Abstract
The sizes and local mobility of nano-sized aggregates formed in aqueous solutions of polydiphenylenesulfophthalide before and after its treatment with alkali metal hydroxides were determined by the method of electron paramagnetic resonance (EPR) spectroscopy and the method of dynamic light scattering. Nano-aggregates possess a dense hydrophobic core whose local mobility is commensurable with the local mobility of solid polystyrene. The solubility of these aggregates is explained by the presence of hydrophilic groups (lyophilizing corona) in their outer layer. The hydrophobic core of nano-aggregates is not uniform; it contains a significant amount of less ordered (“defect”) regions. This is confirmed by the superposition of EPR spectra of spin probes. Dense non-uniform structures of aggregates are formed in diluted solutions and maintain their integrity during gel formation as the concentration of solution increases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The method of electron paramagnetic resonance (EPR) spectroscopy of spin labels and probes gained its place among physical methods in polymer research since the sixties years of the last century [1–3]. Despite its long history, this method continues to be in constant development [4, 5] and active use for investigating polymers in their solid state as well as their gels, solutions, and polymer micelles [6–13].
The specific topic of this study is related to the use of EPR spin probe spectroscopy for the investigation of molecular dynamics and macromolecular structure of aqueous solutions of recently synthesized polymer—polydiphenylenesulfophthalide [14].
The chemical structure of polydiphenylenesulfophthalide (PDPSP) (Structure I) includes a rigid aromatic backbone and side cyclic sulfophthalide groups. The hydrophobic biphenylene groups in PDPSP are able to increase the rigidity of the main chain. The specifics of chemical and physical properties of this polymer are determined by the presence of triphenylmethane fragments and polar ionogenic sulfo groups in sulfophthalide rings.
PDPSP has a high glass transition temperature (~300 °C). Despite its aromatic nature and high rigidity of the macromolecule, it dissolves in tetrachloroethane and high-boiling aprotic solvents, such as DMAA and DMSO [15, 16], owing to the presence of side cyclic sulfophthalide groups.
An interesting property of PDPSP is the opening of the sulfophthalide ring without any change in the backbone resulting from a reaction between the sulfophthalide fragments and alkali metal hydroxides. A possible structure of forming polymer salt is shown below (structure II) [15, 16]: 
The sodium salt (PDPSP-Na), potassium salt (PDPSP-K), and lithium salt (PDPSP-Li) are capable of dissolving not only in tetrachloroethane, DMMA, and DMSO, but also in ethylene glycol and glycerol. Furthermore, PDPSP-Li is soluble in ethanol and water. In diluted solutions, these polymer salts tend to aggregate, while in concentrated solutions, they form gels [15–17].
Investigating the molecular dynamics and the structure of aqueous solutions of polymer salts II as well as poly(sulfonic acid) (PDPSP-H) (III) is of practical interest, given the prospects of using polymer particles for catalysis, modification of surfaces, targeted transport of drugs to cells, and so on.
In this study, we report on the synthesis of water-soluble PDPSP-H and the analysis of nanoparticles structure and properties. Special attention will be devoted to the local molecular mobility of nanoparticles and the local mobility of macromolecules in gels formed in polymer solutions at high concentration.
2 Experimental
The synthesis of PDPSP (M w = 1.58 × 105) was performed according to an earlier published procedure [14]. The formation of polymer salts was accomplished by treating dry PDPSP with 5 mol/L aqueous solutions of LiOH, NaOH, and KOH. The nearly complete opening of the sulfophthalimide ring took place after 7 days of reaction which was monitored by IR spectroscopy [15, 16].
The following procedure was performed to prepare aqueous solutions of PDPSP salts. Placed in dialysis bags, 5 % solutions of these salts in DMAA exhibited their dialysis in water until 100 % removal of DMAA. According to elemental analysis data, after dialysis, about 90 % of the cations can be removed from all salts. After dialysis, the dialysis bags retained transparent aqueous solution of the polymer containing approximately 10 sulfo acid units per one salt unit. In addition to the removal of DMAA, dialysis leads to the deionization of the polymer. This observation is related to the fact that in the process of the replacement of DMAA with water, ion pairs existing in non-aqueous solution gained the ability to be dissociated.
According to IR-spectra data, the main result of dialysis is the formation of the acid form of polymer PDPSP-H due to process of hydrolysis and the opening of the sulfophthalide ring. In the following text, the samples of polyacid prepared via the dialysis of starting polymer are denoted as PDPSP-H (P), and the samples prepared via the dialysis of its salts are denoted as PDPSP-H (M), which includes PDPSP-H (Na), PDPSP-H (K), and PDPSP-H (Li). It is important to emphasize that in all three compounds, the content of alkali metal ions is not higher than 10 % of their amount in polymer salts.
The method of dynamic laser-light scattering (photon-correlation spectroscopy) with the use of a ZetaSizer Nano (ZEN 3600) instrument (Malvern Instrument, United Kingdom) equipped with a 4-mW He–Ne laser (λ 0 = 633 nm) [18] was used for measurements of the hydrodynamic diameters of nano-aggregates of macromolecules in aqueous solutions (C pol = 0.35 wt%).
The prepared solutions of polymers were stored for 24 h before all measurements were started. They were performed at 25 °C at a fixed scattering angle of 173° after filtration of solutions through “Millipore” filter with the average pore diameter of 0.45 μm.
The method of EPR spin probe spectroscopy was used to study the local dynamics of macromolecular nano-aggregates’ associates. Stable nitroxyl radicals (structure below) were chosen as spin probes in this investigation,  where n = 5, R = H (5-DSA), n = 16, R = H (16-DSA), n = 5, R = CH3 (5-DSE), irrespective of the nano-aggregates’ hydrophobic or hydrophilic nature. The presence of hydrophobic groups in these compounds leads to their localization in hydrophobic regions of polymers and biological systems. They have very limited solubility in water. Their use as spin probes allows to determine former case, the probes are located inside the nano-aggregates. In case they are hydrophilic, the probes prefer to remain in aqueous solution or precipitate from the solution.
where n = 5, R = H (5-DSA), n = 16, R = H (16-DSA), n = 5, R = CH3 (5-DSE), irrespective of the nano-aggregates’ hydrophobic or hydrophilic nature. The presence of hydrophobic groups in these compounds leads to their localization in hydrophobic regions of polymers and biological systems. They have very limited solubility in water. Their use as spin probes allows to determine former case, the probes are located inside the nano-aggregates. In case they are hydrophilic, the probes prefer to remain in aqueous solution or precipitate from the solution.
The following procedure was used to introduce probes into aqueous solutions: ethanol solution, containing a calculated amount of the probe was placed in a flask, and the ethanol was fully evaporated. Then, the aqueous solution (or gel), containing a calculated amount of the polymer was placed in the vessel, and the solution was heated to 60–70 °C for 1–2 min followed by stirring at room temperature for 24 h. As a rule, the probe concentration in investigated systems was (3–8) × 10−5 mol/L.
Subsequently, the solutions and gels of polymer with incorporated radicals were placed into thin glass tubes (capillaries). The spectra were recorded on a RADIOPAN spectrometer (Poland) as a rule, a day after the preparation of samples. The modulation amplitude was chosen to be significantly lower than the line width (no higher than 0.5 G in the region of “fast” motions and 1–2 G in the region of “slow” motions) [19, 20]. To avoid saturation effects, the level of microwave power in the resonator did not exceed 3 mW. More details regarding the procedure of selection of experimental conditions for recording the EPR spectra of nitroxyl radicals can be found in the monograph [20].
The spectrum calculation was performed in the framework of Brownian isotropic spin probe rotational reorientation in isotropic environment. The theory of spectrum calculation is given in detailed in Freed’s papers [21, 22].
The program described in [23] was used in calculation of the experimental spectra. The main values of g-tensor: g xx = 2.0088, g yy = 2.0061, and g zz = 2.0027—and the main values of hyperfine interaction A-tensor: A xx = 6, A yy = 5.5, and A zz = 33.5 G—were employed [24].
In the process of simulation, the values of principal components of radicals’ hyperfine tensor were varied in the range ±0.5 G, so that mean value 1/3(A xx + A yy + A zz ) coincided with the experimental isotropic hyperfine coupling constant, a N .
3 Results and Discussion
3.1 Association of PDPSP-H (P) and PDPSP-H (M) Macromolecules in Aqueous Solutions
According to the dynamic light scattering data, there is a unimodal size distribution of particles in the aqueous solution of PDPSP-H (P). These measurements show that the mean hydrodynamic diameter is about 20 nm (Table 1), which corresponds to the dimensions of a coil of an individual macromolecule with M = 1.58 × 105. The coil should contain ~95 vol% solvent. Another possibility for the size of hydrodynamic diameter is the existence of aggregates containing about 20 macromolecules with a dense globular structure. The details about the structure of these particles will be provided according to the results of EPR measurements.
In case when the aqueous solutions of PDPSP-H (M) were prepared via the dialysis of polymer salts, we found a bimodal diameter distribution of particles. This result indicates the presence of macromolecular associates with hydrodynamic diameters of 215–455 nm (Table 1, large mode). The hydrodynamic diameters of a smaller mode are in the range of ~17–25 nm for all PDPSP-H (M) compounds. Thus, they are very close to the hydrodynamic diameters of PDPSP-H (P) particles.
The measurements repeated after 10 days of long storage of PDPSP-H (M) solutions showed very insignificant changes in the dimensions of both kinds of particles. This result supports the conclusion that dynamic equilibrium state between the particles was attained. It is possible that interactions between ion pairs SO3 − M+ that were preserved after dialysis (~10 %) play a considerable role in the aggregation of PDPSP-H (M) macromolecules.
3.2 Local Dynamics of Aggregates
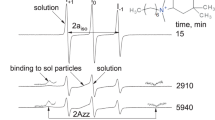
The experimental EPR spectra of 5-DSA and 5-DSE probes measured at 20 °C in dilute (0.35 wt%) solutions of PDPSP-H (M) and corresponding calculated EPR spectra are shown in Fig. 1. It is important emphasize that in all cases, there is superposition of EPR spectra of probes with different correlation times (Table 2). As a rule the correlation times of probes rotating “slowly” are usually τ 1 = (8–9) × 10−9 s, and the correlation times for probes rotating “rapidly” are τ 2 = (1–2) × 10−9 s.
The comparison of observed correlation times of rotation of probes in the aggregates of PDPSP-H (M) macromolecules and the correlation times of rotation of probes in water (<1×10−10 s [25]) revealed that the former are much larger than later. This observation leads to the conclusion that the hydrophobic probe is localized in the hydrophobic regions of aggregates.
According to our experimental data, the local molecular dynamics of macromolecular aggregates of all PDPSP-H (M) is nearly equal. This became evident from almost identical values of correlation times of rotation measured for all studied systems.
It was found that aggregates of macromolecules do not have a uniform structure and the probe is located in different regions which possess different molecular mobility. This conclusion can be made from the observed superposition of the spectra with different rotation correlation times of probes.
There is a close similarity between the EPR spectrum of probe 5-DSE measured in the solution of PDPSP-H (K) and the spectrum of probe 5-DSA in the same solution. The correlation times corresponding to the first spectrum (it is not presented) are τ 1 = 9 × 10−9 s and τ 2 = 1.8 × 10−9 s, and the relative fractions of spin probes with correlation times τ 1 and τ 2 are 87 and 13 %, respectively. The correlation times of rotation of probes 5-DSA and 5-DSE are almost identical. This similarity can be explained by insignificant difference between the chemical structures of these probes.
With rising temperature the mobility of polymer chains increases and rotation correlation times of the probe decrease accordingly. At the same time we did not find significant changes in the relative fractions of each of the spin probes with larger and smaller correlation times (Table 3).
Figure 2 shows the example of the EPR spectra of probe 5-DSA measured in diluted solution of PDPSP-H (P).
The difference in the rotational dynamics of the spin probe in dilute solutions of PDPSP-H (P) (Table 3) and PDPSP-H (M) became the subject of a special investigation. Two important observations were made: (1) the correlation times of the rotation of probes in solutions of PDPSP-H (P) are significantly longer than those in solutions of PDPSP-H (M); (2) the superposition of spectra is observed only at 60 °C, i.e., at a higher temperature than that in the case discussed above.
It is obvious that aggregates of PDPSP-H (P) have more organized structure than similar aggregates formed by macromolecules obtained via the dialysis of polymer alkali salts. An analysis of correlation times allows to refine the structure of aggregates with hydrodynamic diameters of 20 nm that are measured via light scattering in solutions of PDPSP-H (P) and PDPSP-H (M) (small mode). It seems that our preliminary hypothesis that these aggregates may correspond to the coils of individual macromolecules turns out to be wrong.
In reality, the coils of individual macromolecules in solution appear to be labile. Therefore, small molecules, such as the molecules of solvents, freely penetrated them. Accordingly, there is no much difference between the local mobility of probe molecules in the polymer coil and the mobility of this probe in the starting solvent [26, p. 86].
We were able to show that in particles formed in solutions of PDPSP-H (P) and PDPSP-H (M) (the small mode), the local mobility of the probe is much smaller than that in the starting solvent. This leads to the conclusion that the particles are not coils of individual macromolecules. In fact, they are aggregates formed by several macromolecules in the globular state and their local mobility is much smaller than that of solvent molecules.
The results of investigation performed with probe 16-DSA (Table 2) in general are rather close to the results gained with using probe 5-DSA. It should be likely noted that the parameters determined from EPR spectrum of the probe in the solution of PDPSP-H (K) fully coincide with the parameters from the spectrum of the probe in the solution of PDPSP-H (Na).
The only difference in the parameters obtained for 5-DSA and 16-DSA probes is that the correlation times of rotation of probe 16-DSA are generally shorter than those of probe 5-DSA. This experimental observation can be explained by the fact that the paramagnetic fragments of these probes are positioned in various locations of a doxylstearic acid molecule. One substantial difference is that in probe 16-DSA, the paramagnetic fragment is positioned near the end group, while in probe 5-DSA, it takes place in the fifth position of the carboxyl group. Another possible cause is that the paramagnetic fragments of spin probes are positioned in aggregates at different places. An important similarity between two probes is that, as in the case of 5-DSA, in the case of probe 16-DSA, the correlation times of rotation of the probe in PDPSP-H (P) particles are larger than those of the probe in PDPSP-H (M) particles (Table 2).
In all investigated cases for 16-DSA, we found superposition of the spectra (defect structures); however, the fraction of rapidly rotating probes is low (5–9 %). This is not the case, however, of the solution of PDPSP-H (Li), for this polymer salt the fraction of these probes is as high as 40 %. It should be emphasized that the fraction of rapidly rotating 16-DSA probes in PDPSP-H (P) is at least two times lower than that in PDPSP-H (M).
These results should be considered as an experimental confirmation of data obtained with the use of the 5-DSA probe: the aggregates of PDPSP-H (P) are less mobile and have the better organized structure closer than the aggregates of PDPSP-H (M).
It is useful to compare our data on the rotational mobility of spin probes in the studied polymers with the mobility of the same probes in two media. The first of them is solid polystyrene, and the second one is the polystyrene core of the polymer micelle composed of the block copolymer of polystyrene and poly(N-ethyl-4-vinylpyridinium bromide) (PEPB) [27]. In case of polystyrene the correlation times measured at 20 °C for 5-DSA and 16-DSA are 19 × 10−9 and 9.5 × 10−9 s, respectively. In the case of the core of the poly(N-ethyl-4-vinylpyridinium bromide) micelle, τ = 8.0 × 10−9s and τ = 4.2 × 10−9s for 5-DSA and 16-DSA, respectively.
From the comparison of these data with the correlation times presented in Tables 2 and 3, it is possible to conclude that there is no significant difference between the molecular mobility in the hydrophobic cores of aggregates of PDPSP-H macromolecules and the molecular mobility in the solid polystyrene and in the core of the micelle of the block copolymer.
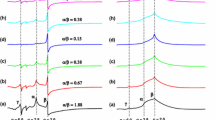
The influence of the PDPSP-H (Li) gel’s formation on the local mobility of spin probes was investigated. Several examples of the EPR spectra are shown in Fig. 3. The values of correlation times, as well as values of relative fractions for spun probes which correspond to different correlation times are presented in Table 4 (similar data for diluted solutions are listed in Table 2).
It was found that during the formation of gel and increasing concentration of polymer up to 11 % by weight, the values of correlation times became larger. This means that local mobility of system experienced some decrease around the location of EPR probe. However, the observed increase in the values of correlation times was rather moderate. It is important to note that in gels as well as in diluted solution, there are two values of the rotational correlation time of EPR probe. It seems that non-uniform structure which corresponds to two values of correlation time is formed in diluted solutions, and it continues to exist in gels. An increase in the concentration of polymer solution causes both types of correlation times (one of which corresponds to “fast” rotation of probe and the one corresponding to its “slow” rotation) to grow simultaneously.
4 Conclusions
The following mechanism of the aggregation of macromolecules of poly(sulfonic acids) PDPSP-H (P) and PDPSP-H (M) in aqueous solutions can be proposed based on the results of this study. The main reason for aggregation is the interaction of hydrophobic biphenylene fragments of macromolecules with their enhanced rigidity. Because of this interaction, the formation of nano-sized aggregates consisting of ~20 macromolecules with the mean hydrodynamic diameter of ~20 nm is taking place. These aggregates have a dense hydrophobic core with packing close to the globular state and they are soluble in water, which can be explained by the presence of the hydrophilic groups –OH, SO3H, and SO3M (lyophilizing corona) in their outer layer.
The hydrophobic core of aggregates is not uniform; it contains a significant amount of less ordered (“defect”) regions. This is confirmed by superposition of EPR spectra of spin probes. The packing density of the hydrophobic core can be influenced by the structure of the lyophilizing corona, and PDPSP-H (P) aggregates feature the highest density in their core.
These nano-sized aggregates seem to be the starting unit blocks that experience further association under conditions of dynamic equilibrium and are able to form large aggregates with the hydrodynamic diameters of 215–455 nm. During this process, the structure of the hydrophobic cores of aggregates and their molecular mobility remain unchanged. Dense non-uniform structure of aggregates is formed in diluted solutions, but it continues to exist during gel formation at increased concentration of solution.
References
A.L. Buchachenko, A.M. Wasserman, Stable Radicals (Khimiya, Moscow, 1973)
A.L. Buchachenko, A.L. Kovarskii, A.M. Wasserman, in Advances in Polymer Science, ed. by Z.A. Rogovin (Halsted Press, Wiley, NewYork, 1974) pp 34–36
P. Tormala, J. Macromol. Sci. Rev. Macromol. Chem. C 17, 297–308 (1979)
G.I. Likhtenstein, J. Yamauchi, S. Nakatsuju, A. Smirnov, R. Namura, Nitroxides (Wiley, Weinheim, 2008)
A.I. Kokorin (ed.), Nitroxides. Theory, Experiment and Applications (InTech, Rijeka, 2012), p. 436
A. Popov, Ju. Zakharova, A. Wasserman, M. Motyakin, V. Kasaikin, J. Phys. Chem. B 116, 12332–12340 (2012)
A.M. Wasserman, M.V. Motyakin, L.L. Yasina, JuA Zakharova, V.M. Matveenko, YuV Shulevich, L.Z. Rogovina, Appl. Magn. Reson. 38, 117–135 (2010)
G. Ionita, A.M. Ariciu, I.M. Turcu, V. Chechnic, Soft Matter 10, 1778–1783 (2014)
A.K. Vorobiev, T.S. Yankova, N.A. Chumakova, Chem. Phys. 409, 61–73 (2012)
Y. Miva, K. Yamamoto, J. Phys. Chem. Ser. B 116, 9277–9284 (2012)
D.A. Chernova, A.K. Vorobiev, J. Appl. Polym. Sci. 121, 102–110 (2011)
R. Shvartzman-Cohen, I. Monje, M. Florent, V. Fridman, D. Goldfarb, R. Yerushalmi-Rozen, Macromolecules 43, 606–614 (2010)
J.S. Lawton, D.E. Budil, Macromolecules 43, 652–661 (2010)
S.N. Salazkin, S.R. Rafikov, G.A. Tolstikov, M.G. Zolotukhin, Dokl. Akad. Nauk SSSR 262, 335–339 (1982)
V.G. Vasil’ev, G.G. Nikiforova, L.Z. Rogovina, L.V. Dubrovina, T.P. Bragina, L.I. Komarova et al., Dokl. Phys. Chem. 382, 51–55 (2002)
L.Z. Rogovina, G.G. Nikiforova, M.I. Buzin, V.G. Vasil’ev, G.I. Timofeeva, L.V. Dubrovina, T.P. Bragina, L.I. Komarova, Polymer Sci. Ser. A 44, 817–825 (2002)
L.A. Wasserman, I.I. Barashkova, V.G. Vasil’ev, V.S. Papkov, S.N. Salazkin, A.M. Wasserman, Polym. Sci. Ser. A 56, 10–16 (2014)
H.G. Barth (ed.), Modern Methods of Particle Size Analysis (Wiley, New York, 1984), p. 309
L.J. Berliner (ed.), Spin Labeling. Theory and Applications (Academic, New York, 1976), p. 592
A.N. Kuznetsov, The Spin Probe Technique (Nauka, Moscow, 1976)
J.H. Freed, in Spin Labeling: Theory and Applications, ed. by L.J. Berliner (Academic Press, New York, 1976) pp 53–132
D.J. Schneider, J.H. Freed, in Spin Labeling: Theory and Applications. Biological Magnetic Resonance, vol. 8, ed. by L.J. Berliner, J. Reuben (Plenum, New York, 1989), pp 399–425
D.E. Budil, S. Lee, S. Saxena, J.H. Freed, J. Magn. Reson. A 120, 155–189 (1996)
B.J. Gafney, H.M. McConnel, J. Magn. Reson. 16, 1–28 (1974)
A.M. Wasserman, V.A. Kasaikin, YuA Zakharova, I.I. Aliev, VYu. Baranovsky, V. Doseva, L.L. Yasina, Spectrochimica Acta A 58, 1241–1255 (2002)
A.M. Wasserman, A.L. Kovarskii, Spin Labels and Probes in Physical Chemistry of Polymers (Nauka, Moscow, 1986)
M.V. Motyakin, E.A. Lysenko, P.S. Cheluskin, L.L. Yasina, A.M. Wasserman, Polym. Sci. Ser. A 52, 235–242 (2010)
Acknowledgments
We highly appreciate the Russian Foundation for Basic Research (project no. 12-03-01023) and the Programme N9 of the Presidium of the Russian Academy of Sciences for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wasserman, L.A., Barashkova, I.I., Vasil’ev, V.G. et al. EPR Spin Probe Study of Molecular Mobility and Structure of Aqueous Solutions and Gels of Polydiphenylenesulfophthalide. Appl Magn Reson 46, 1409–1420 (2015). https://doi.org/10.1007/s00723-015-0702-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-015-0702-3