Abstract
A nuclear magnetic resonance (NMR) based experimental procedure to determine the dynamic viscosity (η) in blood plasma solutions is presented. An equation relating η and the transverse proton magnetic relaxation rate (\(1/T_{2}\)) is obtained after considering plasma an extremely diluted water solution of albumin with no long range hydrodynamics interactions among macromolecules, and a fast exchange of water molecules between the free and associated water. Carr–Purcell–Meiboom–Gill pulse sequence was used to measure the transverse proton magnetic relaxation time (T2) in a magnetic resonance console coupled to one homogeneous magnetic system (0.095 T). A η value of 1.68 ± 0.08 mPa s was obtained in 20 control samples, which statistically matched the value obtained in the same samples using an Ostwald viscometer (1.61 ± 0.04 mPa s). η was determined in 172 patients with multiple myeloma (2.47 ± 0.15 mPa s) and 72 with sickle cell disease (2.45 ± 0.24 mPa s) showing a statistically significant increase over the control individuals. The results show the utility of this NMR method to estimate dynamic viscosity in plasma for medical purposes, and a comparison with other methods is done.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plasma dynamic viscosity (η) measurements have an important role in the clinical management of patients prone to hyperviscosity syndrome [1], which occurs during clinical conditions as: polycythemia, Waldenström macroglobulinemia, multiple myeloma, leukemia, asthma, sickle cell disease (SCD), and stroke [2,3,4]. η can be determined using capillary, falling-body, and rotational viscometers [1], with the Harkness viscometer being the recommended option by the International Committee for Standardization in Hematology from 1984 to 2006 [1, 5]. These instruments generally require a significant amount of sample and those using minimal sample volumes, less than 2 mL, have shortcomings during the measurements or use undesirable chemical compounds as mercury [1, 6]. The turbidity of the plasma samples could disturb the measurement in conventional viscometers (mainly capillary and falling body with low level of automatic function). These devices are time consuming because they must be washed between successive measurements and, despite some improvements being made, frequently the accuracy of the results depends on the skill of the operator [1]. Moreover, traditional viscometers available in the market are more usually oriented to other applications and commonly the companies registered to produce these instruments do not focus on clinical laboratories [1]. On the other hand, not all viscometers measure η directly, being necessary to determine first the sample density (ρ) to calculate η from the kinematic viscosity (ν), which increases the volume of sample and/or the measurement time.
Magnetic resonance (MR) has been employed to measure η in protein solutions [6,7,8,9]. Endre and Kuchel proposed in 1986 the measurement of the rotational correlation time of the 13C-labeled physiological intracellular molecules (glycine and glutathione), starting from the 13C longitudinal relaxation time (T1), which allowed the hemoglobin viscosity determination using spherical or symmetrical ellipsoids configuration [8]. This is a complex method, which needs, at least, 4 h of incubation to allow influx of 13C-glycine and labeling of glutathione.
Peña-Zamora and Fernández [9], in 2005, utilized magnetic relaxation to evaluate ν in plasma samples. They employed a calibration curve of the proton T1 versus ν (determined using the Ostwald viscometer) obtained in glycerin solutions of different viscosities. Nevertheless, to determine η it is necessary to make an independent measurement of ρ in the same experimental conditions, increasing the time for analysis and the volume of the sample needed. Moreover, T1 is magnetic field dependent in those kinds of samples, even for low resonance frequency, making it necessary to obtain a new calibration curve when the external magnetic field changes. On the other hand, the different behaviors of the viscosity in plasma and glycerin solutions could contribute to the error in η determination.
In 2006, Lores et al. employed electronic paramagnetic resonance (EPR) to measure the microviscosity of hemoglobin [7] in normal individuals and SCD patients during hemoglobin S (HbS) polymerization. The results matched with previous studies performed in the intracellular environment of red blood cells (RBC) [8]; nevertheless, this experiment is complex and time consuming because it requires carbonmonoxyhemoglobin (HbCO) labeled with 4-maleimido-2,2,6,6-tetramethyl-piperidine-1-oxyl (4MT).
In 2008, Hong et al. added superparamagnetic particles to artificial serum samples and observed its aggregation under the effect of an external magnetic field [6].The effect of this aggregation process on the proton transverse relaxation time (T2) was measured and plotted versus the reciprocal of serum η determined using a gold standard method. With this calibration curve, it is possible to determine the serum η starting from the T2 measurements. The problems with this method are the addition of external compounds (superparamagnetic particles) to the serum sample without evaluation of the effect on the sample viscosity and the employment of temperature values (40 °C) near the protein denaturalization limit. Moreover, this method has not been validated in real samples of serum or plasma.
The results explained above show the potential of MR, especially magnetic relaxation, to estimate blood plasma η, which is supported by: the direct inverse relation between the nuclear magnetic relaxation times and η, the small amount of sample needed (around 0.5 mL or less) and the possibility of measuring different samples using disposable containers. In this work, we propose a T2-based proton magnetic relaxation method to determine η in plasma samples, which eliminates the magnetic field dependence of the experiment and guarantees an inverse relation between the nuclear magnetic relaxation time and η. The proposed method evaluates η directly from the T2 values, eliminating ρ determination, and does not use external compounds inside the sample avoiding the risk of viscosity change.
2 Materials and Methods
The plasma samples used to obtain the calibration curve and those from control individuals were obtained from voluntary donors at the provincial blood bank. The plasma samples from multiple myeloma and SCD patients were obtained starting from fresh venous whole blood by venipuncture and immediately heparinized. After that the plasma was collected by natural sedimentation and decanting or by centrifugation (2500 rpm, 10 min) and decanting. The plasma samples were immediately measured or stored at 4 °C.
A set of plasma calibration samples was prepared by continuous dilution with buffer phosphate saline (PBS, pH 7.4, Sigma Chemicals Co.).
For reference η measurements, an Ostwald viscometer [1] and a 25 mL pycnometer (20 °C) were used. For MR measurements, plasma samples of 500 μL were used and the experiment was performed in an MR console (MARAN DRX, Oxford Instruments, UK). T2 was measured using Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence with 800 echoes, a resonance frequency of 4.0353 MHz (homogeneous permanent magnet, B0 = 0.095 T), 5 and 10 μs for 90° and 180° pulses, respectively, 32 scans, 50% for the radio frequency power amplifier and the receiver gains, 12 s of relaxation delay and echo time of 5.2 ms.
All the measurements were performed at 20 °C and η values reported in mPa s as usual. A Student’s test (t test) was performed to compare the main values with α = 0.001. The η values for patients and control individuals were estimated using an interval estimation: \(\eta = \bar{\eta } \pm \varepsilon\), where \(\bar{\eta }\) is the main value of η and ε depends on the number of samples (n), the standard deviation (s) and the percentile of the student distribution with n − 1 degree of freedom and α = 0.001.
3 Results and Discussion
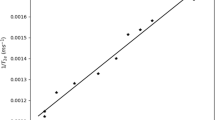
The direct experimental relation between η and the transverse proton magnetic relaxation rate (1/T2), obtained employing the calibration set of plasma samples, showed a linear behavior (see Fig. 1, r2 = 0.99) following the equation:
where T2 and η (Ostwald viscometer measurements) appear in s and Pas, respectively.
This linear behavior can be explained analyzing the proton transverse relaxation in plasma solution. Plasma can be considered a water solution of albumin (albumin: 40 –50 g/L, fibrinogen: 2–5 g/L and α-macroglobulin: 2.2–3.8 g/L), where T2 is affected by the fast exchange of water molecules between the bound (b) and free (f) water compartments [10]:
where Pb and T2b represent the water fraction and the T2 for the bound water population. Pf and T2f represent the water fraction and the T2 for the free water compartment. \(P_{\text{b}} + P_{\text{f}} = 1\).
According to the literature [11], if \(n_{\text{ws}}\) is the number of water accessible sites per protein molecule, V the fraction of the volume occupied by protein, N the molar concentration of protein and Nw the molarity of water, then Pf = 1 and:
Considering this, Eq. 2 can be written as:
Taking into account \(N = \frac{C}{M}\), where C is the protein concentration in gL−1 and M the molecular weight of the protein in g Mol−1:
The experimental behavior of 1/T2 versus C for the calibration set of plasma samples utilized in this work appears in Fig. 2, being consistent with Eq. 5 and with the independence of V, \(n_{ws}\) and T2b with the protein concentration in the employed interval [10]. For the case of C = 0, \(\frac{1}{{T_{2} }} = \frac{1}{{T_{{2{\text{f}}}} }}\approx0.4\;{\text{s}}^{ - 1}\), the proton transverse relaxation rate of PBS, which is in agreement with previous report for albumin solutions [12].
If we consider plasma to be an extremely diluted solution of spherical macromolecules [10] in which no long range hydrodynamics interactions occur among the albumin molecules, then the expression developed by Einstein for η [8] can be employed:
Here, η0 represents the dynamic viscosity of the solvent, \(\bar{v}\) the partial specific volume occupied by proteins in mL g−1 and the units of C are g mL−1 [8].
Using Eqs. 5 and 6, it is possible to obtain:
where \(T_{{ 2 {\text{f}}}} ,\) \(N_{\text{w}}\), η0 and M are constant magnitudes in our experimental conditions. On the other hand, V, \(\bar{v} , n_{\text{ws}}\) and T2b can be considered to be also constant in our plasma calibration set according to the previously shown results (Fig. 2), and \(\bar{v}\) is now in L g−1. Then, Eq. 7 represents a linear dependence of η on \(1/T_{2}\) with positive slope and positive intercept, which allows us to explain the shape and content of the dynamic viscosity calibration curve (Eq. 1).
The NMR method developed was used in 20 control individuals and the obtained results were compared with the values obtained using the Ostwald viscometer for the same samples; see Table 1.
The η values determined by NMR (1.68 ± 0.08 mPa s) match (paired test, \(\alpha = 0.001, p = 0.00215782\)) with those measured utilizing the Ostwald viscometer (1.61 ± 0.04 mPa s). During the measurement of one sample with the NMR method, the standard deviation represents only 1.57% of the main value or less; when different samples from the same individual are analyzed, it reaches only 2.93%. No statistical differences were found in η after separation of RBC and plasma using natural sedimentation and decanting or centrifugation and decanting methods. Until 15 days of storage at 4 °C, the η values determined by NMR remained in its normal range.
To evaluate the utility of the developed NMR method, we used it to determine η in patients suffering from multiple myeloma and sickle cell disease (see Table 2).
The values of η are increased in patients with multiple myeloma (\(\left\lceil T \right\rceil > t_{0.9995}^{190} , \alpha = 0.001\)) and sickle cell disease (\(\left\lceil T \right\rceil > t_{0.9995}^{90} , \alpha = 0.001\)). Patients with multiple myeloma are widely known to have an increased concentration of plasma proteins [9, 11, 13, 14] and the same occurs in those with sickle cell disease [15]. The concentration of proteins defines the viscosity of protein solutions according to Einstein equation for dilute solutions or Mooney Law for concentrated samples [8]. Other parameters like the intrinsic protein viscosity and the crowding factor for interactions also contribute. Nevertheless, in this case the intrinsic protein viscosity is constant, because the samples always contain the same protein types. On the other hand, considering plasma as a diluted protein solution, the crowding factor contribution can be neglected. Normal values of plasma viscosity indicate normal values of the plasma protein concentration. In case of diseases in which plasma viscosity increases, an increase in plasma protein concentration is indicated. The calibration curve (Fig. 1) covers the natural variation of viscosity in plasma samples of healthy individuals (see Table 1) and also the increase of η in some diseases (Table 2). It could be limited when protein–protein interactions become relevant. This point will be an object of deeper analysis in a future paper; here, the goal is only to show the NMR method developed and its potential utility to determine η in some diseases in which it changes.
In this proton magnetic relaxation-based procedure, η can be obtained directly from the T2 measurements (previous ν determinations are not required), the amount of sample and the time needed for measurement are reduced by ten and two times, respectively, the automatic function is guaranteed to contribute to the accuracy of the procedure and the use of disposable containers favors the possibility of measuring different samples daily. In spite of these advantages, NMR is the most expensive method developed for plasma viscosity measurements [1, 8, 9]. Nevertheless, this disadvantage could be overcome considering its multiple potential medical applications [16,17,18,19] in the clinical laboratory in addition to viscosity determination.
To use the developed NMR method, some requirements are needed to avoid undesired changes in T2 values which are not related with η. While obtaining the plasma samples, possible contamination with other materials such as water, proteins, cells or paramagnetic compounds should be avoided. The measurement temperature must be constant inside the sample; actually, since T2 and η are temperature dependent, measuring at another temperature will require a new calibration curve.
According to η values of plasma (Table 1), the bound water correlation time (\(\tau_{c}\)) in this sample has values around 4 × 10−6 s (Debye model). Taking it into consideration, the magnetic resonance experiment should be performed at frequencies (ω) low enough to avoid the T2 dependence on ω. When it cannot be accomplished, the calibration curve must be updated for each ω value.
It is also recommended, considering the values of the water self-diffusion coefficient in plasma [10], to use a homogeneous magnetic field (B0) for the measurements. Effective magnetic field gradient below 20 mT/m (\(G \le 20\;{\text{mT/m}}\)) will guarantee obtaining actual T2 values. For the case in which \(G > 20\;{\text{mT/m}}\), an effective T2 value will be obtained and a new calibration curve must be created in each case. Keeping \(G \le 20\;{\text{mT/m}}\) allows using echo times below 7 ms; otherwise, adequate echo time should be selected to avoid diffusion contribution.
Despite that the effect of molecular oxygen content was not considered in the NMR methods developed by other researchers to determine η in protein solutions [6,7,8,9], its potential influence in our observed T2 values cannot be lightly neglected. Nevertheless, this effect does not affect the strong correlation obtained between the transversal relaxation rate and η (Fig. 1) as predicted by Eq. 7. This effect can be eliminated or minimized using a degassing method or equilibrating the sample with a nitrogen atmosphere, but it could affect the simplicity of the method without any remarkable improvement.
A more detailed analysis concerning sample preparation and magnetic resonance experiment development is expected in future works.
4 Conclusions
An NMR method was developed to estimate dynamic viscosity in blood plasma samples, which statistically agree with the measurements performed with a capillary viscometer (Ostwald viscometer). This NMR method improves the plasma viscosity measurements, because only 250–500 μL of sample is required, no external compounds are added to the sample for the measurement, the instrument does not require cleaning between samples, the measurement is fast (less than 5 min per determination) and it is not affected by the turbidity of the sample. Moreover, the developed NMR method shows utility to determine dynamic viscosity in different diseases in which plasma protein concentration changes.
References
R. Rosencranz, S.A. Bogen, Am. J. Clin. Pathol. A 125, S78 (2006)
D.J. Wright, E. Jenkins, Blood A36, 516 (1970)
M. Harreby, B. Danneskiold-Samsoe, J. Kjer, M. Lauritzen, Ann. Rheum. Dis. A 46, 601 (1987)
O.I. Ajayi, A.A. Famodu, E. Oviasu, Turk J. Hematol. A24, 18 (2007)
International Committee for Standardization in Haematology, J. Clin. Pathol. A37, 1147 (1984)
R. Hong, M.J. Cima, R. Weissleder, L. Josephson, Magn. Reson. Med. A59, 515 (2008)
M. Lores, C. Cabal, O. Nascimento, A.M. Gennaro, Appl. Magn. Reson. A30, 121 (2006)
Z.H. Endre, P.M. Kuchel, Biophys. Chem. A24, 337 (1986)
L. Peña-Zamora, Biology Graduate Thesis, Universidad de Oriente, Santiago de Cuba, Cuba (2005)
R.S. Menon, P.S. Allen, Biophys. J. A57, 389 (1990)
S.H. Koenig, W.E. Schillinger, J. Biol. Chem. A244, 3283 (1969)
P.S. Allen, M.E. Castro, E.O. Treiber, J.A. Lunt, D.P.J. Boisvert, Phys. Med. Biol. A31, 699 (1986)
N. Bloembergen, E.M. Purcell, R.V. Pound, Phys. Rev. A 73, 679 (1948)
S. Kiihne, R.G. Bryant, Biophys. J. A78, 2163 (2000)
S. Chien, S. Usami, J.F. Bertles, J. Clin. Invest. A49, 623 (1970)
A. Fernández, C. Cabal, M. Lores, J. Losada, E. Pérez, Hemoglobin A33, 206 (2009)
A.F. García, C. Cabal, J. Losada, E. Alvarez, C. Soler, J. Otero, Hemoglobin A29, 181 (2005)
M. Lores-Guevara, J. García-Naranjo, Y. Mengana, J. Pereira, Adv. Biol. Chem. A4, 388 (2014)
M.A.L. Guevara, Y.M. Torres, J.C.G. Naranjo, A.R. Aguilera, L.C.S. Beyrio, M.A.M. Felue, T.S. Brada, J. Philippé, J. Biosci. Med. A4, 152 (2016)
Acknowledgements
This work has been supported by the Belgian Development Cooperation through VLIR-UOS (Flemish Interuniversity Council-University Cooperation for Development) in the context of the Institutional University Cooperation program with Universidad de Oriente. The authors also want to thank the MRI RESEARCH CENTRE of the University of New Brunswick, Fredericton, Canada, for all the support received to finalize this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guevara, M.A.L., Torres, Y.M., Naranjo, J.C.G. et al. Plasma Dynamic Viscosity Determined by NMR. Appl Magn Reson 49, 1075–1083 (2018). https://doi.org/10.1007/s00723-018-1026-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-018-1026-x