Abstract
This study was conducted with a swine model to determine the safety of long-term dietary supplementation with l-arginine–HCl or l-arginine free base. Beginning at 30 days of age, pigs were fed a corn- and soybean meal-based diet (31.5 g/kg body weight/day) supplemented with 0, 1.21, 1.81 or 2.42 % l-arginine–HCl (Experiment 1) or with 0, 1, 1.5 or 2 % l-arginine (Experiment 2). The supplemental doses of 0, 1, 1.5, and 2 % l-arginine provided pigs with 0, 315, 473, and 630 mg l-arginine/kg body weight/day, respectively, which were equivalent to 0, 286, 430, and 573 mg l-arginine/kg body weight/day, respectively, in humans. At 121 days of age (91 days after initiation of supplementation), blood samples were obtained from the jugular vein of pigs at 1 and 4 h after feeding for hematological and clinical chemistry tests. Dietary supplementation with l-arginine increased plasma concentrations of arginine, ornithine, proline, albumin and reticulocytes, while reducing plasma concentrations of ammonia, free fatty acids, triglyceride, cholesterol, and neutrophils. l-Arginine supplementation enhanced protein gain and reduced white-fat deposition in the body. Other variables in standard hematology and clinical chemistry tests, serum concentrations of insulin, growth hormone and insulin-like growth factor-I did not differ among all the groups of pigs. These results indicate that dietary supplementation with l-arginine (up to 630 mg/kg body weight/day) is safe in pigs for at least 91 days. Our findings help guide clinical studies to determine the safety of long-term oral administration of l-arginine to humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Arginine has traditionally been classified as a nutritionally nonessential amino acid for healthy adults because it is synthesized in most mammals, including humans, rats and pigs (Flynn et al. 2002; Wu 2010; Wu and Knabe 1995). However, as the common precursor for the synthesis of nitric oxide (NO), creatine, polyamines, and other molecules with enormous physiological importance (Morris 2007; Wu et al. 2009), l-arginine has versatile roles in metabolism and physiology and, therefore, is beneficial for the functions of multiple systems (including the circulatory, gastrointestinal, immune, and reproductive systems) in humans and animals (Creager et al. 1992; Hoang et al. 2013; Wu and Meininger 2000; Wu et al. 2012). Additionally, l-arginine has recently been reported to reduce fat accretion in white adipose tissue of obese rats (Fu et al. 2005; Jobgen et al. 2009; Wu et al. 2007b), obese humans (Hurt et al. 2014; Lucotti et al. 2006), obese sheep (Satterfield et al. 2012), and growing-finishing pigs (Tan et al. 2012). Thus, l-arginine is now recognized to be a potentially attractive ingredient in dietary supplements, functional foods, and beverages (Wu 2013). However, the use of l-arginine for these purposes has been limited primarily due to the concerns of regulatory agencies, policymakers, and consumers over the safety of long-term supplementation because of (1) the lack of clinical data in the literature (Shao and Hathcock 2008); and (2) a reportedly possible increase in the risk of adverse cardiovascular events in patients with acute myocardial infarction (Schulman et al. 2006). At present, the safe upper limit for arginine supplementation to healthy adults is unknown (Boger and Bode-Boger 2001; Evans et al. 2004; Hurt et al. 2014; Tanghao et al. 1999; Wu et al. 2013).
The present study was conducted with pigs to determine their physiological responses to dietary supplementation with graded levels of l-arginine or l-arginine–HCl. The pig is similar to the human with respect to nutrient digestion, absorption, and metabolism, as well as physiology and immunology, and, therefore, is a widely used animal model for studying human nutrition and physiology (Burrin et al. 2014; Suryawan and Davis 2014; Wu et al. 1996). When food protein is hydrolyzed by proteases and peptidases in the gastrointestinal tract, l-arginine or its dipeptide/tripeptides is released and subsequently absorbed by intestinal mucosal cells (Closs et al. 2004; Wu 1998). l-Arginine–HCl, the hydrochloric salt of l-arginine, is often manufactured in human-food, animal-feed, or pharmaceutical grades (McKnight et al. 2010; McNeal et al. 2010; Wu 2009). Thus, we used both l-arginine and l-arginine–HCl in our study because they are commercially available for oral or intravenous administration into humans and animals.
Materials and methods
l-Arginine (free base), l-arginine–HCl, and l-alanine were products of Ajinomoto Inc. (Tokyo, Japan). Their purity was >99.9 %, as analyzed by high-performance liquid chromatography (Wu and Meininger 2008). Pigs used for this study were the offspring of Yorkshire × Landrace dams and Duroc × Hampshire sires, and were maintained at the Texas A&M University Swine Center. The experimental procedures were approved by the Institutional Agricultural Animal Care and Use Committee of Texas A&M University.
Experiment 1: Effects of long-term dietary l-arginine–HCl supplementation on physiological responses in pigs
Animals and diets
During the suckling period, pigs were nursed by sows fed an 18.4 % crude-protein diet (Mateo et al. 2008). Piglets were weaned at 21 days of age to a conventional corn- and soybean meal-based diet (Table 1). This basal diet contained 21.0 % crude protein and 1.35 % l-arginine, which were analyzed, as we described previously (Dai et al. 2014; Li et al. 2011). At 30 days of age, four pigs with similar body weights were selected from each of 12 litters and assigned randomly to one of four treatment groups (0, 1.21, 1.81, and 2.42 % l-arginine–HCl) on the basis of litter origin to reduce variation among the experimental groups. The four different diets were prepared by adding 0, 1.21, 1.81, or 2.42 % l-arginine–HCl to the corn- and soybean meal-based diet at the expense of cornstarch. These doses of l-arginine–HCl provided 0, 1, 1.5, and 2 % l-arginine, respectively. Isonitrogenous amounts of l-alanine were added to the diets containing 0, 1.21 and 1.81 % l-arginine–HCl (Table 2). There were 12 pigs (6 males and 6 females) per dietary group, which were housed individually in pens (23–25 °C and relative humidity of 55–60 %). During the entire experimental period, pigs had free access to drinking water and were offered their respective diets at 31.5 g feed per kg body weight per day (divided in two equal meals at 8:00 am and 5:00 pm). The body weights of pigs were determined weekly. The pigs consumed all the feed provided each day.
Collection of blood samples
At 121 days of age (i.e., 91 days after initiation of arginine supplementation), blood samples (10 ml) were obtained from the jugular vein of pigs at 1 and 4 h after feeding. Hematological and clinical chemistry tests of 6-ml blood samples were conducted at Texas A&M Veterinary Medical Diagnostic Laboratory (College Station, Texas). The remaining 4-ml blood samples in tubes with or without EDTA were immediately centrifuged at 10,000 g for 1 min, and the supernatant fluid (plasma or serum) was stored at −80 °C for subsequent analysis of metabolites and hormones.
Determination of body composition
At the end of the second blood collection, six pigs (3 males and 3 females) were selected randomly from 0 and 2 % l-arginine groups to be humanely euthanized by intracardiac administration of 10 ml saturated KCl after anesthesia induced by intramuscular administration of Telazol (5 mg/kg body weight). Immediately after euthanasia, the abdomen of individual pigs was opened and the whole gastrointestinal tract was isolated. The contents of the digestive tract were removed and its lumen was washed three times with saline. The whole body of each pig (including the gastrointestinal tract) was homogenized using a Seydelmann Cutter K64 (Strasser; Stuttgart, Germany), as described by Satterfield et al. (2012, 2013). The content of water, crude protein, crude fat, ash (minerals), and carbohydrate was determined, as previously described (Dai et al. 2014; Jobgen et al. 2009; Wu et al. 1999).
Analyses of amino acids, glucose, ammonia and urea in plasma
Plasma (0.5 ml) were deproteinized with an equal volume of 1.5 M HClO4, followed by addition of 0.25 ml 2 M K2CO3 (Wu et al. 1994). Amino acids in the neutralized extract were determined by fluorometric HPLC methods involving precolumn derivatization with o-phthaldialdehyde as described previously (Wu et al. 1997; Wu and Meininger 2008). The integration of chromatographic peaks was performed using Millenium-32 Software (Waters, Milford, MA, USA). Glucose was determined enzymatically by a spectrophotometric method involving hexokinase and glucose-6-phosphate dehydrogenase (Satterfield et al. 2013). Ammonia and urea were determined using glutamate dehydrogenase and urease plus glutamate dehydrogenase, respectively (Wu 1995).
Analysis of free fatty acids, triglyceride, and hormones in plasma
Free fatty acids and triglyceride in plasma were analyzed using chloroform extraction and assay kits from Wako Chemicals (Richmond, VA, USA), as we previously described (Jobgen et al. 2009). Serum insulin and growth hormone were determined, respectively, using radioimmunoassay kits for porcine insulin and growth hormone (Linco, St. Louis, MO, USA). Insulin-like growth factor-1 (IGF-1) was analyzed using an assay kit from Diagnostic Systems Laboratories, Inc. (Webster, Texas) for porcine serum.
Experiment 2: Effects of long-term dietary l-arginine supplementation on physiological responses in pigs
Experiment 2 was conducted as Experiment 1, except that 0, 1, 1.5, or 2 % l-arginine was supplemented to the basal diet instead of l-arginine–HCl (Table 2).
Statistical analyses
Results are expressed as mean ± SEM. Statistical analyses of data were performed by one-way analysis of variance using the General Linear Models procedures of the statistical analysis (Assaad et al. 2014). Differences among treatment means were determined using the Student–Newman–Keuls multiple comparison method (Assaad et al. 2014). Comparisons of means between 1- and 4-h time points were analyzed by the paired t test. A probability value ≤0.05 was taken to indicate statistical significance (Wei et al. 2012).
Results
Feed intake of pigs supplemented with l-arginine–HCl or l-arginine
Feed intake by pigs in the experimental groups was 31.5 g/kg body weight per day based on the study’s design. This level of feed intake supplied 425 mg l-arginine/kg body weight/day in the basal diet. The supplemental doses of 0, 1.21, 1.81, and 2.42 % l-arginine–HCl provided the pigs with 0, 315, 472.5, and 630 mg l-arginine/kg body weight/day, respectively. Similarly, the supplemental doses of 0, 1, 1.5, and 2 % l-arginine provided the pigs with 0, 315, 472.5, and 630 mg l-arginine/kg body weight/day, respectively. Based on the conversion ratio of 1.1:1.0 (pigs vs. humans; FDA 2005), the human-equivalent doses of the supplemental arginine were 0, 286, 430, and 573 mg l-arginine/kg body weight/day, respectively, or 20, 30, and 40 g l-arginine/day for a 70-kg person, respectively.
Effects of dietary supplementation with l-arginine–HCl or l-arginine on the growth of pigs
In both Exp. 1 and Exp. 2, supplementing 1.21, 1.81 or 2.42 % l-arginine–HCl (equivalent to 1, 1.5 or 2 % l-arginine) or 1, 1.5 or 2 % l-arginine to the basal diet increased (P < 0.05) the body weight of pigs, as compared with the control group (Table 3). The body weights of pigs did not differ (P > 0.05) either between the 1 and 1.5 % arginine groups or between the 1.5 and 2 % arginine groups. However, in both experiments, the pigs supplemented with 2 % arginine were heavier (P < 0.05) than the pigs supplemented with 1 % arginine.
Effects of dietary supplementation with l-arginine–HCl or l-arginine on concentrations of amino acids, ammonia, and lipids in pig plasma
Plasma concentrations of amino acids, ammonia and lipids in control and arginine-supplemented pigs at 1 and 4 h after feeding are summarized in Table 4 (Exp. 1) and Table 5 (Exp. 2). Compared with values obtained at 4 h after feeding, plasma concentrations of amino acids were higher (P < 0.05) at 1 h after feeding. Dietary supplementation with 0.5–2 % arginine dose-dependently increased plasma concentrations of arginine, ornithine and proline, while reducing plasma concentrations of glutamine, glycine, ammonia, cholesterol, free fatty acids, and triglyceride in both Exp. 1 and Exp. 2. Dietary supplementation with l-arginine as either l-arginine-HCl or l-arginine free base did not affect (P > 0.05) concentrations of lysine, histidine, citrulline, and other amino acids in the plasma of pigs at either 1 or 4 h after feeding in Exp. 1 (Table 6 ) or Exp. 2 (Table 7).
Effects of dietary supplementation with l-arginine–HCl or l-arginine on the hematology of pigs
Compared with the control group, dietary supplementation with 2 % l-arginine increased (P < 0.05) concentrations of total serum protein, albumin, alkaline phosphatase, mean corpuscular volume, reticulocytes, as well as the percentages of monocytes and eosinophils in blood obtained from pigs at both 1 and 4 h after feeding, while reducing the percentage of neutrophils in blood (Tables 8 and 9). Dietary supplementation with l-arginine as either l-arginine-HCl or l-arginine free base did not affect (P > 0.05) concentrations of glucose, electrolytes, select enzymes, insulin, growth hormone, insulin-like growth factor-1 (Table 8), or the numbers of white blood cells, red blood cells, and platelets in blood obtained at both 1 and 4 h after feeding in Exp. 1 or Exp. 2 (Table 9).
Body composition of pigs
Protein was the most abundant component of dry matter in pigs (Table 10). When data from Exp. 1 and Exp. 2 were combined, the body composition of nutrients as the percentage of body weight did not differ (P > 0.05) between male and female pigs at 121 days of age. Dietary supplementation with l-arginine as either l-arginine-HCl or l-arginine free base did not statistically affect (P > 0.05) the percentages of water, crude protein, crude fat, minerals or carbohydrate in the whole body. The total amounts of these nutrients in the pigs were calculated on the basis of their body weights (Table 3). Compared with the control group, dietary supplementation with 2 % l-arginine (either as l-arginine–HCl or l-arginine) increased the total amounts of water (P < 0.01), protein (P < 0.01), and minerals (P < 0.05), while reducing (P < 0.01) the total amounts of crude fat, in the pigs (Table 10). The total amounts of carbohydrate did not differ (P > 0.05) between the control and l-arginine supplemented pigs.
Discussion
l-Arginine is a substrate for the synthesis of protein, NO, ornithine (the precursor of polyamines, proline, and glutamate), creatine, and agmatine in mammals (Wu and Morris 1998). As a major building block for polypeptides, arginine represents 14 % of total nitrogen in body proteins (Wu et al. 1999). Of particular note, each of the l-arginine-derived metabolites has enormous physiological importance (Wu et al. 2009). First, NO regulates many of the cellular and organ functions in the body, including endothelium-dependent relaxation of blood vessels, the immune response, and neurotransmission (Dai et al. 2013; Wu and Meininger 2009). Second, polyamines are required for the synthesis of DNA and protein as well as the proliferation and differentiation of all cell types (Agostinelli 2014). Third, proline is a major amino acid for collagen synthesis and, therefore, plays a key role in remodeling of the extracellular matrix (Phang and Liu 2012). Fourth, glutamate is a neurotransmitter in the central nervous system and the gastrointestinal tract (San Gabriel and Uneyama 2013). Finally, creatine is an antioxidant and participates in energy metabolism in skeletal muscle and nerves (Brosnan and Brosnan 2007).
Besides serving as a substrate for synthetic pathways, arginine also has regulatory functions. For example, arginine is an allosteric activator of N-acetylglutamate synthase (Wu 2013). This enzyme converts glutamate and acetyl-CoA into N-acetylglutamate, which is an essential allosteric activator of carbamoylphosphate synthase-I, a key enzyme in the hepatic urea cycle for detoxification of ammonia (Meijer et al. 1985). Thus, l-arginine is required for maintaining hepatic urea synthesis in an active state. Also, pharmacological levels of l-arginine stimulate the secretion of growth hormone and insulin in mammals, thereby playing an important role in regulating protein metabolism (Flynn et al. 2002; Grimble 2007). Furthermore, through stimulating the expression of peroxisome proliferator-activated receptor γ coactivator-1α (a master activator of mitochondrial biogenesis) and the phosphorylation of AMP-activated protein kinase, l-arginine increases the oxidation of fatty acids and glucose in insulin-sensitive tissues, thereby reducing accretion of fat in white adipose tissue (Fu et al. 2005; McKnight et al. 2010). Finally, l-arginine activates the mammalian target of rapamycin cell signaling pathway and, therefore, protein synthesis in multiple tissues, including skeletal muscle (Yao et al. 2008) and small intestine (Tan et al. 2010). Thus, l-arginine can spare muscle protein in obese subjects consuming a weight-reducing diet (Lucotti et al. 2006).
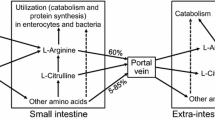
As noted previously, l-arginine had traditionally been classified as a nutritionally nonessential amino acid for healthy adults because it can be synthesized in the body to maintain nitrogen balance (Visek 1986; Wu et al. 2014). However, since the discovery in 1988 that l-arginine is the isonitrogenous precursor for the synthesis of nitric oxide (a signaling molecule and a major vasodilator) in mammalian cells, there has been growing interest in the roles of l-arginine in the functions of multiple systems (including the circulatory, gastrointestinal, immune, and reproductive systems) in humans (Wu et al. 2009). Mean arginine intake by the US adult population is ~5 g/day (Flynn et al. 2002). Approximately 40 % of l-arginine in the diet is catabolized in the first pass by the small intestine, and the remaining 60 % (3 g arginine/day) enters the portal circulation (Castillo et al. 1993; Dai et al. 2011). For comparison, endogenous synthesis of l-arginine from dietary glutamine, glutamate, and proline as well as arterial glutamine provides 1.5 g l-arginine/day to a 70-kg healthy subject (Wu and Morris 1998). Among this total amount of 4.5 g l-arginine from exogenous and endogenous sources, 2.3 g (~50 %) is utilized to synthesize creatine via inter-organ cooperation (the kidneys and liver) (Wu and Morris 1998). The remaining amount of l-arginine (2.2 g/day) is utilized to synthesize protein, ornithine, and nitric oxide. Thanks to the recognition that enhancing the circulating levels of l-arginine can activate key signaling pathways that are beneficial to health, particularly improving cardiovascular function and reducing excessive white adipose tissue in humans and animals (McKnight et al. 2010), l-arginine has now become a potentially attractive ingredient in dietary supplements, functional foods, and beverages (Wu 2013; Hurt et al. 2014). Notably, there are reports that, in healthy adults, increasing l-arginine provision (through supplementation) beyond that in the regular diet can reduce adhesion of platelets to the wall of blood vessels and improve flow-mediated dilation of blood vessels (Gornik and Creager 2004), while enhancing exercise capacity (Colgan 1993), muscle protein synthesis (Cynober 2007; Paddon-Jones et al. 2004), collagen synthesis (Barbul 1986), and fertility (Li et al. 2014; Mateo et al. 2007). This improvement of health and well-being is expected to reduce the risk for chronic disease, including cardiovascular disease (the number one killer in developed countries), obesity, and cancer, further underscoring the importance of l-arginine supplementation in global human health.
Despite its versatile metabolic functions, the use of l-arginine as a dietary or beverage supplement has been limited due to the concerns of regulatory agencies, policymakers, and consumers over the safety of its long-term use as a supplement (i.e., >2 months), because of (1) the lack of clinical data in the literature (Shao and Hathcock 2008); and (2) a possible increase in the risk of adverse cardiovascular events in patients with acute myocardial infarction; Schulman et al. 2006). Results of previous studies indicate the absence of a systematic pattern of adverse effects of oral l-arginine administration in adult humans, which precludes the selection of “No Observed Adverse Effect Level” or “Lowest Observed Adverse Effect Level” as the usual approach to identify a tolerable Upper Level of intake for this dietary supplement (Hayashi 2003; Shao and Hathcock 2008). Thus, investigators have developed a newer method for risk assessment, named the observed safe level (OSL) or the highest observed intake, which is defined as the highest intake level with sufficient evidence of safety (FAO/WHO 2006). In a double-blind, placebo-controlled trial with 16 healthy adult males, oral administration of 20 g l-arginine/day for 4 weeks did not result in any adverse effect as determined using standard clinical chemistry indices (Chin-Dusting et al. 1996). Likewise, healthy adults could tolerate oral administration of 40 g l-arginine/day for 1 week (duration of the study; Beaumier et al. 1995). Similarly, results from other trials indicated no side effects of oral administration of 21 and 42 g l-arginine/day to patients with hypercholesterolemia (Clarkson et al. 1996) or cystic fibrosis (Grasemann et al. 2005) for 4 and 6 weeks, respectively. Based on these findings, an OSL value for oral administration of l-arginine to healthy adults has been suggested to be 20 g/day (Shao and Hathcock 2008). However, the published studies with healthy subjects involved a short duration of l-arginine supplementation (1–4 weeks) and a small number of subjects (5–16) (Beaumier et al. 1995; Chin-Dusting et al. 1996). These concerns limit our confidence in the 20 g/day dose as the OSL value for oral administration of l-arginine to healthy adults and underscore the need for larger and longer studies.
Data from animal studies are much needed to help design clinical trials with humans. Pigs are very similar to humans with regard to digestion, metabolism, and physiology; thus, the pig is a widely used animal model to study human nutrition (Kim and Wu 2004; Pond and Mersmann 2001). The half-lives of intravenously and orally administered l-arginine in the plasma of nonpregnant pigs are 1.06 and 1.86 h, respectively (Wu et al. 2007a). We also reported that healthy adult pigs and rats can tolerate large amounts of supplemental l-arginine (at least 0.21–0.42 and 2.1–5.7 g/kg body weight/day, respectively) (Wu et al. 2007a). Tsubuku et al. (2004) reported that adult rats can tolerate at least 3.6 g l-arginine/kg body weight per day. On the basis of the finding that the intake of dry matter by adult humans is ~ 10 % of that by adult rats, an adult human can likely tolerate an enteral supplemental dose of at least 0.21–0.57 g/kg body weight/day (or 15–40 g/kg body weight/day for a 70-kg subject; Wu et al. 2007a).
Results from the present student provide further evidence that pigs can tolerate high intake of dietary l-arginine. The corn- and soybean meal-based diet used for this research contained 1.35 % l-arginine, supplying 425 mg l-arginine/kg body weight/day. The supplemental doses of 0, 1, 1.5, and 2 % l-arginine provided pigs with 0, 315, 473, and 630 mg l-arginine/kg body weight/day, respectively. Based on the conversion factor adopted by the Food and Drug Administration of the United States (2005), the human-equivalent doses of the supplemental l-arginine were 0, 286, 430, and 573 mg l-arginine/kg body weight/day, respectively, or 20, 30, and 40 g l-arginine/day for a 70-kg person, respectively. Dietary supplementation with l-arginine dose-dependently increased plasma concentrations of arginine, ornithine and proline, while reducing plasma concentrations of ammonia, glycine, glutamine, ammonia, cholesterol, free fatty acids, and triglycerides. This is consistent with the roles for l-arginine in enhancing ornithine formation, ammonia detoxification and muscle protein synthesis in mammals (Wu and Morris 1998; Yao et al. 2008), while stimulating oxidation of fatty acids and inhibiting synthesis of both long-chain fatty acids and triglycerides in a tissue-specific manner (McKnight et al. 2010; Wu et al. 2012). Accordingly, l-arginine-supplemented pigs gained more protein but less fat, as compared with the control group (Table 10). Interestingly, serum levels of alanine transaminase, aspartate transaminase, and lactate dehydrogenase (indicators of integrity of the liver and other tissues) did not differ between control and arginine-supplemented pigs, but serum levels of alkaline phosphatase (released by bone and liver) were elevated in the l-arginine group than in the control. Our study reveals that l-arginine supplementation increases not only tissue protein synthesis but also possibly bone growth in pigs. Of note, all of other variables in standard hematology and clinical chemistry tests did not differ among control and l-arginine-supplemented pigs. Collectively, these results indicate that dietary supplementation with l-arginine or l-arginine–HCl (up to 630 mg l-arginine/kg body weight/day) is safe in pigs for at least 91 days. Our findings help guide clinical studies to determine the safety of long-term oral administration of l-arginine to humans.
In conclusion, supplementing up to 2 % l-arginine (as either l-arginine–HCl or l-arginine base) to the diet for 91 days did not have any adverse effect on postweaning pigs. Our results also indicate a promising effect of l-arginine on improving nutritional status and lean tissue mass, while beneficially reducing plasma levels of ammonia, free fatty acids, triglyceride, and cholesterol, as well as white fat in the body. The data from the present study are helpful in predicting a safe upper limit for oral administration of l-arginine to healthy adults and in guiding clinical studies to determine long-term safety of l-arginine supplementation in humans.
Abbreviations
- Arg:
-
l-Arginine
- HPLC:
-
High-performance liquid chromatography
- NO:
-
Nitric oxide
References
Agostinelli E (2014) Polyamines and transglutaminases: biological, clinical, and biotechnological perspectives. Amino Acids 46:475–485
Assaad H, Zhou L, Carroll RJ, Wu G (2014) Rapid publication-ready MS-Word tables for one-way ANOVA. Springerplus 3:474
Barbul A (1986) Arginine: biochemistry, physiology, and therapeutic implications. J Parenter Enteral Nutr 10:227–238
Beaumier L, Castillo L, Ajami AM, Young VR (1995) Urea cycle intermediate kinetics and nitrate excretion at normal and “therapeutic” intakes of arginine in humans. Am J Physiol Endocrinol Metab 269:E884–E896
Boger RH, Bode-Boger SM (2001) The clinical pharmacology of l-arginine. Annu Rev Pharmacol Toxicol 41:79–99
Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 27:241–261
Burrin DG, Ng K, Stoll B, Sáenz De Pipaón M (2014) Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr 5:82–91
Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR (1993) Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol Endocrinol Metab 265:E532–E539
Chin-Dusting JP, Alexander CT, Arnold PJ, Hodgson WC, Lux AS, Jennings GL (1996) Effects of in vivo and in vitro l-arginine supplementation on healthy human vessels. J Cardiovasc Pharmacol 28:158–166
Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfiled JE (1996) Oral l-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest 97:1989–1994
Closs EI, Simon A, Vékony N, Rotmann A (2004) Plasma membrane transporters for arginine. J Nutr 134:2752S–2759S
Colgan M (1993) Optimum sports nutrition: your competitive edge. Advanced Research Press, Ronkonkoma
Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP (1992) l-Arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest 90:1248–1253
Cynober L (2007) Pharmacokinetics of arginine and related amino acids. J Nutr 137:1646S–1649S
Dai ZL, Wu G, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Wu ZL, Yang Y, Wang JJ, Satterfield MC, Meininger CJ, Bazer FW, Wu G (2013) Nitric oxide and energy metabolism in mammals. BioFactors 39:383–391
Dai ZL, Wu ZL, Jia SC, Wu G (2014) Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J Chromatogr B 964:116–127
Evans RW, Fernstrom JD, Thompson J, Morris SM, Kuller LH (2004) Biochemical responses of healthy subjects during dietary supplementation with l-arginine. J Nutr Biochem 15:534–539
Flynn NE, Meininger CJ, Haynes TE, Wu G (2002) The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother 56:427–438
Food and Agriculture Organization/World Health Organization (FAO/WHO) (2006) A model for establishing upper levels of intake for nutrients and related substances. Technical Workshop on Nutrient Risk Assessment, Geneva
Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), US Department of Health and Human Services (2005) Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Bethesda
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135:714–721
Gornik HL, Creager MA (2004) Arginine and endothelial and vascular health. J Nutr 134:2880S–2887S
Grasemann H, Grasemann C, Kurtz F, Tietze-Schillings G, Vester U, Ratjen F (2005) Oral l-arginine supplementation in cystic fibrosis patients: a placebo-controlled study. Eur Respir J 25:62–68
Grimble GK (2007) Advanced gastrointestinal effects of arginine and related amino acids. J Nutr 137:1693S–1701S
Hayashi Y (2003) Application of the concept of risk assessment to the study of amino acid supplements. J Nutr 133:2021S–2024S
Hoang HH, Padgham SV, Meininger CJ (2013) l-Arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care 16:76–82
Hurt RT, Ebbert JO, Schroeder DR, Croghan IT, Bauer BA, McClave SA, Miles JM, McClain CJ (2014) l-Arginine for the treatment of centrally obese subjects: a pilot study. J Diet Suppl 11:40–52
Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer T, Fried SK, Wu G (2009) Dietary l-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134:625–630
Li XL, Rezaei R, Li P, Wu G (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li XL, Bazer FW, Johnson GA, Burghardt RC, Frank JW, Dai ZL, Wang JJ, Wu ZL, Shinzato I, Wu G (2014) Dietary supplementation with l-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids 46:375–384
Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P (2006) Beneficial effect of a long-term oral l-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab 291:E906–E912
Mateo RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW (2007) Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr 137:652–656
Mateo RD, Wu G, Moon HK, Carroll JA, Kim SW (2008) Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci 86:827–835
McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G (2010) Beneficial effects of l-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids 39:349–357
McNeal C, Wu G, Vasquez S, Wilson DP, Satterfield MC, McKnight JR, Malbari H (2010) The role of arginine for treating obese youth. In: Bagchi D (ed) Global perspectives on childhood obesity. Elsevier, New York, pp 433–442
Meijer AJ, Lof C, Ramos IC, Verhoeven AJ (1985) Control of ureogenesis. Eur J Biochem 148:189–196
Morris SM Jr (2007) Arginine metabolism: boundaries of our knowledge. J Nutr 137:1602S–1609S
Paddon-Jones D, Borsheim E, Wolfe RR (2004) Potential ergogenic effects of arginine and creatine supplementation. J Nutr 134:2888S–2894S
Phang JM, Liu W (2012) Proline metabolism and cancer. Front Biosci (Landmark Ed) 17:1835–1845
Pond WG, Mersmann HJ (2001) Biology of the domestic pig. Cornell University Press, Ithaca
San Gabriel A, Uneyama H (2013) Amino acid sensing in the gastrointestinal tract. Amino Acids 45:451–461
Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G (2012) Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids 43:1593–1603
Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G (2013) Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids 45:489–499
Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gernstenblith G (2006) l-Arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (VINTAGE) randomized clinical trial. JAMA 295:58–64
Shao A, Hathcock JN (2008) Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul Toxicol Pharmacol 50:376–399
Suryawan A, Davis TA (2014) Regulation of protein degradation pathways by amino acids and insulin in skeletal muscle of neonatal pigs. J Anim Sci Biotechnol 5(1):8
Tan BE, Yin YL, Kong XF, Li P, Li XL, Gao HJ, Li XG, Huang RL, Wu G (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Tan BE, Li XG, Yin YL, Wu ZL, Liu C, Tekwe CD, Wu G (2012) Regulatory roles for l-arginine in reducing white adipose tissue. Front Biosci 17:2237–2246
Tanghao O, Chalon S, Moreno H Jr, Hoffman BB, Blaschke TF (1999) Pharmacokinetics of l-arginine during chronic administration to patients with hypercholesterolaemia. Clin Sci (Lond) 96:199–207
Tsubuku S, Hatayama K, Mawatari K, Smriga M, Kimura T (2004) Thirteen-week oral toxicity study of l-arginine in rats. Int J Toxicol 23:101–105
Visek WJ (1986) Arginine needs, physiological states and usual diets. A reevaluation. J Nutr 116:36–46
Wei JW, Carroll RJ, Harden KK, Wu G (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (1995) Urea synthesis in enterocytes of developing pigs. Biochem J 312:717–723
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2013) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
Wu G, Knabe DA (1995) Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol 269:R621–R629
Wu G, Meininger CJ (2000) Arginine nutrition and cardiovascular function. J Nutr 130:2626–2629
Wu G, Meininger CJ (2008) Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol 440:177–189
Wu G, Meininger CJ (2009) Nitric oxide and vascular insulin resistance. BioFactors 35:21–27
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Borbolla AG, Knabe DA (1994) The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124:2437–2444
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Davis PK, Flynn NE, Knabe DA, Davidson JT (1997) Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr 127:2342–2349
Wu G, Ott TL, Knabe DA, Bazer FW (1999) Amino acid composition of the fetal pig. J Nutr 129:1031–1038
Wu G, Bazer FW, Cudd TA, Jobgen WS, Kim SW, Lassala A, Li P, Matis JH, Meininger CJ, Spencer TE (2007a) Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 137:1673S–1680S
Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ (2007b) Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr 137:2680–2685
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, Yin YL (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu ZL, Satterfield MC, Bazer FW, Wu G (2012) Regulation of brown adipose tissue development and white fat reduction by l-arginine. Curr Opin Clin Nutr Metab Care 15:529–538
Wu G, Bazer FW, Satterfield MC, Li XL, Wang XQ, Johnson GA, Burghardt RC, Dai ZL, Wang JJ, Wu ZL (2013) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Yao K, Yin YL, Chu WY, Liu ZQ, Deng D, Li TJ, Huang RL, Zhang JS, Tan BE, Wang W, Wu G (2008) Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr 138:867–872
Acknowledgments
This research was supported by a grant from the International Council of Amino Acid Science (Brussels, Belgium). We thank our graduate students and technicians as well as staff at Texas A&M Veterinary Medical Diagnostic Laboratory for assistance in this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, S., Li, X., Rezaei, R. et al. Safety of long-term dietary supplementation with l-arginine in pigs. Amino Acids 47, 925–936 (2015). https://doi.org/10.1007/s00726-015-1921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-1921-5