Abstract
Intramolecular spin exchange in four flexible PEG-based bis-nitroxides was studied by X-band electron paramagnetic resonance (EPR) spectroscopy as a function of temperature and solvent viscosity. A series of biradicals with different lengths of the ethylene glycol bridge connecting the two nitroxide groups was investigated in aqueous and mixed 1:1 i-propanol:water solutions. Conformational transitions in liquid solutions of the biradicals were analyzed, and thermodynamic parameters of the conformational reorganization were calculated from the EPR spectra. Intramolecular distances r between paramagnetic >N–O• fragments in biradicals in frozen solutions were calculated and compared with dynamic properties of the biradicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Stable nitroxide-free radicals are commonly used as spin probes and labels to report on the microenvironment and molecular interactions in structural biology, supramolecular and nanoscale chemistry. Flexible biradical probes, containing two stable radicals linked via a flexible bridge, significantly expand the scope of the spin label/spin probe technique. The exchange coupling of two unpaired electrons provides additional information about molecular environment in various systems with applications in molecular magnetism [1, 2] or biochemistry [3], as the value of the exchange integral J of a flexible biradical strongly depends on its conformational dynamics.
The synthesis, structures and properties of stable nitroxide biradicals have been previously reported in numerous articles, reviews and books [4–13]. The conformational dynamics of biradicals are affected by solvation of the paramagnetic >N–O• fragment and the structure of the bridge connecting the two nitroxide rings. Most literature reports analyzed the behavior of long-chain (e.g., with the nitroxide units separated by at least 10 bonds) flexible nitroxide biradicals either in organic solvents or ionic liquids [14–23]. The electron paramagnetic resonance (EPR) spectra of the long-chain biradicals in fluid solutions with low viscosity can be described in terms of the co-existence of three interconnected conformations [15]. All published experimental results are in good agreement with this model.
To the best of our knowledge, only two flexible biradicals have been studied by EPR technique in aqueous solution: (CH2)5(CONH-R6)2 [20], and R6-NHCH2CH2NH-R6 [24]. Here, R6 is a TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) unit substituted in the 4-position. EPR studies of biradicals in aqueous solutions are important due to the applications of their compounds as spin probes in molecular biology and biophysics [25].
The R6-NHCH2CH2NH-R6 biradical is flexible but the bridge connecting two radicals is quite short as it consists of only four atoms (five bonds). This biradical was studied in a range of solvents at different temperatures [24]. The authors observed well-resolved EPR spectra with a value of the exchange integral |J| ≥ a, where a is the isotropic hyperfine splitting (hfs) constant, sensitive to the interaction between the >N–O• group of the radical with solvent molecules. The temperature changes in EPR spectra were explained by the co-existence of two conformations of the biradical with |J 1| < < a, |J 2| > a, and fast transitions between these conformations [24]. The Arrhenius dependencies of |J/a| in aqueous solution were strongly pH dependent. These data were used to calculate the ∆S and ∆H parameters, although Arrhenius plots showed some deviations from linearity [24].
This paper reports on a quantitative investigation of four homologous, water-soluble nitroxide biradicals PnT2 (Fig. 1) [26] linked by bridges with a different number of ethylene glycol residues. The effect of solvent viscosity on intramolecular dynamic processes is discussed in terms of Arrhenius and the Debye–Stokes–Einstein laws. The PnT2 biradicals were previously used to probe formation of host–guest complexes with β-cyclodextrin [26] and diffusion of low molecular weight spin probes in covalent gels based on polyethylene glycol/β-cyclodextrin network [27]. The hydration of ethylene glycol groups results in good solubility in water and the biocompatibility of the ethylene glycol chains makes these biradicals attractive for applications in biotechnology.
2 Experimental
2.1 Materials and Methods
Biradicals PnT2 (where n = 2–5) with different lengths of the ethylene glycol chain connecting two nitroxide rings (Fig. 1) were synthesized as described previously [26].
Biradical samples for EPR studies were prepared from 10−2 M stock solutions in ethanol. In each case, an aliquote of the stock solution was added to a vial followed by solvent evaporation. The biradicals were then re-dissolved in appropriate volume of water or 1:1 (v/v) water/i-propanol to give a final concentration of approximately 5 × 10−4 M, to eliminate intermolecular exchange broadening [28].
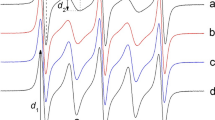
The X-band EPR spectra of PnT2 radicals were recorded on a Bruker ESP-300E spectrometer equipped with a temperature control system (accuracy ± 0.5 K). The sample temperature was varied in the range 294–353 K. Each sample was left to equilibrate at a particular temperature in the instrument resonator for 10 min prior to measurements. For each experimental spectrum, the hfs constant a, amplitudes d k , and widths ∆B k , of lines with k = 1 and 2 (Fig. 2) were measured. In addition, EPR spectra of each biradical in the water/i-propanol mixture were recorded at 120 K.
2.2 Data Analysis
The EPR spectra of flexible long-chain biradicals were analyzed in terms of the “cage effect” [7, 9]. This model assumes strong (|J| > > a) exchange between three effective conformations: an “elongated”, out-of-cage conformation A with J A = 0, and lifetime τ A, and two conformations B and C with total lifetime τ cage, in which the radical fragments are positioned close to each other in the “cage” of the solvent molecules, but J B = 0, and |J C| > > a [9, 15]. The interconversions of the conformations B and C inside the cage are fast, and transitions from outside to inside the cage (e.g., A to B or A to C) are slow, i.e., a·max (τ A, τ cage) >1 [9, 15]. This model makes it possible to obtain the thermodynamic parameters for the intramolecular transitions in long-chain biradicals from the temperature dependence of the EPR spectra of biradicals. The ratio τ cage/τ A can be calculated from the integral intensities I 2 and I 1 of the lines 2, 2′ and 1, 1′, respectively, in the experimental EPR spectra [15]:
The ratio of intensities I 2/I 1 can be estimated from the amplitudes d 1 and d 2 and widths ∆B 1 and ∆B 2 of lines 1 and 2, averaged over the lines 1 and 1′ or 2 and 2′ [9, 15]:
Assuming Arrhenius behavior for the τ cage/τ A ratio, the values of thermodynamic parameters ∆H and ∆S could be obtained. These values correspond to the enthalpy and entropy differences between the out-of-cage and in-cage conformations, respectively
In addition, analysis of the narrowing of exchange lines 2 and 2′ with increased temperature allows one to make conclusions about the motion of the radical fragments inside the cage. In the case of fast modulation of the exchange interaction, the exchange broadening 1/T 2 of these lines is described by the following expression [9, 15]:
Here, τ eff is a complex combination of the modulation parameters and its value is close to the longest characteristic time of the intramolecular motions in the solvent cage. For the Lorentzian lines, this parameter can be determined from Eq. (5) [5]:
where a is taken in frequency units and γ e is the magnetogyric ratio of the free electron.
Assuming the Arrhenius behavior for τ eff, the following equation should be valid:
In this equation, parameters ε and τ 0 characterize movement inside the cage, τ 0 is the characteristic time of motion leading to the transition between effective conformations B and C, and ε is the corresponding activation energy barrier.
3 Results and Discussion
The EPR spectra of biradicals at 323 K are given in Fig. 2. The spectra show quintet patterns with different values of ∆B k and d 2/d 1 parameters. In particular, the EPR spectrum of biradical P3T2 shows significantly more intense lines 2 and 2′ as compared to the other biradicals. We tentatively assign this to the more favorable in-cage conformations for this biradical. The conformations of the short chain oligo-(ethylene glycol) are affected by the degree of hydration. The anti-gauche-anti conformation around the successive O–C–C–O bonds is characterized by the highest hydration and hence is most stable [29, 30]. In the case of P3T2, this brings the paramagnetic moieties closer to each other, which makes cage conformations more stable and hence results in an increase of the intensity of the 2 and 2′ lines.
The interaction of polar solvent molecules with the >N–O• groups of nitroxides leads to a slight decrease of the hfs value a with increased temperature (Fig. 3). The slope of the plot of a vs T is −(27 ± 5)×10−4 G/K for all biradicals dissolved in water, and −(35.6 ± 4)×10−4 G/K for water-i-propanol mixtures. These values are close to those listed in the literature [13].
Experimental plots of aτ eff as a function of temperature for the biradicals dissolved in water are given in Fig. 4 in the Arrhenius coordinates. These linear plots were used to calculate the effective values of the activation energy barrier ε and parameter τ 0 (Table 1). The close inspection of data in Table 1 suggests that:
-
(a)
all ε values measured in both solvents practically coincide within the error limit, and are close to the literature values determined for biradical (CH2)5[CONHR6]2: ε = 18.9 ± 0.4 kJ/mol, and logτ0 = −12.0 [20];
-
(b)
all parameters τ0 are in a reasonable range for intra-cage motion between 10−12 and 10−13 s;
-
(c)
activation barrier ε for P3T2 is somewhat smaller than that for the other biradicals probably due to different stability of the in-cage and out-of-cage conformations (see above);
-
(d)
the ε and τ0 parameters are nearly identical for both solvents, e.g., water and i-propanol-water mixtures (Table 1), thus suggesting that the solvent cage around conformations B and C includes only water molecules even in the presence of i-propanol.
The activation energy for the microscopic in-cage movement τ eff (or experimentally measured aτ eff value) can be compared with the effective activation energy value E η for water viscosity η, a macroscopic parameter which characterizes the bulk of the solvent: Using viscosity values η(T) for H2O taken from Ref. [31], the E η value for water was calculated as 15.3 kJ/mol, which is very close to the value of ε
The interconversion between conformations B and C presumably takes place by a rotational movement or librations of the nitroxide rings. Such rotational diffusion motion can be described by Debye–Stokes–Einstein law. To test whether biradicals PnT2 in aqueous solutions follow this classical approach, the microscopic parameter (aτ eff)−1 was plotted as a function of the macroscopic Stokes parameter T/η for all studied biradicals dissolved in water (Fig. 5). All plots showed good linearity. This is rather unexpected, as the small rotational movement inside the “cage” of solvent molecules is really a microscopic process, and its correlation with a macroscopic parameter T/η is not obvious.
As described in the experimental section, analysis of biradical spectra gives the ratio of in- and out-of-cage residence times τ cage/τ A, which allows one to determine the differences of enthalpies ∆H, and entropies ∆S, between effective out-of-cage and in-cage conformations. A typical temperature dependence of τ cage/τ A in the Arrhenius axes for some biradicals in water is given in Fig. 6, and the corresponding thermodynamic parameters are listed in Table 1. In all cases, the τ cage/τ A parameter increases slightly with increased temperature. The temperature dependence of τ cage/τ A is very shallow, because the enthalpy difference between the in/out-of-cage conformations is very small for the long-chain biradicals. It is interesting to compare these values to those for a biradical with a short alkyl linker (CH2)5[CONHR6]2 in toluene which shows an even smaller enthalpy difference: ∆H and ∆S are 3.6 kJ/mol and 16.7 J mol−1 K−1, respectively [20].
The effect of the length of the bridge connecting the two radical centres on the thermodynamic parameters for the different conformations shows a similar trend for both solvents (Table 1). The elongation of the oligo-(ethylene glyclo) chain in aqueous solutions results in noticeable increase in ∆S and ∆H, while the intra-cage parameters stay almost unchanged.
Apart from temperature, the EPR line shape of biradical spectra depends on solvent viscosity. This is illustrated in Fig. 7 which compares the EPR spectra of P3T2 in water and in i-propanol/water mixture at 295 and 353 K. At the same temperature, the lines 2 and 2′ have lower intensities in i-propanol/water solution than in water due to an increased macroscopic viscosity. This behavior was observed for all PnT2 biradicals.
The thermodynamic properties of biradicals PnT2 are related to their structural features which can also be assessed from the EPR spectra. The most thermodynamically stable conformation of all biradicals (e.g., dominant at low temperatures) has J/a = 0 as evident from Fig. 7. The structure of this conformation is characterized by the distance r between two unpaired electrons of the biradical which is almost equal to the distance between the centres of N–O• groups. These distances can be determined from the EPR parameter d 1/d as described in detail in [32]. Analysis of EPR spectra recorded in frozen i-propanol-water mixtures at 120 K confirmed that J = 0 for all PnT2 biradicals thus making it possible to calculate interspin distances r with high accuracy (Table 2) following equation [32]:
Here, Δ = d 1/d − (d 1/d)0 is a ratio of EPR peak intensities corresponding to the contribution of the dipole–dipole interaction between unpaired electrons to the line shape, and (d 1/d)0 is the same ratio in the absence of the dipolar coupling [23]. The d 1/d parameter can be used to correctly estimate interspin distances in the range 1.2 ≤ r ≤ 2.5–2.7 nm.
Interestingly, biradical P2T2 shows the longest interspin distance of 2.03 nm, despite having the shortest bridge. We conclude, therefore, that P2T2 has the most extended structure, e.g., the effective conformation A has the longest distance between the nitroxide groups. For the longer chain biradicals, the distance between nitroxide groups increases with the elongation of the bridge length from P3T2 to P5T2 which correlates with the increased enthalpy and entropy differences ∆H and ∆S between the out-of-cage and in-cage conformations.
4 Conclusions
The intramolecular spin exchange in four flexible biradicals was studied by X-band EPR spectroscopy in aqueous and 1:1 (v/v) i-propanol:water solutions. The biradicals had different lengths of the ethylene glycol bridge connecting the two nitroxide groups. The EPR spectra were analyzed assuming co-existence of three effective conformations: an extended conformation and two rapidly interconverting “in-cage” conformations. The relative lifetimes of these conformations showed Arrhenius behavior. The enthalpy and entropy gap between the out-of-cage and in-cage conformations was compared with the effective distances between the two nitroxide groups in biradicals, which were calculated using frozen solution EPR spectra. Both the interspin distances and thermodynamic parameters generally increased with the increased ethylene glycol bridge length. However, the exchange properties were affected by the conformational space of the oligo-(ethylene glycol) bridge: the biradical with the shortest bridge (just one ethylene glycol unit) showed the largest effective distance between the nitroxides in the out-of-cage conformation, and the next homologue containing two ethylene glycol groups showed strong preference of the in-cage conformation. Interestingly, the comparison of thermodynamic parameters for the two solvents suggests that the solvent cage in which fast strong exchange is realized contains only water molecules even in the presence of i-propanol.
References
K.C. Ko, D. Cho, J.Y. Lee, J. Phys. Chem. A 117, 3561–3568 (2013)
E. Coulaud, D. Hagebaum-Reigner, D. Siri, P. Tordo, N. Ferre, Phys. Chem. Chem. Phys. 14, 5504–5511 (2012)
I. Krstic, R. Hansel, O. Romainczyk, J.W. Engels, V. Dotsch, T.F. Prisner, Angew. Chem. Int. Ed. 50, 5070–5074 (2011)
E.G. Rozantsev, Free Nitroxyl Radicals (Plenum Press, New York, 1970)
A.L. Buchachenko, A.M. Wasserman, Stable Radicals (Khimiya, Moscow, 1973)
L.J. Berliner (ed.), Spin Labeling: Theory and Applications (Academic Press, New York, 1976)
V.N. Parmon, A.I. Kokorin, G.M. Zhidomirov, Russ. J. Struct. Chem. 18, 104 (1977)
L.J. Berliner (ed.), Spin Labeling II: Theory and Applications (Academic Press, New York, 1979)
V.N. Parmon, A.I. Kokorin, G.M. Zhidomirov, Stable Biradicals (Nauka, Moscow, 1980)
E.G. Rozantsev, R.I. Zhdanov (eds.), Nitroxide Radicals. Chemistry, Applications, Synthesis (Nauka, Moscow, 1987)
L.B. Volodarsky (ed.), Imidazoline Nitroxides. Synthesis, Properties, Applications Vol. 1, 2 (CRC Press, Boca Raton, 1988)
A. Rassat, Pure Appl. Chem. 62, 223 (1990)
A.I. Kokorin, Appl. Magn. Reson. 26, 253 (2004)
A.B. Shapiro, K. Baimagambetov, M.G. Goldfield, E.G. Rozantsev, Zh. Org. Khimii 8, 2263 (1972)
V.N. Parmon, A.I. Kokorin, G.M. Zhidomirov, K.I. Zamaraev, Mol. Phys. 30, 695 (1975)
S.V. Kozlov, A.I. Kokorin, A.B. Shapiro, E.G. Rozantsev, Vysokomol. Soed. 23B, 323 (1981)
S.A. Dikanov, G.I. Shchukin, I.A. Grigor’ev, S.I. Rukin, L.B. Volodarsky, Izv. AN. SSSR Ser. Khim. 34, No. 3, 565 (1985)
S. Sankarapandi, J.M. Rifkind, P.T. Manoharan, Proc. Indian Acad. Sci. Chem. Sci. 106, 1329 (1994)
S. Sankarapandi, M. Sukumar, P. Balaram, P.T. Manoharan, Biochem. Biophys. Res. Comm. 213, 439 (1995)
V.A. Tran, K. Rasmussen, G. Grampp, A.I. Kokorin, Appl. Magn. Reson. 32, 395 (2007)
V.A. Tran, K. Rasmussen, G. Grampp, A.I. Kokorin, Mol. Phys. 105, 2119 (2007)
V.A. Tran, A.I. Kokorin, G. Grampp, K. Rasmussen, Appl. Magn. Reson. 35, 389 (2009)
A.I. Kokorin, in Ionic liquids. Theory and Applications, ed. by A.I. Kokorin (InTech Publ, Rijeka, 2011), pp. 183–200
E. Alster, B.L. Silver, Molec. Phys. 58, 977 (1986)
G.I. Likhtenstein, J. Yamauchi, S. Nakatsuji, A.I. Smirnov, R. Tamura, Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science (Wiley-VCH, New York, 2008)
G. Ionita, V. Meltzer, E. Pincu, V. Chechik, Org. Biomol. Chem. 5, 1910–1914 (2007)
G. Ionita, V. Chechik, Chem. Commun. 46, 8255–8257 (2010)
Yu.N. Molin, K.M. Salikhov, K.I. Zamaraev, Spin Exchange (Springer, Berlin, 1980)
R. Begum, H. Matsuura, J. Chem. Soc. Faraday Trans. 93, 3839–3848 (1997)
K. Holmberg, B. Jonsson, B. Kronberg, B. Lindman, Surfactants and Polymers in Aqueous Solutions, 2nd edn. (Wiley, West Sussex, 2003)
C.H. Cho, J. Urquidi, S. Singh, G.W. Robinson, J. Phys. Chem. B 103, 1991 (1999)
A.I. Kokorin, in Nitroxides: Theory, Experiment and Applications, ed. by A.I. Kokorin (InTech Publ, Rijeka, 2012), pp. 113–164
Acknowledgments
AIK and GAV thank the Russian Foundation for Basic Research (Grant 12-03-00623-a) for financial support of the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ionita, G., Vorobieva, G.A., Chechik, V. et al. Intramolecular Spin Exchange in Flexible PEG-based Nitroxide Biradicals in Aqueous Solutions. Appl Magn Reson 46, 251–260 (2015). https://doi.org/10.1007/s00723-014-0636-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-014-0636-1