Abstract
In mammalian tissues, taurine is an important natural component and the most abundant free amino acid in the heart, retina, skeletal muscle, brain, and leukocytes. This study is to examine the taurine’s protective effects on neuronal ultrastructure, the function of the mitochondrial respiratory chain complex, and on cerebral blood flow (CBF). The model of traumatic brain injury (TBI) was made for SD rats by a fluid percussion device, with taurine (200 mg/kg) administered by tail intravenous injection once daily for 7 days after TBI. It was found that CBF was improved for both left and right brain at 30 min and 7 days post-injury by taurine. Reaction time was prolonged relative to the TBI-only group. Neuronal damage was prevented by 7 days taurine. Mitochondrial electron transport chain complexes I and II showed greater activity with the taurine group. The improvement by taurine of CBF may alleviate edema and elevation in intracranial pressure. Importantly taurine improved the hypercoagulable state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is an important clinical problem, brings not only high mortality but much severe sequelae, such as movement obstacles and cognitive deficits. Acute TBI is characterized by a primary and a secondary injury. Primary brain injury is the direct injury to the brain parenchyma at the time of the initial impact, which can be both focal and diffuse depending on the biomechanics of the impact. The secondary brain injury is caused by a combination of neuronal and vascular damage, proteolytic pathways, excitotoxicity, oxygen-free radicals, apoptosis, inflammatory processes, and ischemia (Graham et al. 1993; McIntosh 1994; Werner and Engelhard 2007). In fact, the time-window between primary and secondary damage may offer a valuable opportunity for treatment for patients to be administered. Therefore, the fast and appropriate drug and nutritional intervention is beneficial to improve survival rates and prognosis.
Taurine (2-aminoethane sulfonic acid) is a conditionally essential, non-protein amino acid present in blood and tissues of mammals. It exists in the neurons by free form, which is an important natural nutritional factor in the process of brain growth and development. Natural taurine has emerged as an alternative candidate for therapeutic intervention since it is involved in neuroprotection and regeneration after injury in the nervous system (Rak et al. 2014; Menzie et al. 2014). Many studies have demonstrated that taurine plays critical role in the aspects of detoxification, membrane stabilization, osmoregulation, neurotransmission, calcium homeostasis, and antioxidant (Huxtable 1992; Schaffer et al. 2000; Georgia et al. 2003; El Idrissi 2008). A recent study suggested that supplementation with taurine and other nutrients are helpful for patients’ recovery from TBI (Curtis and Epstein 2014). Except for TBI model, based on many other experimental models, taurine was also widely studied. Sun et al. (2012) demonstrated that treatment with taurine markedly reduced neurological deficits, lessened brain swelling, attenuated cell death, and decreased the infarct volume 72 h after ischemia in a rat model of stroke. They also demonstrated the dose-dependent protection of taurine against experimental closed head injury in rats (Sun et al. 2015). Yang et al. (2013) reported that taurine had beneficial effects on inhibiting mitochondria-dependent cell apoptosis in rat cardiomyocytes.
Our previous paper has investigated the interaction of taurine with the cascade of inflammation and examined the impact of taurine on cerebral edema, astrocyte activity and neurological function in a rat model of TBI (Su et al. 2014). In the present paper, we evaluated the multifaceted protective effects of taurine based on TBI model, which refer to the ultrastructure of nerve cells in cortex and hippocampus, function of mitochondria respiratory chain complex, state of hemodynamics and change of cerebral blood flow (CBF).
Materials and methods
Reagents
Taurine, defatted BSA and coomassie brilliant blue were purchased from Sigma (St. Louis, MO, USA). Janus Green B was from Fluka. The kits of tissue mitochondria isolation (GMS10006.3) and mitochondria Complex I (GMS50007), II (GMS50008), IV (GMS500010) reagents were all purchased from GenMed Company (Shanghai, China). Thrombelastography (TEG) kit was from Haemoscope Corporation. Sucrose, chloral hydrate, paraformaldehyde and glutaraldehyde were of analytical grade.
TBI model
Adult male Sprague–Dawley rats, weighing between 280 ± 20 g were obtained from the Laboratory Animal Center of Academy of Military Medical Sciences (Beijing, China). Animals were maintained under specific pathogen-free conditions (25–28 °C, relative humidity 55 ± 15 %, 12-h light/dark cycles). Thirty rats were randomized into three groups (ten rats each group). (1) Sham group: rats were subjected to identical surgical procedures except for brain injury, and administered saline only; (2) TBI group: rats were subjected to lateral fluid percussion injury (FPI) in the left brain and received saline only; (3) taurine group: rats were administered with taurine (200 mg/kg) by tail intravenous injection, once daily for 7 days after TBI. All rats were killed 7 days after TBI for further analysis.
TBI model was modified from previous study (Kelley et al. 2006). Rats were intraperitoneally anesthetized with 10 % chloral hydrate (10 μl/100 g), and then immobilized in a stereotaxic frame. After exposing the skull, a 5.0-mm craniotomy was performed over the left parietal bone, 4.5 mm posterior from bregma and 2.5 mm lateral to the sagittal suture, ensuring the integrity of the dura. A female Luer-Lock fitting was then cemented to the skull with craniofacial cement. The rats were connected to the fluid percussion device (University of Virginia, USA) via the Leur-Lock fitting, and an overpressure of 1.8 atmosphere (atm) was delivered to cause a moderate brain injury. Body temperature was monitored by a rectal probe, maintaining at 37 ± 1 °C with a heating pad during the experiment. After surgery, the skin was closed with staples and rats were allowed to recover from anesthesia.
Monitor of cerebral blood flow
A laser Doppler device (LDF, Periflux System 5000, Perimed AB, Sweden) was used to measure CBF. After exposing the skull, two 1.0 mm bone windows were performed over the left and right skull, which were 2 mm posterior from bregma and 2 mm lateral to the middle line, ensuring the integrity of the dura. Two 403 probes were then symmetrically fixed on the left and right bone windows for CBF monitoring. There are three monitoring time points including pre-injury, 30 min and day 7 of post-injury, lasting 30 min for every point. The mean CBF values (Perimed Unit, Pu) for the three time points were automatically calculated by the LDF software.
Detection of hemodynamics
Before animals were killed, sodium citrate anti-coagulated whole blood from carotid vein blood was obtained. The changes of hemodynamics were detected by Thrombelastography (TEG®5000 Hemostatic Analyzer, Haemoscope Corporation, USA). The detection was according to the instruction of TEG. The parameters include reaction time (R), clot kinetics (K, α), maximum amplitude (MA), time to MA and coagulation index (CI). The implications of the six coagulation parameters were given in the Table 1.
Transmission electron microscopy (TEM) of neuron in cortex and hippocampus
Two rats in each group were for TEM detection. The cerebral cortex and hippocampus tissues of injured left brain were separated quickly and cut into 1 mm3 sections. The specimen was first fixed with 2.5 % glutaraldehyde in phosphate buffer saline (PBS) for more than 4 h, washed three times with PBS, then post-fixed with 1 % OsO4 in PBS for 1 h and washed. The specimen was then dehydrated by gradient concentration of ethanol (30, 50, 70, 80, 90, 95 and 100 %) for about 15–20 min at each step, transferred to absolute acetone for 20 min. The specimen was placed in the mixture of acetone and resin and kept for appropriate temperature and time. Specimen was placed in capsules contained embedding medium and heated at 70 °C for about 9 h. The specimen sections were stained by uranyl acetate and alkaline lead citrate for 15 min, respectively, and finally observed by the transmission electron device (Philips EM208s, Philips Co Holland).
Detection of mitochondrial respiration chain enzyme activity
Eight rats in each group were used for enzyme detection, which were killed by decapitation under anesthesia. The brains were separated quickly on ice, then washed with cold PBS and weighed. The brains were put into the cold GenMed lysis buffer (5 ml/g) and vortexed for 5 s. The tissues were then homogenized for 8–10 times by electro-homogenizer (ULTRA-TURRAX T8, IKA-Werke Co., Germany). The homogenate was centrifuged at 1500g for 10 min at 4 °C. The supernatant was collected and centrifuged at 10,000g for 10 min at 4 °C. The pellet (crude mitochondria) was collected and resuspended with 3 ml GenMed store medium, centrifuged at 10,000g for 5 min at 4 °C. The acquired pellet was the purified mitochondria. It was resuspended with 1 ml GenMed store buffer. The activity and purity of mitochondrial isolation were identified by Janus green B stain and electro-scope detection. The mitochondrial protein concentration was determined using the Bradford method. Finally, the activities of Complex I (reduced nicotinamide adenine dinucleotide dehydrogenase, NADH dehydrogenase), Complex II (succinate dehydrogenase, SDH) and Complex IV (cytochrome C oxidoreductase, CCO) were detected according to the instructions based on enzyme-substrate reaction.
Statistical analysis
Data were analyzed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). All values are expressed as mean ± SD. Comparisons between groups were statistically evaluated by Student’s test or one way ANOVA with a post hoc Fisher’s test. Graphpad Prism 5.0 was used to create the artwork. A probability of P < 0.05 was considered to be statistically significant.
Results
Comparison of CBF
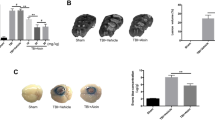
Compared with the sham group, CBF of the left brain cortex obviously decreased for TBI and taurine groups 30 min after injury. After taurine treatment for 7 days, CBF of the left brain cortex in the taurine group became significantly higher than in the TBI group (Fig. 1a). The change trend of CBF in the right brain cortex was similar as that in the left brain (Fig. 1b). However, CBF of the right brain cortex was significantly increased than that of the left brain cortex for TBI and taurine group 30 min after injury (P < 0.05). There was no significant difference to be monitored for CBF of the left and right brain cortex 7 days after injury.
CBF changes of the left and right brain cortex. Three monitoring time points were pre-injury, 30 min and day 7 after injury, lasting 30 min for every point. a CBF change of the left brain cortex; b CBF change of the right brain cortex. Data are expressed as mean ± SD, n = 10. *P < 0.05 vs. sham group, # P < 0.05 vs. TBI group
Changes of hemodynamics
Reaction time in taurine group was significantly prolonged compared with TBI group (P < 0.05). Compared with the sham group, Time to MA was significantly shortened in TBI group. There was no difference to be observed for the other parameters (Table 1).
The ultrastructure of nerve cells
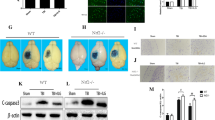
Compared with the sham group, the ultrastructure of some cells in cortex and hippocampus was severely damaged in the TBI group characterized by cell shrinkage and denaturalization, mitochondrion swelling, reticulum expansion and late-onset hematoma (Fig. 2b, e, h, k). After taurine treatment for 7 days, the nerve cells almost returned to normal with the performance of integrated nuclear membrane, normal organelles structure and uniform chromatin density (Fig. 2c, f, i, l).
The ultrastructure changes of neurons in cortex and hippocampus. The sham group, TBI group and taurine group were in turn listed from left to right for each row. a–f The low-power (×6000) and high-power (×16,000) fields of neurons in cortex, respectively. g–l The low-power (×6000) and high-power (×16,000) fields of neurons in hippocampus, respectively
The enzyme activity of mitochondrial respiratory chain
Complex I activity of taurine group significantly increased than that of TBI group for the left brain (Fig. 3a). Complex II activities of taurine group significantly increased than that of TBI group for both the left and right brain (Fig. 3b). There was no difference to be detected for Complex IV activity among the three groups, while Complex IV activity for the left brain was significantly higher than the right brain (P < 0.05) (Fig. 3c).
The brain tissue mitochondria respiration chain complexes activities. Mitochondria were exacted by differential centrifugation at 4 °C, and protein concentration was measured by the Bradford method. a Complex I (NADH dehydrogenase); b Complex II (SDH); c Complex IV (CCO). Data are expressed as mean ± SD, n = 8. *P < 0.05 vs. sham group, # P < 0.05 vs. TBI group
Discussion
CBF reduction appears at the early stage of TBI. CBF decreases and brain swelling after TBI is the result of edema, which results in elevation of the intracranial pressure (ICP) with subsequent impairment of cerebral perfusion and metabolism (Jaggi et al. 1990; Soustiel and Sviri 2007). Our result showed that CBF immediately decreased at 30 min post-injury, which was coincided with some other studies (Yuan et al. 1988; Hlatky et al. 2004; Dagal and Lam 2011). Regulation of CBF is extremely complex. Studies have identified three main regulatory paradigms involved in the regulation of CBF: cerebral autoregulation, flow-metabolism coupling, and neurogenic regulation. (Peterson et al. 2011). Cerebral autoregulation can be impaired in any degree of TBI, which is one of the multiple factors involved in the pathophysiology of TBI. In fact, reduced CBF in clinical and experimental TBI settings is well known and can contribute to ischemia and brain damage (Marion et al. 1991a; Rangel-Castilla et al. 2008; Kallakuri et al. 2015). Taurine, as a neuroprotective agent, has been reported to protect from TBI through several different inhibitory mechanisms referring to inhibiting cytotoxicity and apoptosis, reducing edema and inflammation, lowing oxidative stress, and depressing mitochondria-mediated cell death, etc. (Aly and Khafagy 2014; Bouma et al. 1991; Kim and Cha 2014; Setyarani et al. 2014; Zhou et al. 2006). Our data showed that taurine treatment significantly improved CBF of injured ipsilateral and contralateral brain cortex at 30 min post-injury. CBF increased more obviously after taurine treatment for 7 days. We consider the synthesizing effects of taurine make it beneficial to improve CBF and further alleviate edema, ICP elevation and the other brain dysfunctions.
Coagulopathy is a common phenomenon in TBI and a major contributor to a poor outcome, therefore, early diagnosis and intervention is important. At present, TEG has resurfaced as an ideal test in the trauma population to help guide the clinician in the administration of blood components in a goal directed fashion (Luddington 2005; Windeløv et al. 2011; Johansson et al. 2013). TBI is associated with the hypercoagulable state, the mechanism and duration of which remain unclear. Taurine is known for its beneficial effects in hypercoagulable state (Guan et al. 2011; Roşca et al. 2013). Recently, Tapia et al. (2013) have utilized TEG to analyze the influence of taurine on hemostatic profile in rats, which suggested that taurine exerts a protective effect against the hypercoagulable state. In this study, we sought to observe whether taurine could improve the hypercoagulable state for TBI by detecting parameters of the coagulation profile. The data showed that taurine significantly increase reaction time compared with TBI group, which suggested that taurine could improve the hypercoagulable state by exerting the similar effect as anticoagulants.
Mitochondria are the important intracellular organelles of eukaryote that generate energy for cellular processes by producing ATP (Papa 1996). Abnormalities in mitochondrial morphology cause key events in the progression of neuronal injury and are commonly observed in TBI (Balan et al. 2013; Hiebert et al. 2015). The ultrastructural figures in our study showed that mitochondria became swelling both in cortex and hippocampus after TBI, whereas taurine treatment for 7 days could alleviate the swelling of mitochondria and other organelles.
The mitochondrial respiratory chain is located on the inner membrane of mitochondria and comprised of four large trans-membrane protein complexes (Complexes I–IV). After TBI, mitochondria early happen to pathological changes accompany with energy metabolism dysfunction (Chen and Chan 2009; Liesa et al. 2009; Jahani-Asl et al. 2011). Studies have demonstrated that the respiratory enzyme activity decreased after TBI (Xiong et al. 1997) and ischemic-hypoxia brain injury (Keelan et al. 1999). TBI can also influence the enterocyte mitochondrial respiratory function and enzyme activities leading to gastrointestinal dysfunction (Zhu et al. 2014). A recent study supposed a hypothesis that post-traumatic cytotoxic edema is directly related to mitochondrial function (Vlodavsky et al. 2015). Taurine has been proven to improve the activity of respiratory chain complex by depressing mitochondria-mediated cell death (Sun et al. 2011), removing free radicals and lowering oxidative stress (Sun et al. 2014). The study based on taurine-deficient heart model showed that the taurine-deficient heart is energy starved primarily because of impaired respiratory chain function (Schaffer et al. 2016). Schaffer et al. found that taurine deficiency reduced the biosynthesis of mitochondria encoded proteins ND5 and ND6, which was consistent with their finding that respiratory chain complex I activity was also reduced by taurine deficiency. Since the assembly of the respiratory chain complexes depends upon an abundant supply of mitochondria encoded proteins. They therefore suggested a novel mechanism that taurine specifically alters complex I through alterations in mitochondrial protein biosynthesis, which provides an explanation for the antioxidant activity of taurine (Jong et al. 2012; Schaffer et al. 2014a, b). Our results showed that taurine significantly increased the activities of mitochondrial respiratory Complexes I and II, which was consistent with the studies of Schaffer et al. We considered it might be also related with the effect of taurine on regulating CBF and alleviating mitochondria swelling. Because of the importance of mitochondrial respiratory chain complex in oxygen supply, these results gave more evidence for taurine functioning as an indirect antioxidant.
It has been evidenced that increased oxidative stress was associated with mitochondrial dysfunction characterized by enhanced superoxide and reactive oxygen species generation, the inactivation of the oxidant sensitive enzyme, and the oxidation of glutathione. Some studies have reported the underlying mechanism about the anti-oxidative ability of taurine. The results from Schaffer et al. demonstrated that taurine’s antioxidant activity is linked to improved mitochondrial function, which diminishes mitochondrial superoxide generation. They also supplied that taurine could regulate mitochondrial protein synthesis, thereby enhancing electron transport chain activity and protecting the mitochondria against excessive superoxide generation (Schaffer et al. 2014a, b; Shimada et al. 2015). According to our results, we conclude that taurine may exert antioxidant effect by improving the damaged activities of mitochondrial respiratory chain caused by TBI.
The inhibition on neuronal cell apoptosis is another important effect of taurine. High levels of oxidants generated by mitochondrial dysfunction following with TBI are key contributors to neuronal cell death through necrotic and apoptotic mechanisms. High levels of oxidants elicit neuronal apoptosis through the actions of proapoptotic Bcl-2 family members resulting in mitochondrial permeability transition pore opening. In addition, accumulation of misfolded proteins and high levels of oxidants can elicit endoplasmic reticulum (ER) stress pathways which may also contribute to induction of apoptosis. Actually we have studied the anti-apoptosis effect of taurine on hypoxic glial cells. We detected the apoptosis rate and relative mRNA expression level of Bax and Bcl-2 by flow cytometry and RT-PCR methods, respectively. Our data showed that taurine was beneficial to low the apoptosis rate and increase Bcl-2 mRNA expression (data not shown in the present manuscript). To protect against extraneous stresses, cells possess quality control processes, such as the ubiquitin–proteasome system (UPS) and autophagy, which can rejuvenate cells through the degradation of damaged proteins and organelles. Jong et al. (2015) demonstrated that taurine deficiency triggers the induction of the UPS and autophagy but the degradation activity that mediates cellular clearance is defective. Wu et al. demonstrated that taurine could prevent calcium overload and was also capable of preventing ER stress by inhibiting specific ER stress pathways (Pan et al. 2012; Gharibani et al. 2013; Prentice et al. 2015).
Cerebral hypoxia and/or ischemia following with brain injury elicit neuronal glutamate release increasing. Glutamate excitotoxicity is known to result in calcium overload, mitochondrial dysfunction, high level generation of oxidants and a loss of mitochondrial membrane potential. Wu et al. have done a great deal of work about the anti-glutamate toxicity and calcium overload effects of taurine based on animal and neuronal cell stroke models. They found that taurine protected against glutamate excitotoxicity and cellular damage through the regulation of three ER stress pathways, i.e., suppressing ATF6 and IRE1 pathways (Pan et al. 2012; Gharibani et al. 2013; Prentice et al. 2015). Our results showed that taurine could alleviate mitochondria swelling and increase the activity of mitochondrial respiratory complex. Since mitochondrial function is closely linked to glutamate toxicity, calcium overload and oxidative stress, we concluded taurine also exerted the anti-glutamate toxicity effect by keeping the normal mitochondria morphological structure and respiratory complex activity.
Inflammation is also an important event in brain injury. TBI can also elicit a complex inflammatory responses characterized by glial activation, neutrophil and macrophage recruitment, upregulation of adhesion molecules, and secretion of cytokines (Werner and Engelhard 2007; Lotocki et al. 2009), which cause diffuse brain edema and neurological functional deficits. Taurine has been reported to have potent anti-inflammation effects by diminishing the productions of cytokines, such as IL-1b, IL-6 and TNF-α in the spinal cord injury and ischemic stroke models (Nakajima et al. 2010; Sun et al. 2012). Our previous study showed that taurine could reduce inflammation in TBI by decreasing the expressions of pro-inflammatory cytokines including IL-1α, IL-1β, TNF-α, IFN-γ, IL-6, GM-CSF, etc. (Su et al. 2014). Our present study showed that taurine was beneficial to alleviate mitochondria swelling which may be associated with its function on diminishing the productions of pro-inflammatory cytokines.
In summary, we demonstrated the protective effects of Taurine on SD rats following with TBI referring to regulating coagulation and CBF state, reducing neuron damage in cortex and hippocampus and improving enzyme activity of mitochondria. The data provided evidences to support the hypothesis that taurine exerts protective function through various pathways. Further studies are needed to elucidate the detailed mechanism by which taurine protects the brain from traumatic injury.
References
Aly HA, Khafagy RM (2014) Taurine reverses endosulfan-induced oxidative stress and apoptosis in adult rat testis. Food Chem Toxicol 64:1–9
Balan IS, Saladino AJ, Aarabi B, Castellani RJ, Wade C, Stein DM, Eisenberg HM, Chen HH, Fiskum G (2013) Cellular alterations in human traumatic brain injury: changes in mitochondrial morphology reflect regional levels of injury severity. J Neurotrauma 30:367–381
Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF (1991) Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg 75:685–689
Chen H, Chan DC (2009) Mitochondrial dynamics-fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18:169–176
Curtis L, Epstein P (2014) Nutritional treatment for acute and chronic traumatic brain injury patients. J Neurosurg Sci 58:151–160
Dagal A, Lam AM (2011) Cerebral blood flow and the injured brain: how should we monitor and manipulate it. Curr Opin Anaesthesiol 24:131–137
El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328
Georgia B, Schuller L, Eunkyue P (2003) Taurine: new implications for an old amino acid. FEMS Microbiol Lett 226:195–202
Gharibani PM, Modi J, Pan C, Menzie J, Ma Z, Chen PC, Tao R, Prentice H, Wu JY (2013) The mechanism of taurine protection against endoplasmic reticulum stress in an animal stroke model of cerebral artery occlusion and stroke-related conditions in primary neuronal cell culture. Adv Exp Med Biol 776:241–258
Graham DI, Adams JH, Doyle D, Ford I, Gennarelli TA, Lawrence AE, Maxwell WL, McLellan DR (1993) Quantification of primary and secondary lesions in severe head injury. Acta Neurochir Suppl (Wien) 57:41–48
Guan W, Zhao Y, Xu C (2011) A combined treatment with taurine and intra-arterial thrombolysis in an embolic model of stroke in rats: increased neuroprotective efficacy and extended therapeutic time window. Transl Stroke Res 2:80–91
Hiebert JB, Shen Q, Thimmesch AR, Pierce JD (2015) Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci 350:132–138
Hlatky R, Contant CF, Diaz-Marchan P, Valadka AB, Robertson CS (2004) Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit Care 1:69–83
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW (1990) Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. J Neurosurg 72:176–182
Jahani-Asl A, Pilon-Larose K, Xu W, MacLaurin JG, Park DS, McBride HM, Slack RS (2011) The mitochondrial inner membrane GTPase, optic atrophy 1 (Opa1), restores mitochondrial morphology and promotes neuronal survival following excitotoxicity. J Biol Chem 286:4772–4782
Johansson PI, Sørensen AM, Larsen CF, Windeløv NA, Stensballe J, Perner A, Rasmussen LS, Ostrowski SR (2013) Low hemorrhage-related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion 53:3088–3099
Jong CJ, Azuma J, Schaffer S (2012) Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42:2223–2232
Jong CJ, Ito T, Schaffer SW (2015) The ubiquitin-proteasome system and autophagy are defective in the taurine-deficient heart. Amino Acids 47:2609–2622
Kallakuri S, Bandaru S, Zakaria N, Shen Y, Kou Z, Zhang L, Haacke EM, Cavanaugh JM (2015) Traumatic brain injury by a closed head injury device induces cerebral blood flow changes and microhemorrhages. J Clin Imaging Sci 5:52
Keelan J, Timothy EB, Clark BJ (1999) Heightened resistance of the neonatal brain to ischemia-reperfusion involves a lack of mitochondrial damage in the nerve terminal. Brain Res 821:124–133
Kelley BJ, Farkas O, Lifshitz J, Povlishock JT (2006) Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol 198:350–360
Kim C, Cha YN (2014) Taurine chloramine produced from Taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 46:89–100
Liesa M, Palacín M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89:799–845
Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM, Dietrich WD (2009) Alterations in blood–brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J Neurotrauma 26:1123–1134
Luddington RJ (2005) Thrombelastography/thromboelastometry. Clin Lab Heamatol 27:81–90
Marion DW, Darby J, Yonas H (1991) Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg 74:407–414
McIntosh TK (1994) Neurochemical sequelae of traumatic brain injury: therapeutic implications. Cerebrovasc Brain Metab Rev 6:109–162
Menzie J, Pan C, Prentice H, Wu JY (2014) Taurine and central nervous system disorders. Amino Acids 46:31–46
Nakajima Y, Osuka K, Seki Y, Gupta RC, Hara M, Takayasu M, Wakabayashi T (2010) Taurine reduces inflammatory responses after spinal cord injury. J Neurotrauma 27:403–410
Pan C, Prentice H, Price AL, Wu JY (2012) Beneficial effect of taurine on hypoxia- and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43:845–855
Papa S (1996) Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim Biophys Acta 1276:87–105
Peterson EC, Wang Z, Britz G (2011) Regulation of cerebral blood flow. Int J Vasc Med 2011:823525
Prentice H, Modi JP, Wu JY (2015) Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev 2015:964518
Rak K, Völker J, Jürgens L, Scherzad A, Schendzielorz P, Radeloff A, Jablonka S, Mlynski R, Hagen R (2014) Neurotrophic effects of taurine on spiral ganglion neurons in vitro. Neuroreport 25:1250–1254
Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS (2008) Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus 25:E7
Roşca AE, Badiu C, Uscătescu V, Stoian I, Mirică R, Braga RI, Pavel B, Zăgrean L (2013) Influence of chronic administration of anabolic androgenic steroids and taurine on haemostasis profile in rats: a thrombelastographic study. Blood Coagul Fibrinolysis 24:256–260
Schaffer S, Takahashi K, Azuma J (2000) Role of osmoregulation in the actions of taurine. Amino Acids 19:527–546
Schaffer SW, Jong CJ, Ito T, Azuma J (2014a) Effect of taurine on ischemia-reperfusion injury. Amino Acids 46:21–30
Schaffer SW, Shimada K, Jong CJ, Ito T, Azuma J, Takahashi K (2014b) Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids 46:1147–1157
Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K (2016) Impaired energy metabolism of the taurine-deficient heart. Amino Acids 48:549–558
Setyarani M, Zinellu A, Carru C, Zulli A (2014) High dietary taurine inhibits myocardial apoptosis during an atherogenic diet: association with increased myocardial HSP70 and HSF-1 but not caspase 3. Eur J Nutr 53:929–937
Shimada K, Jong CJ, Takahashi K, Schaffer SW (2015) Role of ROS production and turnover in the antioxidant activity of taurine. Adv Exp Med Biol 803:581–596
Soustiel JF, Sviri GE (2007) Monitoring of cerebral metabolism: non-ischemic impairment of oxidative metabolism following severe traumatic brain injury. Neurol Res 29:654–660
Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, Huang H (2014) Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 266:56–65
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of Taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429
Sun M, Zhao Y, Gu Y, Xu C (2012) Anti-inflammatory mechanism of Taurine against ischemic stroke is related to down-regulation of PARP and NF-κB. Amino Acids 42:1735–1747
Sun Q, Hu H, Wang W, Jin H, Feng G, Jia N (2014) Taurine attenuates amyloid β1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem Biophys Res Commun 447:485–489
Sun M, Zhao Y, Gu Y, Zhang Y (2015) Protective effects of taurine against closed head injury in rats. J Neurotrauma 32:66–74
Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, Mattox KL, Suliburk J (2013) TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg 74:378–386
Vlodavsky E, Palzur E, Shehadeh M, Soustiel JF (2015) Post-traumatic cytotoxic edema is directly related to mitochondrial function. J Cereb Blood Flow Metab. doi:10.1177/0271678X15621068
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesthesiol 99:4–9
Windeløv NA, Welling KL, Ostrowski SR, Johansson PI (2011) The prognostic value of thrombelastography in identifying neurosurgical patients with worse prognosis. Blood Coagul Fibrinolysis 22:416–419
Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP (1997) Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma 14:23–34
Yang Y, Zhang Y, Liu X, Zuo J, Wang K, Liu W, Ge J (2013) Exogenous Taurine attenuates mitochondrial oxidative stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta Biochim Biophys Sin (Shanghai) 45:359–367
Yuan XQ, Prough DS, Smith TL, Dewitt DS (1988) The effects of traumatic brain injury on regional cerebral blood flow in rats. J Neurotrauma 5:289–301
Zhou F, Guo J, Yang R, Gu J, Jin H, Wu G, Cheng J (2006) Effects of Taurine on cerebral blood flow perfusion, cell apoptosis, and infarct volume in acute cerebral ischemic rats. Adv Exp Med Biol 583:353–358
Zhu KJ, Huang H, Chu H, Yu H, Zhang SM (2014) Alterations in enterocyte mitochondrial respiratory function and enzyme activities in gastrointestinal dysfunction following brain injury. World J Gastroenterol 20:9585–9591
Acknowledgments
The authors would like to thank support from the National Natural Science Foundation of China (Grant Nos. 30973089 and 81571216).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Ethical standards
This study was approved by Institutional Ethics Review Committee of Tianjin Neurosurgical Institute, China. All procedures performed in studies involving animals were in accordance with the ethical standards of Tianjin Neurosurgical Institute at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Wang, Q., Fan, W., Cai, Y. et al. Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids 48, 2169–2177 (2016). https://doi.org/10.1007/s00726-016-2244-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2244-x