Abstract
The aim of this study was to investigate the effect of the chronic administration of methionine (Met) and/or its metabolite, methionine sulfoxide (MetO), on the behavior and neurochemical parameters of young rats. Rats were treated with saline (control), Met (0.2–0.4 g/kg), MetO (0.05–0.1 g/kg), and/or a combination of Met + MetO, subcutaneously twice a day from postnatal day 6 (P6) to P28. The results showed that Met, MetO, and Met + MetO impaired short-term and spatial memories (P < 0.05), reduced rearing and grooming (P < 0.05), but did not alter locomotor activity (P > 0.05). Acetylcholinesterase activity was increased in the cerebral cortex, hippocampus, and striatum following Met and/or MetO (P < 0.05) treatment, while Na+, K+-ATPase activity was reduced in the hippocampus (P < 0.05). There was an increase in the level of thiobarbituric acid reactive substances (TBARS) in the cerebral cortex in Met-, MetO-, and Met + MetO-treated rats (P < 0.05). Met and/or MetO treatment reduced superoxide dismutase, catalase, and glutathione peroxidase activity, total thiol content, and nitrite levels, and increased reactive oxygen species and TBARS levels in the hippocampus and striatum (P < 0.05). Hippocampal brain-derived neurotrophic factor was reduced by MetO and Met + MetO compared with the control group. The number of NeuN-positive cells was decreased in the CA3 in Met + MetO group and in the dentate gyrus in the Met, MetO, and Met + MetO groups compared to control group (P < 0.05). Taken together, these findings further increase our understanding of changes in the brain in hypermethioninemia by elucidating behavioral alterations, biological mechanisms, and the vulnerability of brain function to high concentrations of Met and MetO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypermethioninemia is a metabolic disorder characterized by the accumulation of the essential amino acid, methionine (Met), in tissues (Mudd et al. 2001). Six genetic conditions can lead to elevated Met levels; however, methionine adenosyltransferase I/III (MAT I/III) deficiency is the most common cause of isolated and persistent elevated Met levels (Couce et al. 2008; Mudd 2011). Hypermethioninemics patients can present hepatic and neurological manifestations, such as cognitive deficits, demyelination, memory impairments, and cerebral edema (Chamberlin et al. 1996; Mudd et al. 2000, 2001; Couce et al. 2008; Mudd 2011). Recently, it was demonstrated that the secondary metabolite, methionine sulfoxide (MetO), is closely associated with alterations in hypermethioninemia (Costa et al. 2013; Dos Santos et al. 2016; Soares et al. 2017a). Studies have shown that the formation of MetO via reactive oxygen species (ROS)-induced oxidation of Met modifies its physicochemical properties, alters its function, and leads to a loss of protein biological activity (Tarrago et al. 2015; Suzuki et al. 2016).
There is an intrinsic relationship between Met, MetO, and oxidative stress because they are all sulfur amino acids (Tarrago et al. 2015; Suzuki et al. 2016). Oxidative stress is a component of the pathophysiology of several human diseases and contributes to the development and/or progression of these pathologies (Patel 2016, Suzuki et al. 2016). High levels of Met and/or MetO alter the oxidative status in the liver (Stefanello et al. 2009; Costa et al. 2013; Soares et al. 2017a), kidney (Soares et al. 2017a), macrophages (Dos Santos et al. 2016), skeletal muscle (Schweinberger et al. 2015), and brain (Stefanello et al. 2005, 2007a, b; Soares et al. 2017b) of rats.
Cholinergic signaling is mediated by the neurotransmitter, acetylcholine (ACh), which is essential for cognitive performance, such as learning, behavior, and memory (Ferreira-Vieira et al. 2016; Roy et al. 2016). Inactivation of this signaling is promoted by acetylcholinesterase (AChE), which cleaves ACh into choline and acetate (Ferreira-Vieira et al. 2016). Stefanello et al. (2007a, b) have demonstrated that acute and chronic treatment with Met increases AChE activity in the cerebral cortex (Stefanello et al. 2007a, b). However, little is known about the effects of MetO on AChE activity.
Sodium–potassium adenosine triphosphatase (Na+, K+-ATPase), an essential transmembrane enzyme responsible for the electrochemical gradient across the cell membranes (Moseley et al. 2007), is also important for learning and memory-related processes. High levels of Met inhibit the Na+, K+-ATPase activity but increase the expression and immunocontent of Na+, K+-ATPase α1, α2, and α3 subunits in the synaptic plasma membrane and brain of rat offspring (Stefanello et al. 2011; Schweinberger et al. 2018a, b).
We have previously demonstrated that acute administration of Met and/or MetO cause oxidative stress, reduce cell viability, induce DNA damage, and cause cell death by apoptosis in the cerebral cortex of young rats (Soares et al. 2017b); however, the precise mechanisms involved in the pathophysiology of chronic hypermethioninemia remain unclear. Based on these studies, we aimed to investigate the effect of chronic administration of Met and/or MetO on memory, AChE, Na+, K+-ATPase activity, and redox status, brain-derived neurotrophic factor (BDNF) and neuronal cell number in the cerebral cortex, hippocampus, and striatum of young rats.
Materials and methods
Chemicals
Met and MetO were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents used in the experiments were of analytical grade and the highest purity.
Animals and ethical approval
All animal experimental protocols in this work were approved by the Committee of Ethics and Animal Experimentation of the Federal University of Pelotas, RS, Brazil (protocol number: CEEA 3527). Wistar rats, male and female (6 days old) weighing 5–10 g, were obtained from the Central Animal House of the Federal University of Pelotas. The litters (6–10 animals) and the progenitor were kept in a density of four rats per cage (acrylic boxes measuring 40cmx34cmx17cm) in the same room under a constant temperature (23 ± 2 °C), humidity (50–65%), and illuminated from 7:00 a.m. to 7:00 p.m., with food and water available ad libitum. All animals were arbitrarily assigned before starting treatment without specific tools to achieve randomization in the animal facility room. The use of the animals was in accordance with the Brazilian Guidelines for the Care and Use of Animals in Scientific Research Activities (DBCA), National Council of Control of Animal Experimentation (CONCEA), and with the NIH Guide for Care and Use of Laboratory Animals. This study was not pre-registered.
Hypermethioninemia protocol
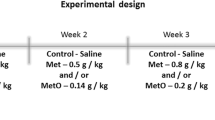
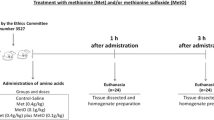
Forty male and female Wistar rats (6 days old) were divided into four groups: Group I (Control/saline), Group II (Met), Group III (MetO), and Group IV (Met + MetO). Met and MetO were dissolved in 0.9% NaCl and were administered by subcutaneous injection twice a day at 8 h intervals between postnatal day 6 (P6) and P28 (Stefanello et al. 2007a, b; Costa et al. 2013; Soares et al. 2017a, b). From P6 to P14, groups II and III received 0.2 g/kg Met and 0.05 g/kg MetO, respectively. From P15 to P21, groups II and III received 0.3 g/kg Met and 0.075 g/kg MetO, respectively. From P22 to P28, groups II and III received 0.4 g/kg Met and 0.1 g/kg MetO, respectively. Group IV received a combination of the concentrations of Met + MetO in the same concentrations. Group I was administered an equivalent volume of saline from P6 to P28 (Fig. 1). The doses of Met and MetO were based on those reported in previous studies (Stefanello et al. 2007a, b; Costa et al. 2013; Soares et al. 2017a, b). Animals were directly killed when they met the exclusion criteria: weight loss in combination or not with behavioral impairment indicating pain and/or stress and suffering. No animals had to be excluded before the end of the study due to complications.
Behavioral procedure
Open-field test
All behavioral tests were performed by a blinded experimenter. Behavioral tests were performed between P23 and P25. Locomotor behavior was evaluated using open-field apparatus, which consisted of a wooden box. The floor of the arena was divided into 16 equal squares (18 × 18 cm) and placed in a sound free room. Rats were placed in the rear left square and allowed to explore freely for 5 min. The total number of squares crossed with all paws (crossing) and fecal droppings were manually counted; the degree of grooming and rearing was also evaluated. The apparatus was cleaned and dried with a 40% alcohol solution after testing with each rat. This test was carried out to identify motor disabilities, which might influence the other behavioral tests performed.
Object recognition test
Rats were habituated to the experimental arena 24 h before the test. Objects, made of waterproof plastic, were placed on the sand floor. This test was performed in two stages. During training, rats were placed in the arena with two identical objects (A1 and A2) and allowed to explore for 5 min. The session was valid if rats explored each object for at least 30 s. Following this, rats were tested 120 min after training to evaluate their short-term memory. For the testing, one of the objects was changed to a different, novel object (B). The rats were introduced into the arena for 5 min and allowed to explore freely. The positions of the objects (familiar or novel) were randomly exchanged. Exploration was defined as smelling or touching the object with the nose and/or forelegs. Sitting on or around the object was not considered exploratory behavior. The apparatus and the objects were cleaned and dried with a 40% alcohol solution after each rat. In this task was evaluated the total exploration time (s) in the training and test; time spent on each object—training (s) (A1, A2); time spent on each object—test (s) (A1 and B); exploratory preference time for the novel object expressed as a percentage evaluated in the object recognition test (Rossato et al. 2007).
Y-maze test
This test measures spatial recognition memory. The apparatus has three arms: start arm, in which rats were placed to start to explore (always open); novel arm, which was blocked during the first trial, but open during the second trial; and other arm (always open).
Briefly, the task was performed as follows: first, rats underwent training: they were placed in the start arm and could explore the start and other arm. The third arm (novel arm) remained closed during training. After 2 h, testing was performed as follows: rats were placed in the start arm with free access to all three arms for 5 min. The number of entries in each arm and time spent exploring each arm (expressed as a percentage of the time spent in each arm) was recorded (Dellu et al. 1997). The apparatus was cleaned with 40% alcohol solution and dried after each session with each animal. Rats were killed by cardiac puncture 12 h after the final amino acid or saline injection. The brain was removed and quickly dissected to obtain the cerebral cortex, hippocampus, and striatum samples. Biochemical analyses were performed by a blinded experimenter, and all samples were run in duplicate.
Acetylcholinesterase activity
Brain samples were placed in a solution of Tris–HCl 10 mM, pH 7.4, homogenized and centrifuged at 1300 g for 10 min at 4 °C. The supernatant was used for the AChE assay, as previously described by Ellman et al. (1961). The reaction mixture comprised 10 mM 5,5-dithiobis(2-nitrobenzoic acid), 100 mM phosphate buffer (pH 7.5), 15 μl supernatant, and 0,8 mM acetylthiocholine. The absorbance at 412 nm was read on a spectrophotometer at 30-s intervals for 2 min at 27 °C. AChE activity was expressed as μmol AcSCh/h/mg of protein.
Na+, K+-ATPase activity
Brain sections were homogenized (1/10 w/v) in 0.32 mM sucrose containing 5.0 mM HEPES and 1.0 mM EDTA, pH 7.5. The homogenates were centrifuged at 1000 g for 10 min, and the supernatants were removed for further analysis. The reaction mixture for this assay contained 5 mM MgCl2, 80 mM NaCl, 20 mM KCl, and 40 mM Tris–HCl (pH 7.4). The reaction was initiated by the addition of ATP to a final concentration of 3 mM. The control samples were generated under the same conditions with the addition of 1 mM ouabain. Na+,K+-ATPase activity was calculated by measuring the difference in absorbance between the study samples and the controls, as described by Wyse et al. (2007). The level of released inorganic phosphate (Pi) was measured, as described by Chan et al. (1986). Specific enzyme activity was expressed as nmol Pi released/min/mg of protein.
Oxidative stress parameters in the brain structures
Brain regions were homogenized (1/10 w/v) using 20 mM sodium phosphate buffer, pH 7.4 containing 140 mM KCl. The homogenates were centrifuged at 2500g for 10 min at 4 °C. The supernatants were collected and used in further analyses.
Reactive oxygen species (ROS) assay
The oxidation of DCFH-DA to fluorescent 2′,7′-dichlorofluorescein (DCF) was measured. Briefly, DCF fluorescence intensity emission was recorded at excitation wavelengths of 525 and 488 nm 30 min after the addition of DCFH-DA to the medium. ROS formation was expressed as µmol DCF/mg of protein (Ali et al. 1992).
Thiobarbituric acid reactive substances (TBARS) assay
For this assay, the supernatants were mixed with 10% TCA and centrifuged. The supernatant was collected and mixed with TBA (0.67%) and incubated in a dry block at 100 °C for 30 min. TBARS levels were determined by absorbance at 535 nm and reported as nmol of TBARS/mg of protein (Esterbauer and Cheeseman 1990).
Total sulfhydryl content assay
Supernatants were added to PBS buffer (pH 7.4) containing EDTA. The reaction was started by the addition of DTNB. One hour after incubation in the dark, DTNB reduced by thiol groups was oxidized (disulfide) and a yellow derivative (TNB) was generated, whose absorbance at 412 nm was measured. The results were reported as nmol TNB/mg of protein (Aksenov and Markesbery 2001).
Nitrite quantification
Nitrite was measured by Griess reaction (Stuehr and Nathan 1989). In brief, 50 μl homogenate was incubated with 50 μl 1% sulfanilamide and 50 μl 0.3% N-1-naphthylethylenediamine dihydrochloride at room temperature (RT) for 10 min. Nitrite was measured at 540 nm using sodium nitrite as standard. Results were expressed as μM nitrite/mg of protein.
Superoxide dismutase (SOD) activity
This assay is based on the inhibition of superoxide-dependent adrenaline auto-oxidation to adenochrome. The intermediate in this reaction is superoxide, which is scavenged by SOD, and the absorbance is measured at 480 nm on a spectrophotometer. SOD activity was measured, as previously described by Misra and Fridovich (1972), and reported as units/mg of protein.
Catalase (CAT) activity
The decomposition of 30 mM H2O2 in 50 mM potassium phosphate buffer (pH 7.0) was continuously monitored at 240 nm for 180 s at 37 °C, as previously reported by Aebi (1984). CAT activity was reported as units/mg of protein.
Glutathione peroxidase (GPx) activity
GPx activity was determined according to manufacturer instructions (RANSEL®; Randox Lab, Antrim, United Kingdom). GPx activity was reported as units/mg of protein.
Protein determination
Protein concentration was measured as previously described by Lowry et al. (1951), except for AChE activity, which used the method previously described by Bradford (1976).
Brain-derived neurotrophic factor (BDNF) assay
Hippocampus BDNF concentration was measured using the ChemiKine BDNF Sandwich ELISA Kit, CYT306 (Chemicon/Millipore, Billerica, MA, USA), according to the manufacturer’s instructions. The results were expressed as pg BDNF/mg protein.
Histological procedures and immunohistochemistry techniques
Four rats from each group were killed by transcardial perfusion. Briefly, rats were anesthetized with isoflurane and perfused with 0.9% saline for 10 min, followed by 4% paraformaldehyde diluted in phosphate-buffered saline (PBS; 0.1 M, pH 7.2–7.4) for 30 min. The brain of the animals was removed and stored for 24 h in 4% buffered paraformaldehyde followed by 70% ethanol for at least 24 h. Following this, the brain samples were embedded in paraffin. Coronal sections were cut using a rotary microtome (4 μm thickness) in the hippocampus. Six sections were used for the analysis (12 hippocampi), at a distance of 50 μm between each cut.
After 30 min at 80 °C, sections prepared for NeuN staining. Briefly, they were cleared using xylene (10 min, 5 min, and 5 min), followed by dehydration with ethanol 100% (5 min), 95% (5 min), 70% (5 min), 50% (5 min), and distilled water. Following this, the sections were rehydrated in PBS (pH 7.4) with 0.5% Triton X-100 (PBS-TX) for 15 min and then incubated in citrate buffer (pH 6.00) for 20 min at 98 °C. Endogenous peroxidase was blocked using 5% hydrogen peroxide (30 V) in methanol for 10 min (3 times). Nonspecific proteins were blocked with 1% BSA (Sigma®) in PBS-tx for at least 1 h at RT. Then, the sections were incubated with primary monoclonal antibody (NeuN: Dako® 1:1000) 1 h RT and overnight at 4 °C. After 3 × PBS for 15 min, sections were incubated with conjugated secondary antibody for 40 min. Finally, the immunohistochemical reaction was revealed with 0.06% 3.3′-diaminobenzidine (DAB; Dako®) in PBS-tx for 5 min and mounted on slides using Entellan® (Merck®). NeuN-positive cells were visualized in the CA1, CA3, and dentate gyrus (DG) (Fig. 5a). The number of positive cells within a 10.083 × 150 μm2 square (Fig. 5b) was counted using Image-Pro Plus® 6.3 (Media Cybernetics®) software.
Statistical analysis
A formal power calculation was not performed, and the sample size (n = 10 animals per group) was based on experience from previous experiments. Data were analyzed by one- or two-way analysis of variance (ANOVA) followed by Tukey or Bonferroni post hoc tests using GraphPad Prism 5.0 (Intuitive Software for Science, São Diego, CA, USA). P < 0.05 (single animal) was considered statistically significant. All data were expressed as mean ± standard error of the mean (SEM).
Results
Methionine and/or methionine sulfoxide does not alter locomotion, but reduces grooming and rearing
The parameters evaluated in the open-field test are listed in Table 1. Locomotion was not significantly different in any experimental group compared with that in the control group (F3,36 = 0.52, P > 0.05). However, MetO and Met + MetO reduced the amount of grooming (F3,36 = 3.78, P < 0.05), while Met, MetO, and Met + MetO decreased the amount of rearing when compared with the control group (F3,36 = 13.50, P < 0.05). There was no difference in the number of fecal droppings among the groups (F3,36 = 2.66, P > 0.05; Table 1).
Methionine and/or methionine sulfoxide impairs short-term and spatial memories
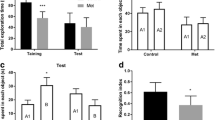
Figure 2a shows that all rats showed increased total exploration time in the testing vs. training phase of the object recognition test. Interestingly, MetO- and Met + MetO-treated rats had longer total exploration times when compared with the control group. During the training phase, there was no difference in the exploration times between objects in all groups (Fig. 2b). During testing, Met-, MetO-, and Met + MetO-treated rats explored the familiar object (A1) more than the rats in the control group. Furthermore, the MetO group showed reduced exploration of the novel object (B) compared with the control group (Fig. 2c). We found that the control group explored the novel object more than the familiar object (Fig. 2d). In contrast, the MetO group explored the novel object significantly less compared with the familiar object (Fig. 2d). Taken together, these findings indicated that Met, MetO, and Met + MetO treatment impaired short-term object recognition memory (F3,36 = 5.12, P < 0.05; Fig. 2e).
Methionine (Met) and/or methionine sulfoxide (MetO) impairs both short-term and spatial memories in young rats 21 days after treatment. a Total exploration time (s); b time spent on each object-training (s); c time spent on each object-test (s); d total time spent during the test session on each object; e exploratory preference time for the novel object expressed as a percentage evaluated in the object recognition test. f Exploratory preference time in the novel, start, and other arms, expressed as a percentage of the total exploration time in the Y-maze test. (g) Number of entries in the novel, start, and other arms evaluated in the Y-maze test. Two-way ANOVA and post hoc Bonferroni multiple comparisons test were used for a–d. One-way ANOVA and post hoc Tukey’s multiple comparisons tests were performed for e–g. A1 and A2 report the familiar object and B refers to new objet. Bars represent mean and ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, compared with control group (n = 9–10). In the a, and b denote a significant difference between training and test *P < 0.05
Similar results were observed in the Y-maze test, indicating an impairment in spatial memory; Met, MetO, and Met + MetO treatment decreased exploratory time (F3,36 = 6.72, P < 0.05) and number of entries (F3,36 = 3.30, P < 0.05) into the novel arm compared with those in the control group (Fig. 2f). There were no differences in the exploratory time in the start (F3,36 = 0.51, P > 0.05) or other (F3,36 = 0.70, P > 0.05; Fig. 2f) arms or in the number of entries in the start (F3,36 = 2.00, P > 0.05) and other (F3,36 = 0.90, P > 0.05; Fig. 2g) arms.
Methionine and/or methionine sulfoxide alter acetylcholinesterase and Na+,K+ -ATPase activity
AChE activity was increased in the cerebral cortex (F3,18 = 10.77, P < 0.05) and hippocampus (F3,13 = 6.39, P < 0.05) following Met, MetO, and Met + MetO treatment when compared with control rats (Fig. 3). Furthermore, there was an increase in AChE activity in the MetO and Met + MetO groups in the striatum compared with the control group (F3,15 = 4.79, P < 0.05, Fig. 3).
Acetylcholinesterase and Na+, K+-ATPase activity in the cerebral cortex, hippocampus, and striatum of young rats 21 days after treatment with methionine (Met) and/or methionine sulfoxide (MetO). AChE activity is expressed in μmol AcSCh/h/mg protein and Na+, K+-ATPase activity as nmol/Pi released/min/mg protein. One-way ANOVA and post hoc Tukey’s multiple comparisons test were performed. Bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, compared with the control group (n = 4–7)
As shown in Fig. 3, Na+,K+-ATPase activity was significantly decreased in the MetO and Met + MetO groups in the hippocampus (F3,17 = 8.91, P < 0.05, Fig. 3). However, no change was observed in Na+,K+-ATPase activity in the cerebral cortex (F3,17 = 1.88, P > 0.05) and striatum (F3,17 = 0.17, P > 0.05) following Met and/or MetO treatment (Fig. 3).
Methionine and/or methionine sulfoxide induces oxidative stress in the cerebral cortex, hippocampus, and striatum
Table 2 shows the results of the oxidative stress analyses. Met, MetO, and Met + MetO treatment increased the level of TBARS in the cerebral cortex (F3,22 = 5.23, P < 0.05); however, there was no change in ROS (F3,15 = 0.42, P > 0.05), total thiol (F3,17 = 3.13, P > 0.05), or nitrite (F3,18 = 0.15, P > 0.05) levels. Furthermore, there was no difference in antioxidant enzyme activity, such as SOD (F3,20 = 0.95, P > 0.05, Fig. 4a), CAT (F3,21 = 1.51, P > 0.05, Fig. 4b), and GPx (F3,23 = 0.87, P > 0.05, Fig. 4c), compared with the control group.
Superoxide dismutase (SOD) catalase (CAT) and glutathione peroxidase (GPx) activity in the cerebral cortex (a–c), hippocampus (d–f), and striatum (g–i) of young rats 21 days after treatment with methionine (Met) and/or methionine sulfoxide (MetO). CAT, SOD, and GPx activity are reported as units/mg of protein. One-way ANOVA and post hoc Tukey’s multiple comparisons test were performed. Bars represent mean ± SEM. *P < 0.05, compared with the control group (n = 4–7)
In the hippocampus Met, MetO, and Met + MetO treatment increased ROS (F3,14 = 8.42, P < 0.05), and TBARS (F3,19 = 8.29, P < 0.05) levels, while MetO and Met + MetO treatment reduced the total thiol (F3,19 = 6.05, P < 0.05) and nitrite (F3,17 = 7.40, P < 0.05; Table 2) content. In addition, administration of Met/MetO combined or alone reduced SOD (F3,19 = 6.66, P < 0.05, Fig. 4d), and GPx (F3,16 = 12.30, P < 0.05, Fig. 4f) activity in the hippocampus. CAT activity was reduced in the rats that received MetO or Met + MetO (F3,15 = 8.69, P < 0.05, Fig. 4e) when compared with the control group.
Met, MetO, and Met + MetO enhanced ROS levels (F3,15 = 34.5, P < 0.05) in the striatum (Table 2). MetO and Met + MetO treatment increased TBARS levels (F3,16 = 8.86, P < 0.05) and reduced total thiol content (F3,19 = 7.93, P < 0.05) when compared with the control group (Table 2). Furthermore, reduced nitrite was observed in the striatum in all treatment groups (F3,19 = 5.19, P < 0.05, Table 2). Figure 4 shows that MetO and Met + MetO reduced SOD (F3,17 = 3.92, P < 0.05, Fig. 4g) and CAT (F3,16 = 4.24, P < 0.05, Fig. 4h) activity in the striatum; however, no changes were observed in GPx activity (F3,20 = 2.16, P >0.05, Fig. 4i).
Effect of methionine and/or methionine sulfoxide on BDNF in the hippocampus of young rats
Figure 5 shows that the concentration of BDNF was reduced in the hippocampus in MetO and Met + MetO groups when compared with the control group (F3,17 = 6.84, P < 0.05, Fig. 5).
Concentration of brain-derived neurotrophic factor (BDNF) in the hippocampus of young rats 21 days after treatment with methionine (Met) and/or methionine sulfoxide (MetO). BDNF is reported as pg/mg of protein. One-way ANOVA and post hoc Tukey’s multiple comparisons test were performed. Bars represent mean ± SEM (n = 4–7). *P < 0.05, **P < 0.01 compared with the control group (n = 5–6)
Methionine and/or methionine sulfoxide reduces the number of NeuN-positive cells in the CA3 and DG
Figure 6a shows the CA1, CA3, and DG (area within the red square, objective lenses 5 × scale bar 500 μm): CA1 (objective lenses 20 × , scale bar 100 μm), CA3 (objective lenses 20 × , scale bar 100 μm), and DG (objective lenses 10 × , scale bar 250 μm). Areas were delimited in the hippocampus, and the number of NeuN-positive cells were counted (objective lenses 40 × , scale bar 50 μm, orange square [area 10,083 × 150 μm2], Fig. 5b). Quantitative analysis of NeuN + cells in the CA1 (Fig. 5c), CA3 (Fig. 6d), and DG (Fig. 6e). We found a significant reduction in NeuN + cells in the CA3 following Met + MetO treatment when compared with controls (F3,12 = 4.26, P < 0.05, Fig. 6d). In addition, there was a reduction in NeuN + cells in the DG in Met, MetO, and Met + MetO groups when compared with the control group (F3,12 = 7.52, P < 0.05, Fig. 6e). No changes was founded in NeuN + cells in the CA1 (F3,12 = 0.10, P > 0.05, Fig. 6c).
Number of NeuN-positive cells in the CA1, CA3, and DG from young rats 21 days after treatment with methionine (Met) and/or methionine sulfoxide (MetO). Data are reported as mean ± SEM. One-way ANOVA and post hoc Tukey’s multiple comparisons test were performed. *P < 0.05 and **P < 0.01, compared with the control group (n = 4)
Discussion
Hypermethioninemia is characterized by high levels of Met and its metabolite, MetO. In MAT I/III deficiency, Met plasma levels increase to up to 30-fold higher, and MetO levels may increase to 460 μM in the plasma (Gahl et al.1988). Although global epidemiological data have not been obtained to date, previous studies have shown that the incidence of MAT I/III deficiency is 1 in 27,000 newborns in the Iberian Peninsula (Marcão et al. 2015). We have previously assessed the effects of acute and chronic exposure of high levels of Met and MetO in experimental models. Based on these studies, we have shown the toxic potential of these amino acids in in vitro and in vivo (Costa et al. 2013; Dos Santos et al. 2016; Soares et al. 2017a, b; Stefanello et al. 2005, 2007a, b, 2009, 2011; Schweinberger et al. 2015, 2018a, b). In this study, we have demonstrated that Met and/or MetO induced memory deficits and biochemical alterations in important brain structures.
First, we assessed whether treatment affected behavior using an open-field test for motor abnormalities evaluation (Belzung and Griebel 2001). We found that Met and/or MetO did not alter the spontaneous locomotor activity of the animals. However, Met, MetO, and Met + MetO showed reduced rearing, indicating a lower exploratory activity. Rearing behavior is associated with improvements in spatial cognitive map formation and space defense (Borta and Schwarting 2005), which can facilitate learning and spatial memory. Therefore, it is plausible that a decrease in rearing may explain the findings of the Y-maze and object recognition tests (Pawlak and Schwarting 2002). The decrease in rearing and grooming may be associated with Met- and MetO-induced anxiogenic behavior. In line with this, Hrncic et al. (2016) demonstrated that methionine-enriched diet reduces rearing and promotes anxiety-like behavior.
Our results have shown that administration of Met and MetO alone, or in combination, impaired short-term object recognition memory. In addition, impairment in the Y-maze test was observed. Our results are in agreement with other studies that demonstrate memory impairment with long-term Met exposure in rats (Stefanello et al. 2007a, b) and zebrafish (Vuaden et al. 2012). The brain is organized into multiple memory systems that are associated with different brain structures. The cerebral cortex, and more specifically, the perirhinal cortex and hippocampus contribute to recognition memory (Vann and Albasser 2011; Antunes and Biala 2012). In addition, the hippocampus plays a crucial role in spatial memory (Bast et al. 2009; Vann and Albasser 2011). Corroborating this evidence, Bast et al. (2009) demonstrated that animals with hippocampal damage were not able to learn new spatial locations within a familiar environment, similar to the results in the present study (Bast et al. 2009). Furthermore, the interaction between the hippocampus and striatum significantly contributes to the initial learning and sequential motor behavior (Ghiglieri et al. 2011; Albouy et al. 2013). Thus, damage to the cerebral cortex, hippocampus, and striatum can directly affect neural memory systems.
Our results showed that Met, MetO, and Met + MetO induced oxidative damage in the brain. This treatment increased ROS and TBARS levels and decreased the SH content and SOD, CAT, and GPx activity in the hippocampus and striatum. The enzyme and non-enzyme antioxidant defenses play an important role as ROS and reactive nitrogen species (RNS) scavengers. ROS and RNS may contribute to brain injury, activating several intracellular signaling cascades, such as mitochondrial and proteasomal dysfunction, and inflammation (Patel 2016; Suzuki et al.2016). In this context, it is plausible that a Met- and/or MetO-induced decrease antioxidant enzymatic activity could lead to an excess of reactive species, such as anion superoxide and hydrogen peroxide, resulting in lipid peroxidation and a disruption of cellular homeostasis in brain structures.
MetO formation from Met is known to be dependent on ROS levels. Also, MetO can also be oxidized by ROS to other metabolites such as methionine sulfone and homocysteic acid (Mudd 2011; Tarrago et al. 2015; Suzuki et al. 2016). Thus, MetO metabolism may happen differently between brain structures since it is dependent of ROS production which is not altered in the cerebral cortex but is increased in the striatum and hippocampus.
Furthermore, nitrite levels were reduced in the hippocampus and striatum of young rats by Met and/or MetO treatment. Nitrite is the product of oxidation of nitric oxide (NO), which is an important intra- and extracellular signaling molecule involved in learning and memory-related processes and mediation of excitatory responses (Paul and Ekambaram 2011; Gasparovic et al. 2016). Decreased nitrite levels, as found in this study, may be an indirect measure of reduced levels of NO. This may be associated with the increase in ROS levels, leading to a reduction in the bioavailability of NO. This is associated with neurodegeneration, cognitive failure, and memory loss, similar to the behavior found in this present investigation (Toda and Okamura 2016).
We have evaluated the effect of chronic treatment of Met and/or MetO on AChE activity. AChE is a crucial enzyme in synaptic transmission as it rapidly hydrolyzes acetylcholine at cholinergic synapses. In addition, AChE has several other important roles in the central nervous system, such as glial activation, postsynaptic differentiation, cell adhesion, and the activation of dopaminergic neurons (Silman and Sussman 2005). Here, we showed that Met and MetO when administered alone or in combination altered the AChE activity in all brain structures evaluated. Similar results were founded by Stefanello et al. (2007a, b), which showed an increase in AChE activity in the cerebral cortex of rats treated with Met.
The increase in the AChE activity in the cerebral cortex and hippocampus may reduce acetylcholine levels, leading to the memory deficits we observed in this study. In addition, data have demonstrated that dysfunctions on cholinergic signaling in the striatum are associated with the pathophysiology of Parkinson’s disease and dystonia (Bohnen and Albin 2010). Considering the key physiological role of AChE, alterations in this enzyme may represent an important mechanism associated with the symptoms, such as cognitive deficits, delayed psychomotor development, and dystonia, noted in patients with hypermethioninemia (Mudd 2011; Schweinberger and Wyse 2016; Nashabat et al. 2018).
The rats treated with MetO and Met + MetO showed a decrease in Na+, K+-ATPase activity in the hippocampus. This alteration may be associated with high ROS levels or reduced the neuron number observed in the same groups. High ROS production can lead to a conformational change in Na+, K+-ATPase and induce the endocytosis, thereby reducing plasma membrane density, and Na+, K+-ATPase activity at the cell surface (Zhang et al. 2008). Schweinberger et al. (2015) showed that although high Met levels inhibited Na+, K+-ATPase activity, the expression and content of this enzyme is increased in the brain of offspring (Schweinberger and Wyse 2016). This result indicates that high ROS can directly influence Na+, K+-ATPase activity.
The Na+, K+-ATPase α3 isoform is highly expressed in neuronal projections, which suggest that it may be the main isoform affected by MetO and Met + MetO, because there is a reduction in the number of neurons (Clausen et al. 2017). Also, it was demonstrated that the Na+, K+-ATPase α3 isoform has functional significance in the control of spatial learning and memory (Holm et al. 2016).
In addition to the decrease in Na+, K+-ATPase activity in the hippocampus, we have demonstrated that MetO and Met + MetO treatment reduced the levels of BDNF. BDNF is essential for hippocampal long-term potentiation (LTP) (Leal et al. 2014, 2015). This is the main form of synaptic plasticity and is indicative of the efficacy of the synaptic information storage, which is the main cellular mechanism correlated learning and memory (Leal et al. 2014, 2015). In addition, BDNF can control differentiation and neuronal survival. In this sense, a decreased hippocampal BDNF concentration corroborates the reduction in NeuN, which also have an important cognitive function (Leal et al. 2014, 2015). Schweinberger et al. (2018a, b) also demonstrated that Met administration in pregnant rats alters the ultrastructure of neurons in the brain of offspring, corroborating the data found in this study (Schweinberger et al. 2018a, b).
Previously, it has been shown that spatial memory training modifies BDNF receptor tyrosine kinase expression in young and old rat hippocampus (Silhol et al. 2007). Thus, the reduction in BDNF levels could be contributing to the spatial memory deficit observed in the present study through the interaction of BDNF via tyrosine kinase receptors.
The hippocampal formation is sensitive to pathophysiological changes, which can affect hippocampal-dependent functions and reduce the production of new neurons and structural changes (Huang et al. 2015). The mechanism that leads to cognitive dysfunction is associated with the hippocampus is complex; however, persistent oxidative stress plays an important role in this function (Huang et al. 2015). Another point to be considered is that DG is a region with the presence of precursor cells of neurons which seems to be the most affected, demonstrating reduction of neurons and increase of apoptosis under toxicological conditions (Rahmeier et al. 2016; Huf et al. 2018). Thus, alterations in this region, as observed in this work, could interfere in the other regions of the hippocampus.
The neurons generated postnatally in the subgranular region of the hippocampus are involved in spatial learning and memory. The generation of new neurons in dentate gyrus is important for hippocampal functioning and has been implicated in spatial memory. Furthermore, it has been demonstrated that MetO and Met + MetO reduce the neurons number in the DG, suggesting that these amino acids may impact hippocampus neurogenesis (Erasso et al. 2013).
Carrasco et al. (2014) reported that MAT1A knout mice did not demonstrate spatial memory deficit or neurogenesis reduction in the hippocampus of mice (Carrasco et al. 2014). This discrepancy in the protocol at least it explains the differences in the results obtained since in our study MAT activity is not reduced and probably there are the production of others metabolites. In addition, unlike the animal model used here, the authors used mice adults while we used young rats which also can be influenced in the results.
In conclusion, the results obtained in the present study showed that chronic exposure to Met and/or MetO induced memory deficits in young rats via increased oxidative stress and AChE activity in the cerebral cortex, hippocampus, and striatum. MetO alone or in combination with Met also decreased Na+, K+-ATPase and BDNF levels, and the number of neurons in the hippocampus (Fig. 7). These data assist with understanding the neurological changes found patients with hypermethioninemia. In addition, these results provide new perspectives for future studies that seek therapeutic targets for this pathology.
Effect of chronic administration of methionine (Met) and methionine sulfoxide (MetO) on the behavioral and neurochemical parameters in young rats. SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, ROS reactive oxygen species, SH-total thiol content, TBARS thiobarbituric acid reactive species, AChE acetylcholinesterase, BDNF Brain-derived neurotrophic factor
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145. https://doi.org/10.1016/S0304-3940(01)01636-6
Albouy G, King BR, Maquet P, Doyon J (2013) Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus 23:985–1004. https://doi.org/10.1002/hipo.22183
Ali SF, LeBel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13:637–648
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110. https://doi.org/10.1007/s10339-011-0430-z
Bast T, Wilson IA, Witter MP, Morris RG (2009) From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol 7:e1000089. https://doi.org/10.1371/journal.pbio.1000089
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149. https://doi.org/10.1016/S0166-4328(01)00291-1
Bohnen NI, Albin R (2010) The cholinergic system and Parkinson disease. Behav Brain Res 22:564–573. https://doi.org/10.1016/j.bbr.2009.12.048
Borta A, Schwarting RKW (2005) Post-trial treatment with the nicotinic agonist metanicotine: differential effects in Wistar rats with high versus low rearing activity. Pharmacol Biochem Behav 80:541–548. https://doi.org/10.1016/j.pbb.2004.12.014
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Carrasco M, Rabaneda LG, Murillo-Carretero M, Ortega-Martínez S, Martínez-Chantar ML, Woodhoo A, Luka Z, Wagner C, Lu SC, Mato JM, Micó JA, Castro C (2014) Glycine N-methyltransferase expression in the hippocampus and its role in neurogenesis and cognitive performance. Hippocampus 24:840–852. https://doi.org/10.1002/hipo.22274
Chamberlin ME, Ubagai T, Mudd SH, Wilson WG, Leonard JV, Chou JY (1996) Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J Clin Invest 98:1021–1027. https://doi.org/10.1172/JCI118862
Chan K, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+-ATPase activity. Anal Biochem 157:1375–1378. https://doi.org/10.1016/0003-2697(86)90640-8
Clausen MV, Hilbers F, Poulsen H (2017) The structure and function of the Na, K-ATPase Isoforms in health and disease. Front Physiol 8:371. https://doi.org/10.3389/fphys.2017.00371
Costa MZ, Silva TM, Flores NP, Schmitz F, Scherer EBS, Viau CM, Saffi J, Barschak AG, Wyse AT, Spanevello RM, Stefanello FM (2013) Methionine and methionine sulfoxide alter parameters of oxidative stress in the liver of young rats: in vitro and in vivo studies. Mol Cell Biochem 384:21–28. https://doi.org/10.1007/s11010-013-1777-5
Couce ML, Bóveda MD, Castiñeiras DE, Corrales FJ, Mora MI, Fraga JM, Mudd SH (2008) Hypermethioninaemia due to methionine adenosyltransferase I/III (MAT I/III) deficiency: diagnosis in an expanded neonatal screening programme. J Inherit Metab Dis 2:S233–S239. https://doi.org/10.1007/s10545-008-0811-3
Dellu F, Fauchey V, LeMoal M, Simon H (1997) Extension of a new two-trial memory task in the rat: influence of environmental context on recognition processes. Neurobiol Learn Mem 67:112–120. https://doi.org/10.1006/nlme.1997.3746
Dos Santos LM, da Silva TM, Azambuja JH, Ramos PT, Oliveira PS, da Silveira EF, Pedra NS, Galdino K, Couto CA, Soares MS, Tavares RG, Spanevello RM, Stefanello FM, Braganhol E (2016) Methionine and methionine sulfoxide treatment induces M1/classical macrophage polarization and modulates oxidative stress and purinergic signaling parameters. Mol Cell Biochem 424:69–78. https://doi.org/10.1007/s11010-016-2843-6
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 17:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Erasso DM, Camporesi EM, Mangar D, Saport S (2013) Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats. Brain Res 1530:1–12. https://doi.org/10.1016/j.brainres.2013.07.035
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115. https://doi.org/10.2174/1570159X13666150716165726
Gahl WA, Bernardini I, Finkelstein JD, Tangerman A, Martin JJ, Blom HJ, Mullen KD, Mudd SH (1988) Transsulfuration in an adult with hepatic methionine adenosyltransferase deficiency. J Clin Invest 81:390–397. https://doi.org/10.1172/JCI113331
Gasparovic AC, Zarkovic N, Zarkovic K, Semen K, Kaminskyy D, Yelisyeyeva O, Bottari SP (2016) Biomarkers of oxidative and nitro-oxidative stress: conventional and novel approaches. Br J Pharmacol 174:1771–1783. https://doi.org/10.1111/bph.13673
Ghiglieri V, Sgobio C, Costa C, Picconi B, Calabresi P (2011) Striatum–hippocampus balance: from physiological behavior to interneuronal pathology. Prog Neurobiol 94:102–114. https://doi.org/10.1016/j.pneurobio.2011.04.005
Holm TH, JostIsaksen T, Glerup S, Heuck A, Bottger P, Füchtbauer E, Nedergaard S, Nyengaard JR, Andreasen M, Nissen P, Lykke-Hartmann K (2016) Cognitive deficits caused by a disease-mutation in the α3 Na+/K+-ATPase isoform. Sci Rep 6:31972. https://doi.org/10.1038/srep31972
Hrncic D, Mikić J, Rasic-Markovic A, Velimirović M, Stojković T, Obrenović R, Rankov-Petrović B, Šušić V, Djuric D, Petronijević N, Stanojlovic O (2016) Anxiety-related behavior in hyperhomocysteinemia induced by methionine nutritional overload in rats: role of the brain oxidative stress. Can J Physiol Pharmacol 94:1074–1082. https://doi.org/10.1139/cjpp-2015-0581
Huang T, Leu D, Zou Y (2015) Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch Biochem Biophys 576:2–7. https://doi.org/10.1016/j.abb.2015.03.014
Huf F, Bandiera S, Müller CB, Gea L, Carvalho FB, Rahmeier FL, Reiter KC, Tortorelli LS, Gomez R, Fernandes MC (2018) Comparative study on the effects of cigarette smoke exposure, ethanol consumption and association: behavioral parameters, apoptosis, glial fibrillary acid protein and S100β immunoreactivity in different regions of the rat hippocampus. Alcohol. https://doi.org/10.1016/j.alcohol.2018.08.009
Leal G, Comprido D, Duarte CB (2014) BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76:639–656. https://doi.org/10.1016/j.neuropharm.2013.04.005
Leal G, Afonso PM, Salazar IL, Duarte CB (2015) Regulation of hippocampal synaptic plasticity by BDNF. Brain Res 1621:82–101. https://doi.org/10.1016/j.brainres.2014.10.019
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marcão A, Couce ML, Nogueira C, Fonseca H, Ferreira F, Fraga JM, Bóveda MD, Vilarinho L (2015) Newborn screening for homocystinuria revealed a high frequency of MATI/III deficiency in Iberian Peninsula. JIMD Rep 20:113–120. https://doi.org/10.1007/8904_2014_400
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moseley AE, Williams MT, Schaefer TL, Bohana CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB (2007) Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27:616–626. https://doi.org/10.1523/JNEUROSCI.4464-06.2007
Mudd SH (2011) Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet Part C 157:3–32. https://doi.org/10.1002/ajmg.c.30293. (Seminars in Medical Genetics)
Mudd SH, Jenden DJ, Capdevila A, Roch M, Levy HL, Wagner C (2000) Isolated hypermethioninemia: measurements of S-adenosylmethionine and choline. Metabolism 49:1542–1547. https://doi.org/10.1053/meta.2000.18521
Mudd SH, Mudd SH, Skovby F (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1279–1327
Nashabat M, Al-Khenaizan S, Alfadhel M (2018) Methionine adenosyltransferase I/III deficiency: beyond the central nervous system manifestations. Ther Clin Risk Manag 14:225–229. https://doi.org/10.2147/TCRM.S151732
Patel M (2016) Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci 37:768–778. https://doi.org/10.1016/j.tips.2016.06.007
Paul V, Ekambaram P (2011) Involvement of nitric oxide in learning and memory processes. Indian J Med Res 133:471–478
Pawlak CR, Schwarting RKW (2002) Object preference and nicotine consumption in rats with high vs. low rearing activity in a novel open field. Pharmacol Biochem Behav 73:679–687. https://doi.org/10.1016/S0091-3057(02)00852-3
Rahmeier FL, Zavalhia LS, Tortorelli LS, Huf F, Géa LP, Meurer RT, Machado AC, Gomez R, Fernandes MDC (2016) The effect of taurine and enriched environment on behaviour, memory and hippocampus of diabetic rats. Neurosci Lett 630:84–92. https://doi.org/10.1016/j.neulet.2016.07.032
Rossato JI, Bevilaqua LRM, Myskiw JC, Medina JH, Izquierdo I, Cammarota M (2007) On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem 14:36–46. https://doi.org/10.1101/lm.422607
Roy R, Niccolini F, Pagano G, Politis M (2016) Cholinergic imaging in dementia spectrum disorders. Eur J Nucl Med Mol Imaging 43:1376–1386. https://doi.org/10.1007/s00259-016-3349-x
Schweinberger BM, Wyse AT (2016) Mechanistic basis of hypermethioninemia. Amino Acids 48:2479–2489. https://doi.org/10.1007/s00726-016-2302-4
Schweinberger BM, Turcatel E, Rodrigues AF, Wyse ATS (2015) Gestational hypermethioninaemia alters oxidative/nitrative status in skeletal muscle and biomarkers of muscular injury and inflammation in serum of rat offspring. Int J Exp Pathol 96:277–284. https://doi.org/10.1111/iep.12136
Schweinberger BM, Rodrigues AF, Dos Santos TM, Rohden F, Barbosa S, da Luz Soster PR, Partata WA, Faccioni-Heuser MC, Wyse ATS (2018a) Methionine administration in pregnant rats causes memory deficit in the offspring and alters ultrastructure in brain tissue. Neurotox Res 33:239–246. https://doi.org/10.1007/s12640-017-9830-x
Schweinberger BM, Rodrigues AF, Turcatel E, Pierozan P, Pettenuzzo LF, Grings M, Scaini G, Parisi MM, Leipnitz G, Streck EL, Barbé-Tuana FM, Wyse ATS (2018b) Maternal hypermethioninemia affects neurons number, neurotrophins levels, energy metabolism, and Na+, K+-atpase expression/content in brain of rat offspring. Mol Neurobiol 55:980–988. https://doi.org/10.1007/s12035-017-0383-z
Silhol M, Arancibia S, Maurice T, Tapia-Arancibia L (2007) Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience 146:962–973. https://doi.org/10.1016/j.neuroscience.2007.02.013
Silman I, Sussman JL (2005) Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol 5:293–302. https://doi.org/10.1016/j.coph.2005.01.014
Soares MS, Oliveira PS, Debom GN, da Silveira Mattos B, Polachini CR, Baldissarelli J, Morsch VM, Schetinger MR, Tavares RG, Stefanello FM, Spanevello RM (2017a) Chronic administration of methionine and/or methionine sulfoxide alters oxidative stress parameters and ALA-D activity in liver and kidney of young rats. Amino Acids 49:129–138. https://doi.org/10.1007/s00726-016-2340-y
Soares MSP, Viau CM, Saffi J, Costa MZ, da Silva TM, Oliveira PS, Azambuja JH, Barschak AG, Braganhol E, Wyse AT, Spanevello RM, Stefanello FM (2017b) Acute administration of methionine and/or methionine sulfoxide impairs redox status and induces apoptosis in rat cerebral cortex. Metab Brain Dis 32:1693–1703. https://doi.org/10.1007/s11011-017-0054-9
Stefanello FM, Chiarani F, Kurek AG, Wannmacher CMD, Wajner M, Wyse AT (2005) Methionine alters Na+, K+-ATPase activity, lipid peroxidation and nonenzymatic antioxidant defenses in rat hippocampus. Int J Dev Neurosci 23:651–656. https://doi.org/10.1016/j.ijdevneu.2005.06.003
Stefanello FM, Matté C, Scherer EB, Wannmacher CMD, Wajner M, Wyse ATS (2007a) Chemically induced model of hypermethioninemia in rats. J Neurosci Methods 160:1–4. https://doi.org/10.1016/j.jneumeth.2006.07.029
Stefanello FM, Monteiro SC, Matté C, Scherer EB, Netto CA, Wyse AT (2007b) Hypermethioninemia increases cerebral acetylcholinesterase activity and impairs memory in rats. Neurochem Res 32:1868–1874. https://doi.org/10.1007/s11064-007-9464-0
Stefanello FM, Matté C, Pederzolli CD, Kolling J, Mescka CP, Lamers ML, Assis AM, Perry ML, Santos MF, Dutra-Filho CS, Wyse ATS (2009) Hypermethioninemia provokes oxidative damage and histological changes in liver of rats. Biochimie 91:961–968. https://doi.org/10.1016/j.biochi.2009.04.018
Stefanello FM, Ferreira AG, Pereira TC, da Cunha MJ, Bonan CD, Bogo MR, Wyse AT (2011) Acute and chronic hypermethioninemia alter Na+, K+-ATPase activity in rat hippocampus: prevention by antioxidants. Int J Dev Neurosci 29:483–488. https://doi.org/10.1016/j.ijdevneu.2011.02.001
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555. https://doi.org/10.1084/jem.169.5.1543
Suzuki S, Kodera Y, Saito T, Fujimoto K, Momozono A, Hayashi A, Kamata Y, Shichiri M (2016) Methionine sulfoxides in serum proteins as potential clinical biomarkers of oxidative stress. Sci Rep 6:38299. https://doi.org/10.1038/srep38299
Tarrago L, Péterfi Z, Lee BC, Michel T, Gladyshev VN (2015) Monitoring methionine sulfoxide with stereospecific mechanism-based fluorescent sensors. Nat Chem Biol 11:332–338. https://doi.org/10.1038/nchembio.1787
Toda N, Okamura T (2016) Hyperhomocysteinemia impairs regional blood flow: involvements of endothelial and neuronal nitric oxide. Pflugers Arch 468:1517–1525. https://doi.org/10.1007/s00424-016-1849-y
Vann SD, Albasser MM (2011) Hippocampus and neocortex: recognition and spatial memory. Curr Opin Neurobiol 21:440–445. https://doi.org/10.1016/j.conb.2011.02.002
Vuaden FC, Savio LE, Piato AL, Pereira TC, Vianna MR, Bogo MR, Bonan CD, Wyse AT (2012) Long-term methionine exposure induces memory impairment on inhibitory avoidance task and alters acetylcholinesterase activity and expression in zebrafish (Danio rerio). Neurochem Res 37:1545–1553. https://doi.org/10.1007/s11064-012-0749-6
Wyse AT, Streck EL, Barros SV, Brusque AM, Zugno AI, Wajner M (2007) Methylmalonate administration decreases Na+, K+-ATPase activity in cerebral cortex of rats. NeuroReport 11:2331–2334. https://doi.org/10.1097/00001756-200007140-00052
Zhang L, Zhang Z, Guo H, Wang Y (2008) Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol 22:615–621. https://doi.org/10.1111/j.1472-8206.2008.00620.x
Acknowledgements
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Handling Editor: M. Engelmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soares, M.S.P., de Mattos, B.d.S., de Souza, A.Á. et al. Hypermethioninemia induces memory deficits and morphological changes in hippocampus of young rats: implications on pathogenesis. Amino Acids 52, 371–385 (2020). https://doi.org/10.1007/s00726-019-02814-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02814-2