Abstract
The objective of this study was to evaluate effects of dietary crude protein (CP) intake on ileal amino acid digestibilities and expression of genes for digestive enzymes in growing and finishing pigs. In Experiment 1, 18 growing pigs (average initial BW = 36.5 kg) were assigned randomly into one of three treatments (n = 6/treatment group) representing normal (18 % CP), low (15 % CP), and very low (12 % CP) protein intake. In Experiment 2, 18 finishing pigs (average initial BW = 62.3 kg) were allotted randomly into one of three treatments (n = 6/treatment group), representing normal (16 % CP), low (13 % CP) and very low (10 % CP) protein intake. In both experiments, diets with low and very low CP were supplemented with crystalline amino acids to achieve equal content of standardized ileal digestible Lys, Met, Thr, and Trp, and were provided to pigs ad libitum. Daily feed intake, BW, and feed/gain ratios were determined. At the end of each experiment, all pigs were slaughtered to collect pancreas, small-intestine samples, and terminal ileal chymes. Samples were used for determining expression of genes for digestive enzymes and ileal amino acid digestibilities. Growing pigs fed the 12 % CP and 15 % CP diets had lower final body weight (P < 0.01) and ADG (P < 0.0001) when compared with pigs fed the 18 % dietary CP diet. Growing pigs fed with the 12 % CP diet showed higher digestibilities for CP (P < 0.05), DM (P < 0.05), Lys (P < 0.0001), Met (P < 0.01), Cys (P < 0.01), Thr (P < 0.01), Trp (P < 0.05), Val (P < 0.05), Phe (P < 0.05), Ala (P < 0.05), Cys (P < 0.01), and Gly (P < 0.05) than those fed the 18 % CP diet. Finishing pigs fed the 16 % CP diet had a higher (P < 0.01) final body weight than those fed the 10 % CP diet. mRNA levels for digestive enzymes (trypsinogen, chymotrypsin B, and dipeptidases-II and III) differed among the three groups of pigs (P < 0.05), and no difference was noted in the genes expression between control group and lower CP group. These results indicated that a reduction of dietary CP by a six-percentage value limited the growth performance of growing–finishing pigs and that a low-protein diet supplemented with deficient amino acids could reduce the excretion of nitrogen into the environment without affecting weight gain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is growing interest in amino acid (AA) nutrition to enhance the efficiency of global animal production (Wu et al. 2014a, b). Dietary protein is an important source of amino acids for livestock species and, therefore, inadequate protein intake results in their suboptimal growth and health (Noblet et al. 2001; Orlando et al. 2007; Wang et al. 2015b). On the other hand, sufficient provision of dietary protein is necessary to supply balanced amounts of various AA and small peptides in the gastrointestinal tract (Portejoie et al. 2004; Rezaei et al. 2013a, b; Toledo et al. 2014). Adequate understanding of the digestive network for the regulation of protein and AA metabolism in animals (including pigs) is crucial for designing the new generation of diets to feed them. Although studies have been conducted to investigate effects of dietary protein and AA intake on weight gains, little is known about the impact of supplemental AA in diets on AA digestibilities and expression of genes for digestive enzymes in growing–finishing animals.
A reduction in dietary protein level results in a concomitant decrease in the intake of AA and nitrogen (Portejoie et al. 2004; Wang et al. 2012; Zollitschstelzl 1992), thereby limiting de novo synthesis of AA and impairs digestive function (Orlando et al. 2007; Wu et al. 2014c). The digestive tract is principally responsible for the terminal digestion and absorption of nutrients, including protein (Wu 2013a). The digestive network of animals is a process of complex metabolic transformations, which include glucose and AA utilization, intracellular protein turnover and fat deposition, as well as their regulation by hormones and other factors (Jobgen et al. 2006; Navarro-Guillen et al. 2015; Perez-Jimenez et al. 2009). To efficiently extract nutrients from the ingested food, a repertoire of digestive enzymes is required to break-down macronutrients in the diet into a form that can be readily absorbed (Kaji et al. 2013). It is known that proteolytic enzymes are mainly produced by stomach (pepsin), pancreas (trypsin, chymotrypsin, and elastase), and intestine (membranous and cytosolic enzymes) (Infante and Cahu 2007).
Previous studies with pigs have shown that there exists a close relationship between AA nutrition and efficiency of nutrient utilization (Sharma et al. 2014; Wu 2014; Wu et al. 2014c). However, expression of genes for digestive enzymes has been reported to be regulated by the nature and molecular forms of dietary nutrients (e.g., protein and AA) (Bartelt et al. 2002; Wu 2009). Therefore, the objective of the present study was to determine the effect of low-protein diet on ileal AA digestibilities and expression of genes encoding digestive enzymes in growing and finishing pigs.
Materials and methods
Experimental diets and procedure
This study was conducted and approved by the Animal Welfare Committee of the Institute of Subtropical Agriculture, The Chinese Academy of Sciences. Cross-bred pigs (Duroc × Landrace × Yorkshire) were randomly assigned randomly into one of three dietary treatments representing normal (group C), low (group B) and very low (group A) intake of dietary crude protein (CP). Pigs were housed individually in metabolism cages and there were 6 pigs per treatment group. Titanium dioxide (TiO2), which served as a digestion indicator/marker, was added to all the experimental diets. There were 2 separate studies involving eighteen growing pigs (Experiment 1) and eighteen finishing pigs (Experiment 2). In both experiments, diets were formulated according to the National Research Council (NRC 2012) to meet nutrient requirements for growing and finishing pigs. There was a 3-day acclimatization period prior to the commencement of each experiment. Pigs had free access to feed and drinking water throughout the experimental period. Body weight of each pig was recorded at the beginning and end of the study to compute weight gains. Feed intake was calculated on a daily basis as the difference between the feed offered and the feed remained in the feeder.
Experiment 1
Eighteen cross-bred (Duroc × Landrace × Yorkshire) growing pigs with the average body weight of 36.47 ± 0.20 kg were assigned randomly into one of three dietary CP levels: normal (18 % CP; group C), low (15 % CP; group B), and very low (12 % CP; group A) CP. Experimental diets designated as Groups A and B were supplemented with some AA that are not synthesized in the body (l-lysine, l-methionine, l-threonine, and l-tryptophan) to meet the requirements of growing pigs (NRC 2012).The experiment lasted 30 days. The composition of the experimental diets used in this study is shown in Table 1.
Experiment 2
Eighteen cross-bred (Duroc × Landrace × Yorkshire) finishing pigs with the average body weight of 62.30 ± 0.07 kg were assigned randomly into one of three dietary CP levels: normal (16 % CP; group C), low (13 % CP; group B) and very low (10 % CP; group A) CP. Experimental diets fed to pigs in Groups A and B were supplemented with different levels of l-lysine, l-methionine, l-threonine, and l-tryptophan) to meet the requirements of finishing pigs (NRC 2012). This experiment lasted for 50 days. The composition of experimental diets used in this study is shown in Table 2.
Sample preparation
At the end of each experiment, pigs were anesthetized with an intravenous injection of sodium pentobarbital (50 mg/kg BW) and then euthanized. The entire intestine and viscera for each pig was rapidly removed (Wang et al. 2014). The digesta samples were obtained from the ileum for determining the digestibilities of energy (DE), dry matter (DM), crude protein (CP), and AA. Meanwhile, duodenum, jejunum (approximately 3 g from the mid-point of each segment), and pancreas section samples were collected, immediately frozen in liquid nitrogen and stored at −80 °C for subsequent analysis of gene expression.
Analysis of conventional index
Ileal digesta samples were pooled for each pig and homogenized in a blender (Waring Commercial, Torrington, CT), sub-sampled, and freeze-dried. Ileal digesta samples were finely ground in a coffee grinder (CBG5 Smart Grind, Applica Consumer Products Inc., Shelton, CT) and thoroughly mixed for analysis. All samples and experimental diets were analyzed for DM, DE, nitrogen (N), and the content of AA. Dry matter was determined according to the method of AOAC (1990; method 925.09) and gross energy (GE) was determined using an adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, IL). Nitrogen was determined using an N analyzer (Model CNS-2000, Leco Corporation, St. Joseph, MI). Samples for AA analysis were prepared by acid hydrolysis according to the method of AOAC (1984; method 982.30) as modified by Mills et al. (Mills et al. 1989). Briefly, approximately 100 mg of each sample was digested in 4 mL of 6 N HCl for 24 h at 110 °C, followed by neutralization with 4 mL of 25 % (wt/vol) NaOH and further cooled to room temperature. The mixture was then equalized to a 50-mL volume with sodium citrate buffer (pH 2.2) and analyzed using an AA analyzer (Sykam, Eresing, Germany). Samples for analysis of S-containing AA (Met and Cys) were subjected to performic acid oxidation before acid hydrolysis. However, tryptophan was not determined, as it was destroyed during acid hydrolysis (Dai et al. 2014).
Quantification of mRNA and cDNA synthesis by real-time PCR analysis
Primers were designed with the use of Primer 5.0 according to the gene sequence of pigs (http://www.ncbi.nlm.nih.gov/pubmed/) to produce amplification products (Table 3). β-Actin was used as a housekeeping gene to normalize target gene transcript levels. Total RNA was isolated from liquid nitrogen-frozen and ground jejunal tissue with the TRIZOL reagents (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA), according to the manufacturer’s instructions. β -Actin was used as an internal control to normalize target gene transcript levels. Real-time PCR was performed as previously described (He et al. 2013). Briefly, 1 µL cDNA template was added to a total volume of 10 µL assay solution containing 5 µL SYBR Green mix, 0.2 µL Rox, 3 µL deionized H2O, and 0.4 µmol/L each of forward and reverse primers. We used the following protocol: (i) pre-denaturation (10 s at 95 °C); (ii) amplification and quantification, repeated 40 cycles (5 s at 95 °C, 20 s at 60 °C); (iii) melting curve construction (60–99 °C with heating rate of 0.1 °C S-1 and fluorescence measurements). The relative level of a target gene was expressed as a ratio of the target gene to the control gene using the formula 2−(ΔΔCt), where ΔΔCt = (CtTarget − Ctβ-actin)treatment − (CtTarget − Ct β-actin)control. The relative expression of target genes in the control group was set to be 1.0.
Statistical analysis
Data were analyzed using the one-way analysis of variance and the Student–Newman–Keuls multiple comparison test, as described by Assaad et al. (2014). P values <0.05 were considered statistically significant.
Results
Growth performance
As shown in Table 4, growing pigs fed the 12 % CP and 15 % CP diets had a lower BW (P < 0.01) and ADG (P < 0.0001), when compared with pigs fed the 18 % CP diet. Growing pigs fed the control diet had a better (P < 0.0001) ratio of feed to gain than those fed the 12 and 15 % CP diets. However, the ADFI of growing pigs was not affected (P > 0.05) by altering dietary CP levels.
Finishing pigs fed the control diet had a higher (P < 0.01) final BW than those fed the 10 % CP diet. The ADG of finishing pigs was reduced (P < 0.0001) from 782 g/d in pigs fed the control diet to 634.33 g/d in pigs fed the 10 % CP diet. Finishing pigs fed the control diet or the 13 % CP diet had a similar ratio of feed to gain, which was higher (P < 0.0001) than that for pigs fed the 10 % CP diet. Dietary treatment had no effect (P > 0.05) on the ADFI of finishing pigs.
Ileal terminal digestibilities of AA
As shown in Table 5, the low CP diet had no effect (P > 0.05) on its digestible energy in growing pigs. Pigs fed the 13 % CP diet showed higher digestibilities of CP (P < 0.05), DM (P < 0.05), Lys (P < 0.0001), Met (P < 0.01), Cys (P < 0.01), Thr (P < 0.01), Trp (P < 0.05), Val (P < 0.05), Phe (P < 0.05), Ala (P < 0.05), Cys (P < 0.01), and Gly (P < 0.05) than pigs fed the 18 % CP diet. Lys digestibility increased (P < 0.0001) from 72.93 % in pigs fed the 18 % CP diet to 80.87 % in pigs fed the 13 % CP diet. Similar results were obtained for Met. However, there were no differences (P > 0.05) in the digestibilities of other AA between the 15 % CP diet and the control diet (Table 5).
Data on ileal AA digestibilities in finishing pigs are summarized in Table 6. There were no differences (P > 0.05) on Leu, Phe, Asp, and His digestibilities among the three groups of finishing pigs. Pigs fed the 10 % protein diet showed higher digestibilities of DE (P < 0.01), CP (P < 0.01), DM (P < 0.01), Ile (P < 0.01), Lys (P < 0.05), Met (P < 0.05), Thr (P < 0.0001), Trp (P < 0.0001), Val (P < 0.05), Ala (P < 0.001), Arg (P < 0.05), Cys (P < 0.001), Glu (P < 0.05), Gly (P < 0.05), Ser (P < 0.01), Tyr (P < 0.01), and Pro (P < 0.05) than those fed the control diet. Ileal digestibilities of protein, DM, Arg, Ile, Thr, Trp, and Pro in finishing pigs did not differ (P > 0.05) between the 13 % CP and 10 % CP groups.
Gene expression of digestive enzymes
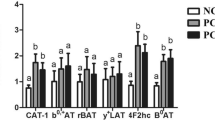
During the growing period, pigs fed the 12 % CP diet showed lower mRNA levels for trypsinogen (P < 0.001), pancreatic α-amylase 2A (P < 0.05), chymotrypsin B (P < 0.01), chymotrypsin C (P < 0.01), duodenal enterokinase (P < 0.01), jejunal dipeptidase-II (P < 0.05), and jejunal dipeptidase-III (P < 0.01), when compared to pigs fed the 18 % CP diet. However, there were no differences in the expression of trypsinogen, pancreatic α-amylase 2A, chymotrypsin B, chymotrypsin C, duodenal enterokinase, jejunal dipeptidase-II, or jejunal dipeptidase-III between the 15 and 18 % CP groups. The mRNA levels for pancreatic α-amylase 2B, pancrelipase, jejunal maltase, or jejunal sucrase did not differ among the three groups of growing pigs (P > 0.05).
During the finishing period, mRNA levels for trypsinogen (P < 0.05), pancreatic α-amylase 2B (P < 0.05), jejunal dipeptidase-II (P < 0.01), and jejunal dipeptidase-III (P < 0.05) were decreased in pigs fed the 12 % CP diet, compared with pigs fed the 16 % CP diet. The lowest mRNA levels for trypsinogen (P < 0.05), jejunal dipeptidase-II (P < 0.01), and jejunal dipeptidase-II (P < 0.05) were observed in pigs fed the 10 % CP diet (Table 7). mRNA levels for pancreatic α-amylase 2A, pancreatic carboxypeptidase B, pancrelipase, jejunal maltase, or jejunal sucrase did not differ (P > 0.05) among the three groups of finishing pigs.
Discussion
A reduction in the N excretion of livestock is a critical issue of environmental protection. Reducing dietary CP intake is an effective way to decrease N excretion of growing and finishing pigs (Dourmad and Jondreville 2007; Gallo et al. 2014). Substantial reductions of protein in diets can be implemented if diets are supplied with nutritionally indispensable AA (Hernandez et al. 2011; Hinson et al. 2009; Kim et al. 2009). However, maximal growth performance and feed efficiency of pigs depend on adequate provision of AA that can be synthesized by animals (Hou et al. 2013, 2015; Wu et al. 2013). In our current study, we hypothesized that addition of certain AA to a low CP diet may improve the growth performance of the growing and finishing pigs. We sought to evaluate the effect of 3- or 6-percentage reduction in dietary protein when supplemented with crystalline AA on the weight gain and digestible ability of the swine. Based on the results from the current study, a reduction in dietary CP by a 3-percentage value with a concomitant addition of “nutritionally essential” AA would support similar growth performance and feed efficiency in finishing pigs but not in growing pigs. However, a further reduction of dietary CP by a 6-percentage value had a negative impact on growth performance in both growing and finishing pigs. Our results are consistent with previous findings (Gallo et al. 2014). Obviously, despite supplementation with nutritionally essential AA, reducing dietary CP by a 3-percentage value reduces ADG, feed efficiency, and the final BW of growing pigs because such a diet cannot provide sufficient “nutritionally nonessential AA” (Hou et al. 2015; Wang et al. 2014, 2015a; Wu et al. 2013, Wu 2014). These findings further underscore the important role for synthesizable AA in maintaining growth and development of growing pigs (Wu 2014).
In previous experiments with pigs fed an “ideal” ration, growth was unaffected by ad libitum feeding experiments until a 100-kg BW (Hinson et al. 2009; Kerr et al. 2003b; Ruusunen et al. 2007). A common feature of these studies was to reduce dietary CP content, while maintaining the same dietary concentration of nutritionally essential AA per kilogram of feed by supplementing with crystalline AA. In this regard, Galassi et al. (2010) found that growth performance and feed efficiency of restricted-fed pigs were unaffected by diets providing only 120 or 99 g CP/kg feed when both diets supplied 6.5, 2.1, 4.5, and 1.4 g/kg feed of total Lys, Met, Thr, and Trp, respectively (Galassi et al. 2010). However, the component of weight gains in growing and finishing pigs was not determined in these studies. It is not known whether the maintenance of weight gains in pigs fed the low CP diet resulted from an increase in whole-body fat with a concomitant decrease in the percentage of protein in the carcass. To date, no studies have been performed to evaluate the effects of low-protein diets with supplemental AA on growing and finishing pigs raised under an ad libitum feeding regimen. Based on the results of our present research and those from other investigators (Gallo et al. 2014; Xiccato et al. 2005), we propose that a reduction in dietary CP along with concomitant supplementation of appropriate amounts of free AA (including synthesizable AA) can provide an effective strategy to improve feed efficiency in swine production while reducing the excretion of urinary and fecal nitrogen from the animals to the environment. This can be applied to both male and female pigs (Guzman-Pino et al. 2014; Hansen et al. 2014; Noblet et al. 2001; Orlando et al. 2007).
The AA content in the diets used in this experiment is consistent with the requirement of growing and finishing pigs (NRC 2012). Designing a low CP diet for growing and finishing pigs requires the use of crystalline AA to maintain an optimal efficiency in the utilization of dietary protein for protein deposition in the body (Kerr et al. 2003a, b). As the costs of crystalline AA (including “nutritionally nonessential AA”) are lowered in the future, low CP diets will be economically attractive to farmers and also environmentally desirable.
The values for the ileal digestibility of CP and some AA in the diets used in our current study differed significantly. Reduction of dietary CP by a 6-percentage value showed the highest ileal digestibility of CP and AA. These results are in agreement with values previously reported by Agyekum et al. (2014). The difference in the response of CP and AA ileal digestibility to diets with different CP levels may be due to relative increases in the rates of protein digestion and absorption of the resultant products (i.e., free AA, dipeptides, and tripeptides) in the small intestine. Feed-grade crystalline AA are fully available to the small intestine, whereas not all AA in dietary protein are released by digestive proteases in the gut lumen (Wu, 2014). As noted previously, it is beneficial to feed a low CP diet to growing and finishing pigs when such a diet is supplemented with crystalline AA. This notion is also supported by a number of published studies (Abbasi et al. 2014; Awad et al. 2014; Hansen et al. 2014; Kulthe et al. 2014; Rodriguez-Gonzalez et al. 2014; Sharma et al. 2014; Xu and Pan 2014). Therefore, our findings raise the possibility that the nutritional needs of swine can be met when dietary CP is reduced by a 3-percentage value, and the low-protein diet is supplemented with adequate amounts of AA. However, when dietary CP is reduced by a 6-percentage value and the low-protein diet is supplemented only with nutritionally essential AA, the growth performance and feed efficiency of growing and finishing pigs cannot be maintained even though the AID of AA are improved. Growth is a complex phenomenon that relies on the digestive capabilities of animals (Gomez-Requeni et al. 2013). Several studies on pigs have suggested that the activity of the main digestive enzymes and their responses to different dietary compositions determine how effectively a given diet may promote whole-body growth (Guzman-Pino et al. 2014; Perez-Jimenez et al. 2009). The digestion of dietary CP and carbohydrates in pigs is accomplished by many different kinds of digestive enzymes in the different parts of the gastrointestinal tract: pancreatic α-amylase 2A, pancreatic α-amylase 2B, pancreatic carboxypeptidase B1, chymotrypsin B, chymotrypsin C, pancrelipase, trypsinogen, enterokinase; maltase, sucrase, dipeptidase-II, and dipeptidase-III. In the current study, the mRNA levels for trypsinogen, pancreatic α-amylase 2A, chymotrypsins B and C, duodenal enterokinase, and jejuna dipeptidase-II and III differed markedly in growing pigs fed the three experimental diets. Likewise, mRNA levels for trypsinogen, pancreatic α-amylase 2B, chymotrypsin B, jejuna dipeptidases-II and III differed among the three groups of finishing pigs. Of note, the values for pigs fed the 13 or 16 % CP diet were higher than the pigs fed the 10 % CP diet. Thus, our results indicate that intestinal expression of the genes for protein digestion is reduced in response to a low-protein diet.
Pancreatic enzymes have been extensively studied (Cahu et al. 2004). Their expression is increased with age. In pigs, the secretion of pancreatic enzymes in the intestinal lumen increases during the first several weeks of postnatal life. This process characterizes the normal maturation of the pancreas and is controlled by cholecystokinin, which in turn is indirectly and positively regulated by the dietary protein level and other nutrients (Abbasi et al. 2014). Intestinal peptide hydrolases are found in two main subcellular locations, the cytosol and the brush border membrane of enterocyte (Wu 2013a, b). Cytosolic enzymes are mainly di- and tripeptidases located in the enterocyte cytosol, completing protein hydrolysis by reducing peptides to free amino acids (Hu et al. 2015; Navarro-Guillen et al. 2015). Protein hydrolysates generated from the diet stimulate the activities of these cytosolic peptidases (Infante and Cahu 2007) and consequently facilitate the utilization of dietary protein by pigs. Interestingly, we observed an increase in intestinal mRNA levels for chymotrypsins B and C as well as duodenal enterokinase in growing and finishing pigs fed low CP diets. It should be borne in mind that expression of digestive enzymes is subjected to complex regulation at both transcriptional and translational levels by a variety of factors, including the composition and balance of dietary AA. Future studies are required to determine protein abundances of digestive enzymes in pigs fed low CP and adequate CP diets.
In summary, low-protein diets supplemented with crystalline AA can maintain expression of digestive enzymes and ileal amino acid digestibility in growing and finishing pigs, and support weight gains and feed efficiency in finishing pigs when dietary CP is reduced by a 3-percentage value. Such low CP diets with supplementation with nutritionally essential AA alone cannot maintain weight gains or feed efficiency in growing pigs, indicating the nutritional importance of adequate amounts of synthesizable AA in animal nutrition. These novel findings have important implications for the development of new interventions to ameliorate dietary protein shortage and environmental pollution from livestock production. Further research is warranted to understand how dietary AA affects the digestible network in growing and finishing pigs.
Abbreviations
- AA:
-
Amino acids
- BW:
-
Body weight
- CP:
-
Crude protein
- DE:
-
Digestible energy
References
Abbasi MA, Mahdavi AH, Samie AH, Jahanian R (2014) Effects of different levels of dietary crude protein and threonine on performance, humoral immune responses and intestinal morphology of broiler chicks. Braz J Poultry Sci 16:35–44
Agyekum AK, Woyengo TA, Slominski BA, Yin YL, Nyachoti CM (2014) Effects of formulating growing pig diet with increasing levels of wheat-corn distillers dried grains with solubles on digestible nutrient basis on growth performance and nutrient digestibility. J Anim Physiol Anim Nutr 98:651–658
Assaad H, Zhou L, Carroll RJ, Wu G (2014) Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 3:474
Awad EA, Fadlullah M, Zulkifli I, Farjam AS, Chwen LT (2014) Amino acids fortification of low-protein diet for broilers under tropical climate: ideal essential amino acids profile. Italian J Anim Sci 13
Bartelt J, Jadamus A, Wiese F, Swiech E, Buraczewska L, Simon O (2002) Apparent precaecal digestibility of nutrients and level of endogenous nitrogen in digesta of the small intestine of growing pigs as affected by various digesta viscosities. Arch Anim Nutr 56:93–107
Cahu C, Ronnestad I, Grangier V, Infante JLZ (2004) Expression and activities of pancreatic enzymes in developing sea bass larvae (Dicentrarchus labrax) in relation to intact and hydrolyzed dietary protein; involvement of cholecystokinin. Aquaculture 238:295–308
Dai ZL, Wu ZL, Jia SC, Wu G (2014) Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J Chromatogr B 964:116–127
Dourmad JY, Jondreville C (2007) Impact of nutrition on nitrogen, phosphorus, Cu and Zn in pig manure, and on emissions of ammonia and odours. Livest Sci 112:192–198
Galassi G, Colombini S, Malagutti L, Crovetto GM, Rapetti L (2010) Effects of high fibre and low protein diets on performance, digestibility, nitrogen excretion and ammonia emission in the heavy pig. Anim Feed Sci Tech 161:140–148
Gallo L, Monta GD, Carraro L, Cecchinato A, Carnier P, Schiavon S (2014) Growth performance of heavy pigs fed restrictively diets with decreasing crude protein and indispensable amino acids content. Livest Sci 161:130–138
Gomez-Requeni P, Bedolla-Cazares F, Montecchia C, Zorrilla J, Villian M, Toledo-Cuevas EM, Canosa F (2013) Effects of increasing the dietary lipid levels on the growth performance, body composition and digestive enzyme activities of the teleost pejerrey (Odontesthes bonariensis). Aquaculture 416:15–22
Guzman-Pino SA, Sola-Oriol D, Figueroa J, Perez JF (2014) Influence of the protein status of piglets on their ability to select and prefer protein sources. Physiol Behav 129:43–49
Hansen MJ, Norgaard JV, Adamsen APS, Poulsen HD (2014) Effect of reduced crude protein on ammonia, methane, and chemical odorants emitted from pig houses. Livest Sci 169:118–124
He LQ, Yang HS, Li TJ, Fang J, Zhou XH, Yin YL, Wu L, Nyachoti MN, Wu G (2013) Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45:383–391
Hernandez F, Martinez S, Lopez C, Megias MD, Lopez M, Madrid J (2011) Effect of dietary crude protein levels in a commercial range, on the nitrogen balance, ammonia emission and pollutant characteristics of slurry in fattening pigs. Animal 5:1290–1298
Hinson RB, Schinckel AP, Radcliffe JS, Allee GL, Sutton AL, Richert BT (2009) Effect of feeding reduced crude protein and phosphorus diets on weaning-finishing pig growth performance, carcass characteristics, and bone characteristics. J Anim Sci 87:1502–1517
Hou YQ, Wang L, Yi D, Ding BY, Yang ZG, Li J, Chen X, Qiu YS, Wu G (2013) N-Acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Hou YQ, Yin YL, Wu G (2015) Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med. doi:10.1177/1535370215587913
Hu HB et al (2015) Effects of dietary xylan on growth performance, digestive enzyme activity and intestinal morphology of juvenile turbot (Scophthalmus maximus L.) Isr J Aquacult Bamid 67:1–10
Infante JLZ, Cahu CL (2007) Dietary modulation of some digestive enzymes and Metabolic processes in developing marine fish: applications to diet formulation. Aquaculture 268:98–105
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Kaji I, Akiba Y, Kaunitz JD (2013) DIGESTIVE PHYSIOLOGY OF THE PIG SYMPOSIUM: involvement of gut chemosensing in the regulation of mucosal barrier function and defense mechanisms. J Anim Sci 91:1957–1962
Kerr BJ, Southern LL, Bidner TD, Friesen KG, Easter RA (2003a) Influence of dietary protein level, amino acid supplementation, and dietary energy levels on growing-finishing pig performance and carcass composition. J Anim Sci 81:3075–3087
Kerr BJ, Yen JT, Nienaber JA, Easter RA (2003b) Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J Anim Sci 81:1998–2007
Kim BG, Petersen GI, Hinson RB, Allee GL, Stein HH (2009) Amino acid digestibility and energy concentration in a novel source of high-protein distillers dried grains and their effects on growth performance of pigs. J Anim Sci 87:4013–4021
Kim JC, Heo JM, Mullan BP, Pluske JR (2011) Efficacy of a reduced protein diet on clinical expression of post-weaning diarrhoea and life-time performance after experimental challenge with an enterotoxigenic strain of Escherichia coli. Anim Feed Sci Tech 170:222–230
Kulthe AA, Pawar VD, Kotecha PM, Chavan UD, Bansode VV (2014) Development of high protein and low calorie cookies. J Food Sci Tech Mys 51:153–157
Mills PA, Rotter RG, Marquardt RR (1989) Modification of the glucosamine method for the quantification of fungal contamination. Can J Anim Sci 69:1105–1106
National Research Council (2012) Nutrient Requirements of Swine: Eleventh Revised Edition. The National Academies Press, Washington, DC
Navarro-Guillen C, Moyano FJ, Yufera M (2015) Diel food intake and digestive enzyme production patterns in Solea senegalensis larvae. Aquaculture 435:33–42
Noblet J, Le Bellego L, Van Milgen J, Dubois S (2001) Effects of reduced dietary protein level and fat addition on heat production and nitrogen and energy balance in growing pigs. Anim Res 50:227–238
Orlando UAD, de Oliveira RFM, Donzele JL, Silva FCD, Generoso RAR, de Siqueira JC (2007) Dietary crude protein levels and amino acid supplementation for gilts from 30 to 60 kg maintained in a high environmental temperature. Rev Bras Zootecn 36:1573–1578
Perez-Jimenez A, Cardenete G, Morales AE, Garcia-Alcazar A, Abellan E, Hidalgo MC (2009) Digestive enzymatic profile of Dentex dentex and response to different dietary formulations. Comp Biochem Phys A 154:157–164
Portejoie S, Dourmad JY, Martinez J, Lebreton Y (2004) Effect of lowering dietary crude protein on nitrogen excretion, manure composition and ammonia emission from fattening pigs. Livest Prod Sci 91:45–55
Rezaei R, Wang WW, Wu ZL, Dai ZL, Wang JJ, Wu G (2013a) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol 4:7
Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G (2013b) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rodriguez-Gonzalez H, Hernandez-Llamas A, Garcia-Ulloa M, Racotta IS, Montoya-Mejia M, Villarreal H (2014) Effect of protein and lipid levels in diets for female red claw crayfish Cherax quadricarinatus on quality of offspring (juvenile), with emphasis on growth performance, biochemical composition and stress resistance to low oxygen, high ammonia and salinity. Aquacult Nutr 20:557–565
Ruusunen M, Partanen K, Poso R, Puolanne E (2007) The effect of dietary protein supply on carcass composition, size of organs, muscle properties and meat quality of pigs. Livest Sci 107:170–181
Sharma VC, Mahesh MS, Mohini M, Datt C, Nampoothiri VM (2014) Nutrient utilisation and methane emissions in Sahiwal calves differing in residual feed intake. Arch Anim Nutr 68:345–357
Toledo JB, Furlan AC, Pozza PC, Carraro J, Moresco G, Ferreira SL, Gallego AG (2014) Reduction of the crude protein content of diets supplemented with essential amino acids for piglets weighing 15 to 30 kilograms. Rev Bras Zootecn 43:301–309
Wang YF, Zhang YZ, Feng ZM, Zhang YG, Li TJ, Huang RL (2012) Measurement of protein and amino acid digestibility for swine diet formulation. J Food Agric Environ 10:650–654
Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, Dahanayaka S, Wu G (2014) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wang H, Zhang C, Wu G, Sun YL, Wang B, He BB, Dai ZL, Wu ZL (2015a) Glutamine enhances tight-junction protein expression and modulates CRF signaling in the jejunum of weanling piglets. J Nutr 145:25–31
Wang H, Ji Y, Wu G, Sun KJ, Sun YL, Li W, Wang B, He BB, Zhang Q, Dai ZL, Wu ZL (2015b) L-Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J Nutr 145:1156–1162
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2013a) Amino Acids: Biochemistry and Nutrition. CRC Press, Boca Raton, Florida
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G, Wu ZL, Dai ZL, Yang Y, Wang WW, Liu C, Wang B, Wang JJ, Yin YL (2013) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Fanzo J, Miller DD, Pingali P, Post M, Steiner JL, Thalacker-Mercer AE (2014a) Production and supply of high-quality food protein for human consumption: sustainability, challenges and innovations. Ann NY Acad Sci 1321:1–19
Wu G, Bazer FW, Cross HR (2014b) Land-based production of animal protein: impacts, efficiency, and sustainability. Ann NY Acad Sci 1328:18–28
Wu G, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL (2014c) Amino acid nutrition in animals: Protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Xiccato G, Schiavon S, Gallo L, Bailoni L, Bittante G (2005) Nitrogen excretion in dairy cow, beef and veal cattle, pig, and rabbit farms in Northern Italy. Italian J Anim Sci 4:103–111
Xu WJ, Pan LQ (2014) Dietary protein level and C/N ratio manipulation in zero-exchange culture of Litopenaeus vannamei: evaluation of inorganic nitrogen control, biofloc composition and shrimp performance. Aquac Res 45:1842–1851
Zollitschstelzl J (1992) Effects of reduced protein-content on performance and N-excretion in pig fattening. Bodenkultur 43:353–362
Acknowledgments
This work was supported by National Basic Research Program of China (2013CB127301, 2013CB127306), National Scientific and Technology Support Project (2013BAD21B04), the Chinese Academy of Sciences through its Hundred Talent Program to Kan Yao, and Texas A&M AgriLife Research (H-8200).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
L. He and L. Wu made equal contributions to this study, so they are joint first authors.
Rights and permissions
About this article
Cite this article
He, L., Wu, L., Xu, Z. et al. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 48, 21–30 (2016). https://doi.org/10.1007/s00726-015-2059-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2059-1