Abstract—
The influence of the concentration of components in a tetraethyl orthosilicate (TEOS)–water–ethanol mixture on the growth of silicate aggregates in a basic environment is studied with small-angle neutron scattering. There is a general increase in the size of aggregates with an increase in the amount of both water and TEOS. At the same time, when the H2O : TEOS molar ratio is 2 : 1, the structure of the aggregates repeats, regardless of the TEOS concentration in the system. The scattering length density of the aggregates is found via hydrogen/deuterium isotopic substitution and contrast variation to analyze the possible inclusion of residual ethyl and hydroxyl groups into their structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Tetraethyl orthosilicate Si(C2H5O)4 (TEOS) is widely used in the synthesis of various organic materials, including catalysts, flavors, dyes, agrochemicals, and medicines [1–3]. Due to its ability to form branched silicon-containing polymers [4, 5], TEOS is an integral part of promising technologies in the thermocatalytic decomposition of methane for hydrogen production [6, 7] and in the manufacture of acid-base sensors [8, 9] and various membrane filters [10], including to protect the environment [11].
Varying the conditions of hydrolysis synthesis (acidity, temperature, and concentration of components) allows various structures to be obtained, from polymers with weak intermolecular bonds to dense colloidal particles [1, 12, 13]. Such structures are actively studied with a small-angle X-ray and neutron scattering (SAXS and SANS) [14], which make it possible to obtain their structural characteristics (size, polydispersity, surface type, etc.) at the nanoscale (1–100 nm). Indeed, previous small-angle scattering curves for silicate aggregates grown in TEOS water–alcohol (ethanol) solutions under basic conditions indicated that fractal structures consisting of silicate aggregates with a diffuse surface were formed [12, 13, 15, 16]. When the influence of the water–TEOS molar ratio (w) on the structure of aggregates was studied with SANS, the critical behavior of their structural parameters (w = 2) was found [15]. The surface properties are independent of w for w > 2, which indicates a situation close to the complete saturation of Si–OH bonds in TEOS at w = 2. The internal structure of aggregates was studied with external and internal variation of the SANS neutron contrast via isotopic hydrogen/deuterium substitution [16]. The authors showed that most hydrolyzed Si–OH bonds are not closed and participate in the condensation reaction to form Si–O–Si bonds.
The aim of this work is to study the structure of such clusters with SANS (including contrast variation) depending on the total concentration of components in the system. Considering that the proportion of TEOS with those of other components is in certain ratios, the concentration of TEOS in the solution is chosen as the parameter determining the structure, which varies in our experiments. Experiments are also performed at earlier cluster growth stages in comparison with previous studies [15, 16] to test the hypothesis about the influence of the amount of TEOS on a sol–gel polymerization processes during the active formation of aggregates.
EXPERIMENTAL
The samples were prepared according to the procedure described in [15, 16]. Aggregates were formed via the sol-gel polymerization of TEOS in aqueous-alcoholic solutions (pH ~7.5) initiated via the addition of ammonium hydroxide to obtain a basic environment (pH ~10.5). Samples containing 5, 10, and 20 wt % TEOS were prepared and measurements were performed at 18°C. The amount of water was determined by the H2O : Si(C2H5O)4 molar ratio (designated as w). Three series of samples corresponded to w = 1, 2, and 10. Aggregation in each sample proceeded for 5 days prior to measurements. Systems were prepared via the addition of heavy water instead of light water to vary the neutron scattering length density of the aggregates.
The SANS experiments were performed on a YuMO time-of-flight small-angle diffractometer located at the IBR-2 pulsed reactor (Joint Institute for Nuclear Research, Dubna, Russia) [17] within an international user program. Neutrons with a de Broglie wavelength of λ = (0.05–0.5) nm were used to obtain the SANS differential cross section per unit volume of the sample I(q) in the modulus range of the transmitted wave vectors q = (4π/λ) sin(θ/2) = (0.08–4) nm–1, where θ is the scattering angle. Scattering was recorded using two circular isotropic scattering detectors located at a distance of 4.5 and 13 m from the sample. Absolute calibration of the scattering intensity was performed with the use of vanadium standards.
Liquid dispersions for the SANS experiments were placed in Helma flat quartz cuvettes with an optical path of 1 mm. The background scattering was measured (with subsequent subtraction) separately on buffer samples. Freshly prepared systems without ammonium hydroxide were used.
RESULTS AND DISCUSSION
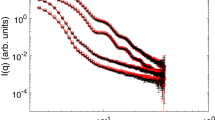
Figure 1 shows how neutron scattering changes with an increase in the TEOS concentration and a different amount of water in the system. The cases under consideration (w-values) lie in the vicinity of the critical value w = 2 found earlier [15]. There is a power-law scattering behavior for large q-values on the SANS curves (Fig. 1):
which is approximated on a double logarithmic scale by linear dependences (Fig. 1). The PS exponent obtained in all cases is greater than four (the value corresponding to Porod’s law in the case of a smooth surface), which corresponds to a diffuse surface of formations [15, 16, 18]. Analysis of Table 1 indicates that an increase in the relative amount of water in the system leads to an increase in the surface diffusivity, whereas an increase in the TEOS concentration smooths the surface (the PS exponent approaches four).
Experimental curves of the small-angle neutron scattering for three series of samples with different initial TEOS concentrations ((squares) 5, (circles) 10, and (rhombuses) 20 wt %) corresponding to different molar ratios between water and TEOS (w): (a) 1, (b) 2, and (c) 10. Dashed lines are the Guinier approximations (2) that determine the sizes of the aggregate and solid lines are power-law dependences corresponding to scattering at the surface of the aggregates (1). The fitting parameters are given in Tables 1 and 2. The dash-dotted line shows the second Guinier level for systems with the highest amount of water (c) with an gyration radius of 8.6(3) and 7.9(3) nm for 10 and 20 wt % respectively; a characteristic kink due to the second level in the curves is indicated by an arrow.
The initial parts of the curves are well approximated with a Guinier law [14]:
where G is the zero-angle scattering intensity found from the product of the concentration, the average square of the volume, and the squared contrast (the scattering length density difference between clusters and solvent), and Rg is the gyration radius, which is the mass-weighted mean square distance from the center of inertia of the aggregate and is an estimation characteristic size of silicate aggregates. Table 2 shows the approximation results of experimental data with Eq. (2) within qRg < 1.5.
The size of aggregates was additionally estimated from the scattering curves in the full q-range and the Porod integral [14]:
which provides the Porod volume:
Table 3 also shows the linear sizes of the aggregates obtained as the cube root of Vp. The experimental spectra were extrapolated by one order of magnitude toward small q-values via the Guinier approximation (2) to increase the accuracy of numerical integration (3). There was a general increase in both the characteristic size parameters of the aggregates with an increase in both the concentration of TEOS and water in the system. The only exception was the case w = 2, when an increase in the TEOS concentration from 10 to 20 wt % did not change the structure of the aggregates. This observation and the fact that the PS exponents for these two samples also coincide within the error indicate that there is a special point on the state diagram of the system in the vicinity of the molar ratio H2O : TEOS (2 : 1) and, therefore, there are special colloidal-aggregation conditions that allow an increase in the production yield of silicate aggregates through an increase in an amount of TEOS while maintaining the same structural characteristics.
It should be noted that two sizes, differing from each other by approximately three times, appear for the highest concentrations in the SANS spectra of the samples. An arrow indicates the corresponding kink in Fig. 1c. This is probably due to the fact that secondary agglomeration into fractal structures proceeds together with aggregation, which is possibly activated at the last growth stages of these colloids. A rather large concentration of “building material” (water and TEOS), however, contributes to these effects at earlier stages.
An experiment on inner contrast variation (analysis of changes in scattering curves with variations in the average scattering density of aggregates) was performed to study the internal structure of the TEOS aggregates. If there were residual ethyl or hydroxyl groups in the aggregate structure, the scattering curves would be very different, in the case that ‒OH is replaced by –OD. The scattering length density of the solvent in this experiment almost remained unchanged due to the low volume fraction of water in the system and was equal to that of ethanol (‒0.345 × 1010 cm–2). The aggregate scattering length density was estimated in [16] and was 4.4 × 1010 cm–2. In the first approximation (when the molar volume was constant), the scattering intensities were estimated and compared for different neutron contrasts for closed –OH and –OD groups in aggregates in the case of one nonhydrolyzed silicon bond among four possible bonds (per Si atom). The calculations showed that if there is at least one hydroxyl group with deuterium in the composition, the scattering intensity should be 3.18 times higher than that of a hydrogen atom. Besides a multiple increase in the signal, the introduction of different isotopes into the structure leads to significant differences in the spectrum for large scattering vectors.
Figure 2 shows the scattering curves in colloidal solutions prepared from light and heavy water for comparison. It is clear that the curves corresponding to the same w-value almost coincide. The slight differences are probably a result of the preparation procedure. First of all, this error appeared because of estimation of the concentrations, as the volume fraction of water is quite low. The data obtained indicate that there are no –OH groups in the cluster structure; in other words, the vast majority of hydrolyzed bonds participate in the condensation reaction to form Si–O–Si bonds. The theory of “poisoned bonds” [12, 13] indicates that silicate aggregates should contain residual ‒C2H5 groups, which did not have time to hydrolyze because of the competition of two chemical reactions (hydrolysis and condensation) and lost access to the dispersion medium because of the fast process of aggregation. The study of outer contrast variation, however, when the solvent scattering density was varied from C2H5OH to C2D5OD mixtures, did not confirm this assumption and showed that the structure of the aggregates is similar to that of amorphous SiO2 [16].
Inner contrast variation in small-angle neutron scattering at silicate aggregates obtained via hydrolysis of TEOS in H2O/C2H5OH (empty symbols) and D2O/C2H5OH (filled symbols) basic solutions with different molar ratios between water and TEOS (w): (squares) 1 and (circles, the I(q)-values are multiplied by 10 for better perception) 2, and (rhombuses, the I(q)-values are multiplied by 100 for better perception) 10. The concentration of TEOS was 10 wt % for all systems.
The most probable reason of the differences in the structure of aggregates with different amount of water and TEOS, therefore, is not the chemical heterogeneity of hydrolysis, but the conformational diversity of polymer structures formed from hydrolyzed TEOS in basic solutions due to the nucleophilic substitution mechanism.
CONCLUSIONS
The dependence of the structure of silicate aggregates in the tetraethyl orthosilicate–water–ethanol system on the synthesis parameters was studied via small-angle neutron scattering. We showed that the aggregation process is critical depending on the amount of water and TEOS in the system and that the most stable structural parameters relative to variations in concentration of system components can be achieved by keeping the H2O : TEOS molar ratio of (2 : 1). In addition, the experimental results on contrast variation refute the concept of “poisoned” bonds, which states that there are residual ethyl and hydroxyl groups in the aggregates.
REFERENCES
R. K. Iler, The Chemistry of Silica (Wiley, New York, 1979).
R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds (Academic Press, New York, 1981).
M. Hudlicky, Oxidations in Organic Chemistry (ACS, Washington, 1990).
C. J. Brinker, J. Non-Cryst. Solids 100, 31 (1988).
K. Sinkó, L. Cser, R. Mezei, et al., Physica B 276–278, 392 (2000).
J. Li, Y. Gong, C. Chen, et al., Fusion Eng. Des. 125, 593 (2017).
M. J. Lázaro, Y. Echegoyen, I. Suelves, et al., Appl. Catal. A 329, 22 (2007).
L. B. Capeletti, C. Radtke, J. H. Z. Dos Santos, et al., Colloids Surf. A 392, 256 (2011).
L. B. Capeletti, F. L. Bertotto, J. H. Z. Dos Santos, et al., Sens. Actuators B 151, 169 (2010).
M. Lavorgna, L. Mascia, G. Mensitieri, et al., J. Membr. Sci. 294, 159 (2007).
G. M. Pajonk, Catal. Today 52, 3 (1999).
K. D. Keefer and D. W. Schaefer, Phys. Rev. Lett. 56 (2199), 2376 (1986).
D. W. Schaefer and K. D. Keefer, Phys. Rev. Lett. 53 (2457), 1383 (1984).
L. A. Feigin and D. I. Svergun, Structure Analysis by Small-Angle X-Ray and Neutron Scattering (Plenum Press, New York, 1987).
M. V. Avdeev, V. L. Aksenov, J. Kohlbrecher, et al., Physica B. 350, e905 (2004).
O. V. Tomchuk, M. V. Avdeev, L. A. Bulavin, et al., Romanian J. Phys. 63, 906 (2018).
A. I. Kuklin, A. V. Rogachev, D. V. Soloviov, et al., J. Phys. Conf. Ser 848, 012010 (2017).
A. V. Tomchuk, M. V. Avdeev, V. L. Aksenov, et al., J. Surf. Invest.: X Ray Synchrotron Neutron Tech. 6 (5), 821 (2012).
Funding
This work was financially supported by the Russian Science Foundation (project no. 18-72-00099).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Tomchuk, O.V., Avdeev, M.V., Ivankov, O.I. et al. Features of Colloidal Aggregation in Tetraethoxysilane-Water-Ethanol Ternary Mixtures by Small-Angle Neutron Scattering. J. Surf. Investig. 13, 1122–1125 (2019). https://doi.org/10.1134/S1027451019060545
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451019060545