Abstract
Analytical techniques in the sucroenergetic sector can now for the first time be supported by an innovative matrix-matching reference material produced from sugarcane leaves. This will further enhance the validity of the measurement results and contribute significantly to the quality assurance of plant nutrition analysis. The candidate reference material obtained had a low residual moisture and appropriate particle size. Neutron activation analysis was used to determine the mass fraction of Al, Br, Ca, Cl, Ce, Co, Cs, Cu, Fe, Hf, K, La, Mg, Mn, Mo, Na, P, Rb, Sb, Sc, Sm, Sr, Th, V and Zn, and the Kjeldahl method for N. Statistical techniques were applied to evaluate minimum sample mass and within-bottle homogeneity (ANOVA, Tukey test and PCA) and between-bottles homogeneity (ANOVA). It is demonstrated in this paper that the material is homogeneous using 50 mg mass sample for most chemical elements. However, sample masses of 200 mg or greater are required for Co, Na, Sb and V.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is one of the most important economic crops in more than 100 countries worldwide [1]. Projections for the next decade show that the world production of sugarcane will need to increase from 1.8 billion tons in 2015 to 2.2 billion tons in 2024, to accommodate the predicted sugar consumption of 214 million tons [2]. Brazil has been the premier producer of sugarcane for decades, being responsible for half of all sugar traded worldwide. The Food and Agriculture Organization of the United Nations (FAO) estimates that by 2024 the sugarcane production in the country needs to reach 884 million tons per year, just to meet the rising demand and to maintain Brazil’s leading position on the sugar market2. More than expanding the area cultivated with sugarcane, it will be necessary to improve the productivity to cope with the market expansion. The efforts for improvement involve the joint action of different research areas and the introduction of new technologies aiming at developing sugarcane varieties including transgenic ones, demanding appropriate nutritional status, sugar content, yield, and resistance to disease, pests and climate change [3, 4].
For optimum growth and production, nutrients should be adequately provided to the sugarcane plants, since either low or high levels of nutrients can cause physiological imbalances and reduction of productivity. Soil analysis is commonly used for evaluating fertility parameters and availability of nutrients before sugarcane cultivation. Foliage analysis is fundamental for evaluating the ongoing nutritional status of sugarcane plants in the various growing stages since it can assess whether the uptake of nutrients is running at adequate levels. For a correct evaluation of the nutritional status, sugarcane foliage analysis, as any other measurement, requires validated methods capable of producing valid results of known accuracy.
Certified reference materials (CRMs) are essential tools for method validation, metrological traceability and quality control. The composition of the CRM selected should match, as closely as possible, the matrix of samples to be analyzed and the properties to be measured [5, 6]. A survey in the international database for CRMs (COMAR) shows that there is no CRM with similar composition of sugarcane leaves for nutritional studies [7]. In fact, due to its different capacity for extracting nutrients from soil, mainly K, Si, Ca and S, sugarcane usually presents higher concentrations of nutrients than those found in other plant leaves [8]. Hence, a candidate reference material of sugarcane leaves was developed in the Radioisotopes Laboratory of the Nuclear Energy Center for Agriculture of the University of São Paulo following the recommendation established by ISO 17034 and ISO Guide 35 [9, 10], to support the analytical measurements performed in the Brazilian laboratories of the sucroenergetic sector.
A critical point in the preparation of a reference material is related to the representativeness of the small analytical portions taken for measurement. Natural matrices are not completely homogeneous, therefore adequate procedures for grinding and mixing solid materials are necessary for improving the homogeneity as much as possible. In general, the homogeneity depends on the distribution, shape, and density of particle size, while small particles improve the sample representativeness and minimize the sampling uncertainty [11,12,13].
The ISO Guide 35 recommends that homogeneity studies of candidate reference materials should comprise the evaluation of variability within and between the units produced (bottles). As a rule, the number of bottles included in the homogeneity study should be representative of the total batch. Because of that, larger batches require the evaluation of more units, with a minimum of ten units for between-bottles and one for within-bottle testing [10, 14, 15]. Plant reference material should also have low residual moisture to avoid degradation and to extend the stability. In general, 10 % of moisture is considered the maximum level to prevent biological degradation and to guarantee the homogeneity and stability [16].
Neutron activation analysis (NAA) is a nondestructive technique widely used for homogeneity evaluation of solid materials due to its multi-element capacity, high metrological quality and well-known sources of error, which allows the determination of the contribution of each component of uncertainty to the variability of results [17, 18]. The Kjeldahl method is the most commonly used for measuring N in certified reference materials [19, 20].
Within this context, this study intended to evaluate the homogeneity of nutritional and trace elements and the minimum sample mass of the candidate reference material of sugarcane leaves to be used to optimize the analytical methods of the sucroenergetic sector.
Experimental
Preparation of the candidate reference material
Leaves were collected in the city of Iracemápolis, São Paulo State, from a field with five-month-old sugarcane plants of the variety RB966928, the fourth most cultivated in Brazil, the country. The material was sampled and prepared following procedures used for sugarcane foliage analysis, described in the literature [21, 22]. Only the top visible dewlap (TVD) leaves were selected, since they are used as reference tissue for evaluating the sugarcane nutritional status (leaf (+1)). The central portion (30 cm) of each leaf was picked out, and the midrib was removed. In total, 35 kg of material was thus generated. The material was stored at − 20 °C and dried in freeze dryer model ModulyoD (Thermo Savant, USA). Sugarcane leaves are difficult to grind due to the high contents of silicon and fiber. Considering the importance of small particle sizes for providing good homogeneity of a reference material, different grinding procedures were tested for the resulting particle sizes. After an initial test, the dry material was successively ground in a knife mill model GM 200 (Retsch, Germany) and in a planetary ball mill model PM 400 (Retsch, Germany), passing through a nylon sieve of 100 µm opening. Following careful mixing for homogenization, the candidate reference material obtained was distributed into 410 amber PET bottles (caps with sealing lip), each bottle contains 20 g. For longer shelf life, all bottles were sterilized using gamma radiation from 60Co at a dose of 25 kGy and stored in controlled environment of temperature (20 °C) and relative humidity (45 %).
Considering that the particle size is an important parameter in the preparation of reference materials and can directly influence the homogeneity, three bottles were randomly selected and analyzed in triplicate for particle size distribution in the equipment Analysette 22 MicroTec Plus, from Fritsch, Germany, using the wet dispersion unit.
Minimum sample mass and within-bottle testing
One bottle was randomly selected to evaluate the variability among measurements from the same bottle and the minimum representative sample mass. Four sample sizes were tested, i.e., 10 mg, 50 mg, 100 mg and 200 mg, taking ten replicates for NAA and Kjeldahl assays.
For NAA, the analytical portions were directly weighed into high-purity polyethylene vials (Posthumus Products, Beverwijk, Netherlands). Empty vials were used as analytical blanks, while 10-mg pieces of a well-characterized Ni–Cr alloy were added between vials for neutron flux monitoring [23]. Certified reference materials NIST SRM 1515 (apple leaves), NIST SRM 1570a (spinach leaves) and INCT-OBTL-5 (tobacco leaves) were included for quality control. Activation was performed in the nuclear research reactor IEA-R1 of the Nuclear and Energy Research Institute, Brazilian Nuclear Energy Commission (IPEN/CNEN), São Paulo, SP. Samples were irradiated at a thermal neutron flux of 9 x 1012 cm−2 s−1 for 4 h. The induced activity of medium- and long-lived radionuclides, i.e, half-lives longer than 12 h, was measured by high-resolution γ-ray spectrometry at the Radioisotopes Laboratory (CENA/USP). Four measurements were performed with decay times from 4 days to 30 days and counting times ranging from 15 min to 2 h. Chemical elements mass fractions and their uncertainties were obtained by k0 method, using the software package Quantu [24].
For the Kjeldahl method, the analytical portions were placed in digestion tubes. The digestion step was performed on a heating block using 5 mL of an acid digestion mixture composed of H2SO4 (c) (9 M), Cu2SO4 (0.04 M), Na2SeO3 (0.04 M) and Na2SO4 (0.38 M), heating at 100 °C (0.5 h), 200 °C (0.5 h) and 350 °C (2 h). All digested samples were diluted using 10 mL of deionized water and 20 mL of NaOH (11 M) to make the pH strongly alkaline (pH > 11) for distillation. The digestion tube was connected to the condenser and heated. The distilled solution was received into Erlenmeyer flask containing 5 mL of H3BO3 (0.32 M), for ammonium absorption, and indicators methyl red and bromocresol green, for identifying the final point of the reaction. The acid mixture was used as a blank and, for quality control, three certified reference materials were analyzed in triplicate with samples, i.e., NIST SRM 1515 (apple leaves), NIST SRM 1570a (spinach leaves) and NIST SRM 1573a (tomato leaves).
Between-bottles testing
Ten bottles were randomly chosen from the total 410 units for evaluating the variability of chemical elements between bottles. The analytical techniques employed were NAA at the National Institute of Standards and Technology, Gaithersburg, MD, USA, and Kjeldahl method at the Nuclear Energy Center for Agriculture, Piracicaba, SP, Brazil.
Analytical portions of 200 mg were pelletized in duplicate with 13-mm-diameter pellet dies using a hydraulic press. Standards were prepared on filter paper from solutions of high-purity metals or compounds, while filter papers were analyzed as blanks. Four certified reference materials were analyzed for quality control, i.e, NIST SRM 1570 (spinach leaves), NIST SRM 1571 (orchard leaves), NIST SRM 1572 (citrus leaves) and NIST SRM 1573 (tomato leaves). These materials were selected because of available literature data for non-certified elements [25]. Packages of standards, SRMs and samples were irradiated in the pneumatic RT-2 irradiation facility of the NIST Center for Neutron Research at a neutron flux of 3.4 x 1013 cm−2 s−1. For short-lived nuclides, samples and standards were irradiated for 2 min, cooled for 2 min and measured for 10 min in a high-resolution γ-ray spectrometer. The neutron flux was monitored with 8-mg titanium foils. For intermediate and long-lived nuclides, the induced activity was measured after 3 days and 15 days of decay with counting time of 2 h and 8 h, respectively. Quantitative results were obtained with the neutron activation analysis software utilizing the equations for comparator analysis and radioactive decay during cooling and measurement [26]. Corrective calculations for pulse pileup and live time extension were not applicable with the spectrometers running in loss-free mode.
For the Kjeldahl assay, analytical portions of 100 mg were weighed in triplicate from the ten bottles selected and put into the digestion tube. For quality control, the certified reference materials NIST SRM 1515, NIST SRM 1570a and NIST SRM 1573a were used. The total nitrogen was measured following the procedure detailed for minimum sample mass and within-bottle testing.
Residual moisture
The residual moisture of candidate reference material was measured in 1 g analytical portions, testing two different conditions for drying, i.e, desiccator with magnesium perchlorate and oven at 80 °C. The drying time of both methods was evaluated weighing the samples periodically until constant weight. The residual moisture was measured in the studies of within-bottle testing carried out at CENA/USP and between-bottles testing at NIST.
Statistical analysis
The results obtained for the minimum sample masses and within-bottle testing (ten replicates per mass) were analyzed using one-way analysis of variance (ANOVA) with SAS 9.3 software (Institute Inc., Cary, NC, USA). The Tukey multiple comparison test was used to determine the significant differences between the means of four masses (10 mg, 50 mg, 100 mg and 200 mg), while principal component analysis (PCA) with StatSoft Statistica 10.0 (Enterprise, USA) was used to show the variance, grouping and classification of the chemical elements in four different masses. For between-bottles testing, only one-way analysis of variance (ANOVA) was used to determine the variation and differences of the chemical elements among the 10 bottles selected.
Results and discussion
Residual moisture
The drying time in desiccator and oven was tested in the candidate reference material of sugarcane leaves; the mass does not change significantly for drying longer than 4 h in an oven at 80 °C and desiccator for one week.
For between-bottles testing, the residual moisture of the candidate reference material of sugarcane leaves for the 10 bottles analyzed was 4.36 % ± 0.03 % (average and standard deviation) when drying in a desiccator and 4.49 % ± 0.08 % when drying in an oven. Both methods presented similar results and were considered appropriate to quantify the residual moisture in the candidate material. For within-bottle testing, the residual moisture for one bottle analyzed in an oven was 4.45 % ± 0.13 %. The uncertainty due to balance was estimated in 0.08 mg, and this value has insignificant contribution in the results. The results obtained were used for mass correction of the within-bottle and between-bottles testing.
Particle size
The results of particle sizes obtained for 10 %, 50 % and 90 % of cumulative volume for different preparation methods are shown in Table 1. Data obtained demonstrated that sieving after milling is not enough for ensuring the desired particle size (< 100 µm), since the material is fibrous and most particles have an elongated shape. The successive use of knife mill, ball mill and 100-μm sieve was the only procedure found adequate for producing the desired particle size. Therefore, the material was prepared using such procedure. The particle size distribution of the candidate reference material of sugarcane leaves is shown in Fig. 1. The candidate material presented 80 % of the volume with particle sizes between 4 μm and 110 μm (n = 3).
Minimum sample mass and within-bottle testing
Four different sample sizes were used to evaluate the within-bottle variability and the minimum sample mass. The k0-method NAA procedure allowed quantification of mass fractions of Br, Ca, Ce, Co, Cs, Fe, Hf, K, La, Na, Rb, Sc, Sm, Sr, Th and Zn, while P was quantified using the bremsstrahlung radiation originating from beta decay [27], and N was measured by the Kjeldahl method. The average results for ten replicates are shown in Table 2. The significance of differences among the results obtained for the different sample masses was evaluated with ANOVA and Tukey test. For Ca, Cs, Hf, N and Rb, ANOVA showed no significant differences among results obtained from the four sample masses, while all the other elements presented significant differences (p < 0.05, F > Fcrit), mainly for 10 mg samples.
The principal component analysis (Fig. 2) provided evidence that the 10 mg samples presented results different from the other sample masses when including all 18 chemical elements. The results of the 10 mg samples also showed a higher dispersion among the ten replicates, while for the other three masses (50, 100 and 200 mg) a smaller dispersion and a general agreement, since all formed only one cluster.
From Table 2 and Fig. 2, it is observed that the mass fractions obtained for 10 mg samples, in general, presented a wide dispersion, i.e, lower precision, when compared to those obtained for larger samples. In fact, the relative standard deviations reached very high values for 10 mg samples, for instance, 81 % for Na and 69 % for Zn. In a first view, such higher variability for a smaller sample could be attributed to the non-homogenous distribution of chemical elements in the material, indicating that the 10 mg sample is not representative. Nevertheless, the measurement uncertainties should be considered for correctly distinguishing how much the non-homogeneity contributed to such variation of the results. NAA allows the estimation of each source of uncertainty contributing to the final experimental variability or experimental uncertainty (uexp), which therefore can be simplified as in Eq. 1, adapted from literature [28]:
where uc is the measurement uncertainty due to counting statistics, uAN is the combination of all other analytical uncertainties contributing to the precision of measurement and uhom is the uncertainty related to the degree of inhomogeneity of the material.
Smaller sample masses can have a wide impact on the counting statistics and on other sources of analytical uncertainty, for instance blank correction, enlarging both uc and uAN, which will cause larger experimental uncertainty (uexp). Figure 3 illustrates such influence, comparing calculated measurement uncertainties (\(u_{meas}^{2} = u_{c}^{2} + u_{AN}^{2}\)) and experimental uncertainties for the determination of Fe, K, Rb and Sc using different sample masses. These four elements were selected because they cover the full range of results, from about 0.05 mg kg−1 (Sc) up to almost 17000 mg kg−1 (K), and presented relative uncertainties lower than 5 %. A reasonable agreement was observed between the calculated measurement uncertainty and the experimental uncertainty for the four elements and four sample masses.
Figure 4 shows the correlation of uexp and umeas for 16 chemical elements determined by NAA using the four different sample masses. In general, a good agreement is observed, with the main exception of Hf determined in 10 mg samples. The larger experimental uncertainty observed in such situation is an indication of non-homogeneity, which can originate from Hf contained in the alumina balls and jars used in the milling process. The uncertainties of Na and Zn for 10 mg samples were not plotted since they were too high, respectively, 15 % and 50 %, due to the uncertainty associated with blank correction. For masses larger than 10 mg, a good agreement was observed between uexp and umeas and the distribution of chemical elements was considered homogeneous. The highest uhom was found for Zn in 10 mg samples (48 %); however, for samples masses from 50 mg the uhom for Zn was lower than 1 %.
Between-bottles testing
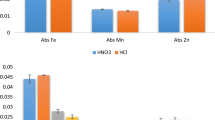
For evaluating the variation among the bottles, the elements determined using sample mass of 200 mg were Al, Br, Ca, Cl, Co, Cs, Fe, K, La, Mg, Mn, Na, Rb, Sb, Sc, Sm, V, Th and Zn. On the other hand, for Cu and Mo, the counting statistics were insufficient for quantification, being found close to the detection limits (Cu < 6.0 mg kg−1 and Mo < 1.4 mg kg−1). The results obtained are shown in Table 3, which shows the mass fractions (mg kg−1) and relative associated uncertainties (uexp, uc, uAN, uhom) in percentage. Uncertainty due to material inhomogeneity (uhom) was calculated using Eq. 1.
The relative experimental uncertainty (uexp) ranged from 0.66 % (Al) to 18.8 % (Sb). The high uncertainties for Sb and V (14.9 %) are mainly due to counting statistics, and conclusions on the homogeneous distribution of these elements cannot be made. In some cases, uhom was not calculated since the combination of uc and uAN was higher than uexp, for instance, Cl had uexp (0.78 %) < uAN (1.21 %). In contrast, uhom of 5.98 % for Co can mostly be attributed to a non-homogeneous distribution of the element using mass of 200 mg. Analysis of variance (ANOVA) was used to corroborate the evaluation of between-bottles homogeneity. The material had significant differences between bottles when F > Fcrit and p < 0.05, which was only the case for Co (p = 0.02, F = 4.90 and Fcrit = 3.50). For N, which was determined by Kjeldahl method, the average mass fraction was 24970 mg kg−1 with uexp = 0.18 % (p = 0.90, F = 0.43 and Fcrit = 2.39) being also considered homogeneously distributed.
Therefore, the elements considered homogeneously distributed between bottles with relative uncertainties (uexp, uc, uAN, uhom) lower than 5 % were Al, Br, Ca, Cl, Cs, Fe, K, La, Mg, Mn, N, Rb, Sc, Sm, Th and Zn.
Conclusion
The candidate reference material of sugarcane leaves has adequate particle size distribution (90 % of volume lower than 110 µm) with residual moisture content lower than 5 %.
The within-bottle homogeneity testing demonstrated that there was no significant differences among the subsamples (p > 0.05; F < Fcrit; uhom< 5 %) for Br, Ca, Ce, Co, Cs, Fe, Hf, K, La, N, Na, P, Rb, Sc, Sm, Sr, Th and Zn. The minimal sample mass of 50 mg is recommended for most of the elements, while 200 mg mass sample is required to assure the homogeneity for Co, Na, V and Sb. The between-bottles homogeneity testing showed no significant differences for Al, Br, Ca, Cl, Cs, Fe, K, La, Mg, Mn, N, Rb, Sc, Sm, Th and Zn, using a sample mass of 200 mg. Considering simultaneously both within- and between-bottles testing, the homogeneity could be confirmed for twelve elements, i.e., Br, Ca, Cs, Fe, K, La, N, Rb, Sc, Sm, Th and Zn.
Stability studies and the final chemical characterization to assign values and uncertainties are being assessed for this innovative material.
References
UNICA - Brazilian Sugarcane Industry Association (2017). http://english.unica.com.br/faq/. Accessed 30 Nov 2017
OECD-FAO - Food and Agriculture Organization of the United Nations (2015). http://www.fao.org/3/a-i4738e.pdf. Accessed 10 Jan 2018
Goes T, Marra R, Araújo M, Alves E, de Souza MO (2011) Sugarcane in Brazil: current technologic stage and perspectives. Rev Polit Agric 1:52–65
Singh RK, Kumar P, Tiwari NN, Rastogi J, Singh SP (2013) Current status of sugarcane transgenic: an overview. Adv Genet Eng 2:1–7
Cali PJ (1979) The role of reference materials in the analytical laboratory. Fresenius Z Anal Chem 297:1–3
King B (2003) The selection and use of reference materials: a basic guide for laboratories and accreditation bodies. Accred Qual Assur 8:429–433
COMAR. International database for certified reference materials (2018) http://www.comar.bam.de/en/ Accessed 25 Jan 2018
Moore PH, Botha FC (2014) Sugarcane: physiology, biochemistry, and functional biology. Wiley, Iowa
ISO 17034 (2016) General requirements for the competence of reference material producers. ISO, Geneva
ISO Guide 35 (2017) Reference materials: general and statistical principles for certification. ISO Geneva
Liu KS (2009) Some factors affecting sieving performance and efficiency. Powder Technol 193:208–213
Rawle AF (2015) Representative sampling: another cinderella of particle size analysis. Procedia Engineering 102:1707–1713
Fajgelj A, Zeisler R, Benesch T, Dekner R (1994) Particle size studies in the preparation of AQCS reference materials. Int Atomic Energy Agency 33:148–157
Pauwels J, Lamberty A, Schimmel H (1998) Homogeneity testing of reference materials. Accred Qual Assur 3:51–55
Stoeppler M, Wolf WR, Jenks PJ (2001) Reference materials for chemical analysis certification: availability and proper usage. Wiley, New York
Quevauviller PH, Maier EA (1999) Interlaboratory studies and certified reference materials for environmental analysis. Springer, Amsterdam
Rossbach M, Grobecker KH (1999) Homogeneity studies of reference materials by solid sampling: AAS and INAA. Accred Qual Assur 4:498–503
Greenberg RR, Bode P, Fernandes EAN (2011) Neutron activation analysis: a primary method of measurement. Spectrochim Acta Part B 66:193–241
Anglov T, Petersen IM, Kristiansen J (1999) Uncertainty of nitrogen determination by the Kjeldahl method. Accred Qual Assur 4:504–510
Chromý V, Vinklárková B, Šprongl L, Bittová M (2015) The Kjeldahl method as a primary reference procedure for total protein in certified reference materials used in clinical chemistry. I. A review of Kjeldahl methods adopted by laboratory medicine. Crit Rev Anal Chem 45:106–111
Malavolta E (2006) Manual de nutrição mineral de plantas. Agronômica Ceres, Piracicaba
Sugar Research Australia (2013) Leaf sampling—A key to improved nutrient management. https://sugarresearch.com.au/wp-content/uploads/2017/02/IS13014-Leaf-Sampling.pdf. Accesed 11 Jun 2013
França EJ, Fernandes EAN, Bacchi MA (2003) Ni–Cr alloy as neutron flux monitor: composition and homogeneity assessment by NAA. J Radioanal Nucl Chem 257:113–115
Bacchi MA, Fernandes EAN (2003) Quantu design and development of a software package dedicated to k0-standardized NAA. J Radioanal Nucl Chem 257:577–582
Roelandts I, Gladney EA (1998) Consensus values for NIST biological and environmental Standard Reference Materials. Fresenius J Anal Chem 360:327–338
Zeisler R, Vajda N, Kennedy G, Lamaze G, Molnar GL (2003) Handbook of nuclear chemistry. Kluwer Academic, Amsterdam, pp 303–362
Matsubara TCM, Bacchi MA, Fernandes EAN (2012) Further investigating the determination of phosphorus in plants by INAA using bremsstrahlung measurement. J Radioanal Nucl Chem 291:201–205
Zeisler R (2002) INAA studies of sampling properties of some natural matrix materials for the development of small sample reference materials. IAEA-TECDOC-1295. International Atomic Energy Agency, Vienna, Austria. 71–76
Acknowledgments
The authors wish to thank the São Paulo Research Foundation (FAPESP) for the financial support (Grant Number 2014/06494-8), the National Institute of Standards and Technology (NIST) for research support and Iracema sugarcane factory for providing the sugarcane leaves for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer
Contributions of the National Institute of Standards and Technology are not subject to copyright in the USA. Certain commercial equipment, instruments or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the NIST nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Rights and permissions
About this article
Cite this article
Martínez, M.I.V., Zeisler, R., De Nadai Fernandes, E.A. et al. Innovative reference material for improving the quality control in the sucroenergetic sector. Accred Qual Assur 23, 329–336 (2018). https://doi.org/10.1007/s00769-018-1348-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-018-1348-2