Abstract
Sickle cell disease (SCD) is a blood disorder, which has been studied using different research methods including linear birefringence, electronic microscopy, viscosity determination, Electron Paramagnetic Resonance and Nuclear Magnetic Resonance. We summarized in this paper the contribution of proton magnetic relaxation, to the study of the behavior of the protons from protein and water as a consequence of hemoglobin S (HbS) polymerization. Spin–Lattice (T1) and Spin–Spin (T2) relaxation times have been sensitive to the agglutination of the HbS, having a decreasing behavior because of this process. T2 has shown a stronger sensitivity, decreasing immediately during deoxygenation in red blood cells (RBC) of patients homozygous for HbS and in pure HbS solution with intracellular concentration. In the HbS solutions with hemoglobin concentration below 208 mg/mL and heterozygous RBC, during induced deoxygenation, the Spin–Spin relaxation time was characterized by a delay time before polymerization, a decrease during aggregation and a further stabilization; the same occurs in the HbS solutions under spontaneous deoxygenation. The fast exchange of water molecules between the bound and free state, as well as the dipolar interaction between the protons inside the water molecules, were utilized to explain the relaxation times behavior; an increase in the correlation time of the strongly bound water was found. Contradictory results were observed between the experimental behavior obtained for T1 and those results predicted theoretically. T2 behavior showed utility to evaluate the clinical state of the SCD patients and the effect of vanillin and Hydroxyurea on the HbS polymerization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Sickle Cell Disease
Sickle cell disease (SCD), also called sickle cell anemia (SCA), is a serious blood disorder that causes acute pain, severe anemia, infections, and vascular blockages that can lead to widespread organ damage and death [1]. The SCD is a genetic disease existing practically in all over the countries as a consequence of the migration processes. Estimates suggest that every year, approximately, 300,000 infants are born with SCD and it will rise to 400,000 by 2050 [2] mainly in countries like: Nigeria, the Democratic Republic of Congo and India. The prevalence of SCA is high throughout large areas in sub-Saharan Africa, the Mediterranean area, the Middle East and India.
In SCD patients, the major percent of hemoglobin (Hb) inside the red blood cells (RBC) is the hemoglobin S (HbS) [3], a mutant Hb in which glutamic acid (a charged amino acid) is substituted for valine (an hydrophobic neutral amino acid) at position 6 of the β chains [4]. Under deoxygenation conditions, an aggregation process of HbS molecules takes place with different phases until the appearance of fibers in the gelation and crystallization stage [5, 6]. Due to the polymerization of HbS, the erythrocytes are affected, changing their shape, flexibility and the permeability of membrane [2, 7], which gives place to vaso-occlusion at the capillary blood vessels [8] and hemolytic anemia. The vaso-occlusions usually provoke acute complications as ischemic damage to the tissues, which causes severe pain and/or organ failure [2].
To explain the SCD physiopathology or to find treatment options [4], different processes have been considered: the RBC abnormal adhesiveness, the contribution of blood cells other than erythrocytes and others. Nevertheless, the HbS aggregation process is still considered as the main molecular process causing the disease and the main focus of interaction when developing a cure or palliative [7]. To study the HbS gelation process, different physical parameters and research methods have been used: Linear Birefringence, Linear Dichroism, X-ray diffraction, Electron Microscopy, viscosity determination, turbidity, heat absorption, electron paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR) [9, 10].
From the 1970s to nowadays, Proton Magnetic Relaxation has been especially useful in the study of agglutination kinetics of the HbS, the vaso-occlusive crisis identification and even the evaluation of treatment options. Nevertheless, this knowledge has not enough been summarized and analyzed together, which is the main goal of this work including the remark of some limitations and new experimental possibilities.
2 Sickle Cell Disease Studied Using Proton Magnetic Relaxation
NMR relaxation has been extensively used to study SCD [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Studies of relaxation dispersion [11], T1 and T2 values [12,13,14, 21, 23, 24], T1- and T2-based kinetics studies [15,16,17, 22, 25, 26, 28, 29], relaxation at the rotating frame [27], as well as the width and amplitude of the water line [18,19,20] have been used to study Hb solutions, RBC and Whole Blood (WB) from sickle cell patients.
2.1 NMR Studies Under Induced Deoxygenation Conditions
One of the options to study Hb, RBC and WB from sickle cell patients has been under induced deoxygenation conditions [11,12,13,14,15,16,17,18,19,20,21,22, 24]. To induce deoxygenation, different options have been used: to use a water saturated gas phase (5–10% of carbon dioxide + 90–95% of nitrogen) and gently shaking for 30–90 min [12,13,14], utilization of a humidified stream of N2/CO2 (95:5, v/v) for 5–10 min [18, 19, 21, 22] and the addition of sodium dithionite (final concentration: 0.05 M) under nitrogen atmosphere [15,16,17,18, 20]. The deoxygenation using sodium dithionite was employed to obtain a total deoxygenation level and the nitrogen + gas mixture was usually used to obtain different deoxygenation degrees [16].
2.1.1 Water Proton Studies
As a consequence of the increasing in deoxyhemoglobin concentration, the homozygous (RBCS) and heterozygous red blood cells for hemoglobin S behaved differently (37 °C, pH 7.4) [14, 22]. T2 decreased immediately in RBCS [14, 22], even for low concentration of deoxyhemoglobin S. In the case of heterozygous RBC [14], the change in T2 was delayed until a determined amount of deoxyhemoglobin S molecules was reached; this amount will decrease with the increasing of HbS concentration inside the erythrocytes. The final values of T2 (100% of deoxygenation) are similar for homozygous and heterozygous RBC, and the first one needs minor amount of deoxyhemoglobin S to reach this final T2 value. Under induced deoxygenation conditions, no large changes in T1 values were observed in the red blood cells of SCD patients [12, 13, 19].
In HbS solutions (37 °C, pH 7.0), the kinetics of polymerization was characterized by a delay time (td) previous to the aggregation process and a T2 decrease during agglutination development [15]. The increase in the total Hb concentration, by increasing the concentration of HbS or other non-gelling Hb (HbA, HbF), causes a decrease in the magnitude of td and an increase in the rate of polymerization, suggesting that aggregation is caused by one non-specific protein–protein interaction. When pure HbS solution, with concentration above 208 mg/ml, is deoxygenated (37 °C, pH 7.0), the kinetics consists in a rapid decrease to a minimum value [15, 16]. With the increase of pH, the rate of polymerization decreases and td increases [16]. In a solution containing both HbA and HbS (equimolar concentration), T2 does not decrease immediately, not changes are observed for approximately 10 s and then T2 decreases slowly, being its final value not different from those obtained in pure HbS solutions [16]. The increase of the fraction of non-gelling Hb inside the solution (maintaining the same total Hb concentration) decreases the rate of polymerization increasing td. No large changes in T1 were observed in the HbS solutions under induced deoxygenation [19].
To analyze the water proton relaxation in Hb solutions, RBC and WB, a fast exchange of water molecules between bulk and associated water compartments was considered [11,12,13,14, 17]. A three-environment model was used including: the bulk (w), hydration (h) and tightly bound (b) water compartments [13]:
where \(f_{w}\), \(f_{h}\) and \(f_{b}\) are the mole fractions of water in each environment. On the other hand, \(\left( {\frac{1}{{T_{1} }}} \right)_{w,h,b}\) and \(\left( {\frac{1}{{T_{2} }}} \right)_{w,h,b}\) represent the longitudinal and transverse water proton relaxation rates, respectively. In the longitudinal relaxation rate, the term related with the tightly bound water was omitted by Cottam and coworkers [13] considering that it is too small.
To evaluate the longitudinal and transverse relaxation rates in the three environments, the dipole–dipole (D–D) interaction between protons was assumed as the main interaction determining the relaxation of the water protons [11,12,13, 17]:
Here, ω is the resonant frequency, \(\tau_{c}\) is the correlation time (being c equal to w, h or b according to the analyzed environment) and C = 7 × 109 s−2 is a constant related to the rigid lattice second moment.
The mole fraction of water at the hydration environment was calculated as \(f_{h} =\) 0.013 ± 0.001 [13]. The value of \(f_{b}\) was estimated first as 0.0019 [13] and further calculated as 0.00465 [14, 17], which corresponds with 10 or less water molecules tightly bound to the Hb (n ≤ 10, 2% of the first hydration layer) [11]. The value of n does not vary with temperature (T), type of RBC (AS, SS or SC), oxygenation state and Hb concentration, but it changes with pH keeping this range (n ≤ 10) [11, 13]. After the HbS polymerization process, n was considered unchanged [14, 17]. From these results, it is clear that \(f_{w}\) = 1 without losing rigor.
Under induced deoxygenation conditions, the main value of \(\tau_{b}\) was found to increase as a result of HbS gelation process [11, 12, 14, 15, 20]. In RBC from sickle cell patients, \(\tau_{b}\) increases from 2.23 × 10−8 s to 2.36 × 10−7 s [14], in HbS solutions from 2.36 × 10−8 to 1.92 × 10−7 s [17] and in Hb mixtures (50% HbS + 50% HbA) from 2.23 × 10−8 to 1.13 × 10−7 s [17]. The correlation time of the hydration water has been estimated as \(2 \times 10^{ - 9} {\text{s}} \le \tau_{h} \le 4 \times 10^{ - 9} {\text{s}}\) and the bulk water is characterized by \(\tau_{w} \approx 10^{ - 11} \,\,{\text{s}}\) [13].
Taking into consideration the three-environment model, as well as the values and variation of the parameters discussed above [13, 14, 17], in Eq. (1) the term corresponding to the tightly bound water cannot be neglected as was considered by Cottam and coworkers [13] because the condition \(\frac{{f_{b} }}{{T_{1b} }} \ll \frac{{f_{h} }}{{T_{1h} }}\) cannot be accomplished for all the range of variations of τb and τh. Then, the theoretical expression for the observed T1 and T2 should be:
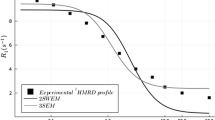
Figures 1 and 2 show the theoretical contributions of hydrated and tightly bound water compartments to the proton relaxations rates according to the three-compartment model developed by Thompson and coworkers [13]. Starting from both figures and the Eqs. (5) and (6), it is possible to observe that, for any values of \(\tau_{h}\) [13], the reported variation of \(\tau_{b}\) [17] will cause a decrease of T2 and an increase of T1. This theoretical approach matches with the experimental results reported for T2 but it is in contradiction with those found for T1.
2.1.2 Protein Proton Studies
The relaxation of protons from the protein has been also analyzed [24]. A temperature- and concentration-dependent increase was found in the longitudinal relaxation rate of the protons belonging to histidine resonances in samples of HbS before gelation with respect to normal Hb. For Hb concentrations of 13.5% or less, and temperature of 25 °C or lower, just the C2 proton of the histidine β2 increases its relaxation rate value. Nevertheless, for temperature higher than 25 °C or Hb concentration greater than 13.5%, several additional histidine resonances in HbS had larger \(T_{1}^{ - 1}\) values than the corresponding ones in normal Hb. It was suggested that this results reflect the formation of small aggregates in the deoxygenated HbS solution before gelation.
2.2 NMR Studies Under Spontaneous Deoxygenation Conditions
Another option to study Hb from patients with SCD has been to study the sample under spontaneous deoxygenation conditions (not induced deoxygenation). A three-region temporal behavior was observed for T1 and T2 in HbS samples with intracellular concentration [25, 26]: a first region in which T1 and T2 do not change, a second region characterized by the relaxation times decreasing (T2: 2.38 ± 0.48 times and T1: 1.29 ± 0.09 times as average) and the third region with a new stabilization. This corresponds with the three characteristic stages of the HbS polymerization (nucleation, polymerization and micro domains formation) [25].
The values of T2 and T1 at the beginning of the obtained behaviors (100% of oxygenation) were minor than those belonging to hemoglobin samples from control individuals in the same experimental conditions. This matches with previous results obtained in experiments under induced deoxygenation [13, 19, 27], and support the suggestions about certain extent of aggregation in HbS samples even in oxygenated state [27].
T2 temporal behavior was different from the behaviors predicted for similar concentrations under induced deoxygenation [15] mainly because of the low values of the polymerization rate and the appearance of one delay time before T2 decreases. Nevertheless, it was similar in shape to the behaviors obtained under induced deoxygenation conditions for samples with concentrations below the intracellular concentration (172 mg/ml) [15], being the values of td (~ 250 min) ten times bigger than those obtained in these samples (~ 25 min) [15]. The absolute value of td is not too much important; the relative value for each patient in the same conditions is really the most important result because it is strongly related with the patient clinical state as will be shown later [32,33,34].
The observed behaviors in the relaxation times were justified considering an increasing in the bound water correlation time from 4 × 10−8 to 8 × 10−8 s under the basis of the experimental T1/T2 behavior [25]. This is coherent with the results obtained using Saturation Transfer EPR (ST-EPR), in which the rotational correlation time of the HbS (τR) changed from 5.1 × 10−8 to 10−7s [28]. In the same work, it was demonstrated that the microviscosity (ημ) inside the HbS solution changed from 2.06 to 3.79 mPas during Hb agglutination, explaining the τR behavior under the basis of the Debye model [28].
Additional relaxation studies were performed during the HbS aggregation [29] with different nuclei (4.42 MHz, 36 °C, intracellular concentration), revealing the same behaviors of T1 and T2 for deuterium and protons inside a partially deuterated HbS solution. This suggested that behaviors of T1 and T2 behaviors are governed by the increase in bound water correlation time and are not related with structural changes inside the solution that could modify the electric contribution to deuterium relaxation.
A two-compartment model, including bound (b) and free (w) water, was used to explain the observed behaviors of T1 and T2 in HbS samples under spontaneous deoxygenation conditions [25]; considering dipolar interaction as the mechanism that contributes the most to proton relaxation in hemoglobin solutions and attributing the dominant role to bound water. If we perform a more detailed analysis, according to what was established in Ref. [25], the theoretical expressions for observed relaxation times are:
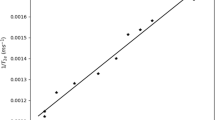
From this theoretical expressions, using ω = 4.42 MHz and C = 7 × 109 s−2, and considering relaxation determined by the extremely low amount of water molecules tightly bound to the protein (\(f_{b} = 0.00465,\,\,\,\tau _{c} = \tau_{b}\)), the dependence of the relaxation times versus \(\tau_{b}\) can be obtained (see Figs. 3, 4).
Starting from Eqs. (7) and (8), as well as the Figs. 3 and 4, again a contradiction appears when the experimental and theoretical behaviors of T1 are compared for the reported range of correlation time variation [17, 25]: from the experimental results, T1 decreases during the HbS polymerization, and this does not match with the theoretical prediction of the two-compartment model used.
Proton relaxation studies performed in RBC samples from SCD patients under spontaneous deoxygenation also allowed to evaluate the time for the transit of water through the membrane of the erythrocyte [30]. A decrease from 20.8 to 15.2 ms was observed, which is coherent with the characteristic loss of water in RBCS because of SCD [2]. These results were in agreement with the increase in the water self-diffusion coefficient observed in HbS solutions after deoxygenation [31].
2.3 Diagnostics and Therapeutics Evaluation Using Water Proton Relaxation in SCD
The existing strong correlation among the MR parameters (T1, T2, \(\tau_{R }\),\(\eta_{\mu }\)) and \(t_{d }\) has allowed to develop some applications related with the clinical evaluation of the sickle cell patients and his treatment [32,33,34]. A decrease of td (36 ± 10% as a main value) was observed during painful crisis in sickle cell patients [32]. It means that a differentiation between both clinical conditions can be established when you compare the td value of one patient during crisis with his own td value during steady state. Furthermore, the utility of the Vanillin as a potential drug for the treatment of SCD was demonstrated “in vivo” in a double blind, placebo-controlled, clinical trial performed in 30 patients (11 belonging to the control group) [34]: the increment of td (1.6 times on average) showed a decrease in the effectiveness of the polymerization process.
In patients under treatment with Hydroxyurea [23], the pretreatment values of 1/T2 in RBCS samples were bigger than those values measured in RBC from control individuals, decreasing with the treatment course (200 days) without reaching the characteristic values of the controls. The red blood cells (25 °C, physiological pH) containing mixtures of HbS and fetal Hb (HbF, range from 0.7 to 8%) showed a continuous T2 (4.42 MHz) decrease until the 60% of the initial value [22] during 24 h of study. This matched with the previous results [14] with the magnitude of T2 decrease being minor. In samples containing 2% of HbF or less, the initial T2 was the same, increasing 1.6 times for samples with 8% of HbF, being this value near to the values of samples from control individuals. The polymerization time (from the initial T2 value to the stabilization value after decreasing) increased its value from 10 to 22 h, when HbF % increased from 0.7 to 8.
3 Conclusions
The relaxation times of protons have been sensitive to the HbS polymerization process in samples of Hb solutions and RBC obtained from patients with SCD. In RBC from patients homozygous for HbS, T2 decreased immediately with deoxygenation even for low concentration of deoxyhemoglobin. Nevertheless, in heterozygous RBC, the change in T2 was delayed until a determined amount of deoxyhemoglobin S molecules was reached.
During induced deoxygenation, for pure HbS solutions with concentrations above 208 mg/ml, the kinetics consists in a rapid T2 decrease to a minimum value. Nevertheless, for HbS solutions with concentrations below the intracellular concentration, the kinetics was characterized by a delay time before the aggregation process, a T2 decrease during agglutination and a further stabilization. The same behavior was obtained for T1 and T2 in samples with intracellular concentration during spontaneous deoxygenation.
Multicompartmental models have been used to explain the proton magnetic relaxation taking into account a fast exchange of water molecules between bound and bulk water and considering dipolar proton–proton interaction as the mechanism contributing the most to this process. The increase in the correlation time of the water tightly bound to the Hb has been used to explain the decrease in relaxation times. For both cases, spontaneous and induced deoxygenation, a contradiction has been found between the theoretical behavior predicted for T1 and the experimental behavior reported. New experiments and the improving of the models will be useful to solve this contradiction.
Proton magnetic relaxation was also able to show modifications in the membrane permeability of RBC from sickle cell patients. Nevertheless, the most useful picture of the SCD effects at molecular level is obtained in the simple case of the HbS solution where the delay time showed remarkable utility to differentiate sickle cell patients in steady state and with vaso-oclusive crisis, as well as, to evaluate the effect of potential and established drugs for SCD treatment.
A more detailed picture of the HbS aggregation process could be obtained using additional methods as: NMR dispersion and NMR spectroscopy. These could be also useful to improve the approaches for patient’s treatment and clinical evaluation.
References
L.Gravitz, S. Pincock, Nature. (2014) https://doi.org/10.1038/515S1a
F.B. Piel, M.H. Steinberg, D.C. Rees, N. Engl. J. Med. A376, 1561 (2017)
E.A. Ajjack, H.A. Awooda, S.E. Adalla, Int. J. Hematol. Disord. A1, 8 (2014)
L.V. Parise, N. Berliner, Blood A127, 789 (2016)
M.J. Rao, K.S. Iyer, A.S. Acharya, J. Biol. Chem. A270, 19250 (1995)
F.A. Ferrone, Experientia A49, 110 (1993)
W.A. Eaton, H.F. Bunn, Blood A129, 2719 (2017)
P.G. Velikov, Br. J. Haematol. A139, 173 (2007)
R.L. Nagel, Hemoglobin Disorders: Molecular Methods and Protocols, 1st edn. (Humana Press, Totawa, 2003), pp. 251–287
R.L. Nagel, H. Chang, Methods Enzimol. A76, 760 (1981)
T.R. Lindstrom, S.H. Koenig, J. Magn. Reson. A15, 344 (1974)
G.L. Cottam, K.M. Valentine, K. Yamaoka, M.R. Waterman, Arch. Biochem. Biophys. A162, 487 (1974)
B.C. Thompson, M.R. Waterman, G.L. Cottam, Arch. Biochem. Biophys. A166, 193 (1975)
G.L. Cottam, M.R. Waterman, Arch. Biochem. Biophys. A177, 293 (1976)
M.R. Waterman, G.L. Cottam, Biochem. Biophys. Res. Commun. A73, 639 (1976)
K. Shibata, M.R. Waterman, G.L. Cottam, J. Biol. Chem. A252, 7468 (1977)
G.L. Cottam, M.R. Waterman, B.C. Thompson, Arch. Biochem. Biophys. A181, 61 (1977)
A. Zipp, I.D. Kuntz, S.J. Rehfeld, S.B. Shohet, FEBS Lett. A43, 9 (1974)
A. Zipp, T.L. James, I.D. Kuntz, S.B. Shohet, Biochim. Biophys. Acta A 428, 291 (1976)
W.A. Eaton, J. Hofrichter, P.D. Ross, R.G. Tschudin, E.D. Becker, Biochem. Biophys. Res. Commun. A69, 538 (1976)
H. Chuang, M.R. Waterman, K. Yamaoka, G.L. Cottam, Arch. Biochem. Biophys. A167, 145 (1975)
J. Losada, F. Gilart, C. Cabal, H.Fleitas, L. Valiente, J. Robier, Proceedings of the 24th Ampere Congress, Magnetic Resonance and Related Phenomena (Poznan, 1988) p. 1027
K.R. bridges, G.D. Barabino, C. Brugnara, M.R. Cho, G.W. Christoph, G. Dover, B.M. Ewestein, D.E. Golan, C.R.G. Guttmann, J. Hofrichter, R.V. MUlkern, B. Zhang, W.E. Eaton, Blood A88, 4701 (1996)
I.M. Russu, C. Ho, Proc. Natl. Acad. Sci. USA A77, 6577 (1980)
M. Lores, C. Cabal, Appl. Magn. Reson. A 28, 79 (2005)
M. Lores, PhD Thesis, Universidad de Oriente, Santiago de Cuba, Cuba (2005)
T.L. James, R. Matthews, G.B. Matson, Biopolymers A18, 1763 (1979)
M. Lores, C. Cabal, O. Nascimento, A.M. Gennaro, Appl. Magn. Reson. A30, 121 (2006)
Y. Cabrales, M. Lores, Y. Machado, Appl. Magn. Reson. A33, 207 (2008)
M.A. Lores-Guevara, Y. Mengana-Torres, J. García-Naranjo, A. Ramírez-Aguilera, L.C. Suárez-Beyrio, M.A. Marichal-Felue, T. Simón-Brada, J. Philippé, J. Biosci. Med. A4, 152 (2016)
M.A. Lores-Guevara, J. García-Naranjo, Y. Mengana-Torres, Adv. Biol. Chem. A4, 388 (2014)
A. Fernández, C. Cabal, M. Lores, J. Losada, E. Pérez, Hemoglobin A33, 206 (2009)
G. Del Toro-García, J.E. Falcón-Dieguez, Y. Alonso-Geli, Y.C. Valdés-Rodríguez, C.A. Cabal Mirabal, Bioquimia A28, 4 (2003)
A. Fernández, C. Cabal, J. Losada, E. Álvarez, C. Soler, J. Otero, Hemoglobin A29, 181 (2005)
Acknowledgements
This work has been supported by the Belgian Development Cooperation through VLIR-UOS (Flemish Interuniversity Council-University Cooperation for Development) in the context of the Institutional University Cooperation program with Universidad de Oriente. The authors also would like to thank the MRI RESEARCH CENTRE of the University of New Brunswick, Fredericton, Canada, for all the support received to finalize this work. Likewise, the authors would like to express their gratitude to Professor V.I. Chizhik for his fundamental contribution to the founding and development of our MR center and to Professor R.N. Muller for the discussion and for the opening of new possibilities in the continuation of these works.
Funding
Funder name: VLIR (Flemish University Council). Grant name: “The Universidad de Oriente promoting the sustainable development in the eastern region of Cuba”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lores Guevara, M.A., Naranjo, J.C.G. & Mirabal, C.A.C. MR Relaxation Studies of Hemoglobin Aggregation Process in Sickle Cell Disease: Application for Diagnostics and Therapeutics. Appl Magn Reson 50, 541–551 (2019). https://doi.org/10.1007/s00723-018-1104-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-018-1104-0