 105:1738–1747. https://doi.org/10.1016/j.foodchem.2007.04.072
Grinberg P, D’Ulivo L, Nadeau K, Gedara Pihillagawa I, Mihai O, LeBlanc K, Yang L, Meija J, Mester Z (2019) Development of low and high level Multivitamin and mineral supplement Certified reference materials: VITA-1 and VITB-1. In Preparation
Güler N, Maden M, Bakırdere S, Ataman OY, Volkan M (2010) Speciation of selenium in vitamin tablets using spectrofluorometry following cloud point extraction. Food Chem 129:1793–1799. https://doi.org/10.1016/j.foodchem.2011.05.007
Institute of Medicine (2000) Dietary reference intakes: vitamin C, vitamin E, selenium, and carotenoids. National Academy Press, Washington, D.C.
Johnson CC, Fordyce FM, Rayman MP (2010) Symposium on ‘geographical and geological influences on nutrition’ factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc Nutr Soc 69:119–132. https://doi.org/10.1017/S0029665109991807
Kozak L, Rudnicka M, Niedzielski P (2012) Determination of inorganic selenium species in dietary supplements by hyphenated analytical system HPLC-HG-AAS. Food Anal Methods 5:1237–1243. https://doi.org/10.1007/s12161-012-9365-y
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443. https://doi.org/10.1126/science.1083516
Kubachka KM, Hanley T, Mantha M, Wilson RA, Falconer TM, Kassa Z, Oliveira A, Landero J, Caruso J (2017) Evaluation of selenium in dietary supplements using elemental speciation. Food Chem 218:313–320. https://doi.org/10.1016/j.foodchem.2016.08.086
Le P-M, Ding J, Leek DM, Mester Z, Robertson G, Windust A, Meija J (2016) Determination of chemical purity and isotopic composition of natural and carbon-13-labeled arsenobetaine bromide standards by quantitative 1H-NMR. Anal Bioanal Chem 408:7413–7421. https://doi.org/10.1007/s00216-016-9827-y
Lipiec E, Siara G, Bierla K, Ouerdane L, Szpunar J (2010) Determination of selenomethionine, selenocysteine, and inorganic selenium in eggs by HPLC–inductively coupled plasma mass spectrometry. Anal Bioanal Chem 397:731–741. https://doi.org/10.1007/s00216-010-3544-8
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Baker LH, Coltman CA (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Am Med Assoc 301:39–51. https://doi.org/10.1001/jama.2008.864
Lopes GS, Silva FLF, Grinberg P, Sturgeon RE (2015) An evaluation of the use of formic acid for extraction of trace elements from Brazil nut and babassu coconut and its suitability for multi-element determination by ICP-MS. J Braz Chem Soc 27:1229–1235. https://doi.org/10.5935/0103-5053.20160018
MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, Burk RF, Dunn JR, Green AL, Hammond R, Schaffner W, Jones TF (2010) Acute selenium toxicity associated with a dietary supplement. Arch Intern Med 170:256–261. https://doi.org/10.1001/archinternmed.2009.495
McGuire MK, Lurgert SL, Milner JA, Glass L, Kummer R, Deering R, Boucek R, Picciano MF (1993) Selenium status of infants is influenced by supplementation of formula or maternal diets. Am J Clin Nutr 58:643–648. https://doi.org/10.1093/ajcn/58.5.643
McSheehy S, Yang L, Sturgeon R, Mester Z (2005) Determination of methionine and selenomethionine in selenium-enriched yeast by species-specific isotope dilution with liquid chromatography-mass spectrometry and inductively coupled plasma mass spectrometry detection. Anal Chem 77:344–349. https://doi.org/10.1021/ac048637e
Mester Z, Willie S, Yang L, Sturgeon R, Caruso JA, Fernández ML, Fodor P, Goldschmidt RJ, Goenaga-Infante H, Lobinski R, Maxwell P, McSheehy S, Polatajko A, Sadi BBM, Sanz-Medel A, Scriver C, Szpunar J, Wahlen R, Wolf W (2006) Certification of a new selenized yeast reference material (SELM-1) for methionine, selenomethinone and total selenium content and its use in an intercomparison exercise for quantifying these analytes. Anal Bioanal Chem 385:168–180. https://doi.org/10.4224/crm.2010.selm-1
National Institutes of Health (2018) Selenium: fact sheet for health professionals https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/. Accessed May 29, 2018
Niedzielski P, Rudnicka M, Wachelka M, Kozak L, Rzany M, Wozniak M, Kaskow Z (2016) Selenium species in selenium fortified dietary supplements. Food Chem 190:454–459. https://doi.org/10.1016/j.foodchem.2015.05.125
Palmer IS, Herr A, Nelson T (1982) Toxicity of selenium in brazil nuts to rats. J Food Sci 47:1595–1597. https://doi.org/10.1111/j.1365-2621.1982.tb04990.x
Pilon-Smits EAH, Quinn CF (2010) Selenium metabolism in plants. In: Hell R, Mendel R-R (eds) Cell biology of metals and nutrients. Springer-Verlag, Berlin, pp 225–241
Rao Y, McCooeye M, Windust A, Bramanti E, D’Ulivo A, Mester Z (2010) Mapping of selenium metabolic pathway in yeast by liquid chromatography-orbitrap mass spectrometry. Anal Chem 82:8121–8130. https://doi.org/10.1021/ac1011798
Rayman MP (2004) The use of high-selenium yeast to raise selenium status: how does it measure up? Br J Nutr 92:557–573. https://doi.org/10.1079/BJN20041251
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Rayman MP, Goenaga-Infante H, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100:238–253. https://doi.org/10.1017/S0007114508922522
Simpson RJ, Neuberger MR, Liu T-Y (1976) Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem 251:1936–1940
Sloth JJ, Larsen EH, Büget SH, Moesgaard S (2003) Determination of total selenium 77Se in isotopically enriched human samples by ICP-dynamic reaction cell-MS. J Anal At Spectrom 18:317–322. https://doi.org/10.1039/b209585h
Uden PC, Boakye HT, Kahakachchi C, Hafezi R, Nolibos P, Block E, Johnson S, Tyson JF (2004) Element selective characterization of stability and reactivity of selenium species in selenized yeast. J Anal At Spectrom 19:65–73. https://doi.org/10.1039/B307508G
Ullah H, Liu G, Yousaf B, Ali MU, Abbas Q, Munir MAM, Mian MM (2018) Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis. Ecotoxicol Environ Saf 149:291–306. https://doi.org/10.1016/j.ecoenv.2017.11.056
Whanger P (2001) Commentary: selenium. J Trace Elem Exp Med 14:221–226. https://doi.org/10.1002/jtra.1031
Wolf W, Zainal H, Yager B (2001) Selenomethionine content of candidate reference materials. Fresenius J Anal Chem 370:286–290. https://doi.org/10.1007/s002160100829
Wolf WR, Zainal H (2002) Methylseleno-amino acid content of food materials by stable isotope dilution mass spectrometry. Food Nutr Bull 23:120–123
Yang L, Sturgeon RE, McSheehy S, Mester Z (2004) Comparison of extraction methods for quantitation of methionine and selenomethionine in yeast by species specific isotope dilution gas chromatography-mass spectrometry. J Chromatogr A 1055:177–184. https://doi.org/10.1016/j.chroma.2004.09.018
Funding
M. Kawamoto is grateful for the grants and fellowships provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brazilian Federal Agency for Support and Evaluation of Graduate Education) under the Process PDSE-88881.131772/2016-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kelly L. LeBlanc declares that she has no conflict of interest. Mayumi S. Kawamoto declares that she has no conflict of interest. Phuong-Mai Le declares that she has no conflict of interest. Patricia Grinberg declares that she has no conflict of interest. Kenny Nadeau declares that he has no conflict of interest. Lu Yang declares that she has no conflict of interest. Ana Rita De Araújo Nogueira declares that she has no conflict of interest. Zoltán Mester declares that he has no conflict of interest.
Ethical Approval
This study did not involve any experiments with humans or animals.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
LeBlanc, K.L., Kawamoto, M.S., Le, PM. et al. Quantitation of Selenomethionine in Multivitamins and Selenium Supplements by High Performance Liquid Chromatography Inductively-Coupled Plasma Mass Spectrometry. Food Anal. Methods 12, 1316–1326 (2019). https://doi.org/10.1007/s12161-019-01442-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01442-6