Summary
Background
To measure intralenticular biometrical changes during accommodation.
Material and methods
This prospective study included two different age groups of volunteers (18–25 years and 30–39 years) and in each age group three different groups of ametropia (emmetropic, myopic, and hyperopic volunteers). All volunteers were measured using partial coherence interferometry (ACMaster, Carl Zeiss Meditec, Germany) in an unaccommodated and an accommodated state. Intralenticular biometric changes concerning anterior and posterior cortex and lens nucleus were analysed.
Results
In total, 104 eyes of 104 volunteers were included in this study. During accommodation in the group of subjects in their 20s, the following changes were observed: In the emmetropic group, the nucleus thickness increased by +0.414 mm, whereas the anterior cortex and posterior cortex thickness remained relatively constant at −0.006 mm (−0.186 mm to 0.046 mm) and +0.008 mm (−0.008 mm to 0.024 mm), respectively.
Conclusions
The increase of the human crystalline lens with age is characterised by a higher increase in cortex thickness than in nucleus thickness. During accommodation, the thickening of the lens is primarily explained by the thickening of the lens nucleus.
Zusammenfassung
Hintergrund
Vermessung der intralentikulären Veränderungen während der Akkommodation mittels partieller Kohärenz-Interferometrie bei verschiedenen Refraktionsgruppen und unterschiedlichen Altersgruppen.
Material und Methode
Diese prospektive Studie inkludierte 2 verschiedene Altersgruppen von Freiwilligen (18–25 Jahre und 30–39 Jahre). In beiden Altersgruppen wurden 3 verschiedene Refraktionsgruppen inkludiert (emmetrope, myope und hypermetrope Augen). Alle Studienteilnehmer wurden mittels partieller Kohärenz-Interferometrie (PCI, ACMaster, Carl Zeiss Meditec, Deutschland) mehrmals gemessen, nämlich im nichtakkommodierten und im akkommodierten Zustand. Die intralentikulären biometrischen Veränderungen bezüglich des vorderen und hinteren Linsenkortex (AC und PC) sowie des Linsenkerns wurden analysiert.
Ergebnisse
Insgesamt wurden 104 Augen von 104 Freiwilligen in der Studie vermessen. In der Gruppe der 18- bis 25-Jährigen wurden während der Akkommodation folgende Veränderungen beobachtet: In der Gruppe der emmetropen Augen nahm die Linsenkerndicke während der Akkommodation um +0,414 mm zu, wohingegen AC und PC kaum eine Veränderung zeigten −0,006 mm (−0,186 bis 0,046) und +0,008 mm (−0,008 bis 0,024). Die Zunahme der Linsendicke war in der Gruppe der 30- bis 39-Jährigen signifikant geringer ausgeprägt (p = 0,002).
Schlussfolgerung
Während der Akkommodation findet die Expansion der Linse vorwiegend im Bereich des Kortex und weniger im Bereich des Linsenkerns statt. Beim Vergleich von verschiedenen Altersgruppen fällt auf, dass die Zunahme der Linsendicke im Alter auch eher durch eine Veränderung im Linsenkortex gekennzeichnet ist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Restoring accommodation in presbyopic patients is still one of the unachieved goals in ophthalmology. Although there are several approaches, such as so-called accommodative intraocular lenses [1], lens refilling [2], and lens regrowing techniques [3], none has been shown to result in relevant accommodation. Within the last decades, knowledge of lens changes during accommodation has increased [4], but there are still uncertainties concerning the mechanism of presbyopia. One of the remaining questions concerns what changes are found within different parts of the crystalline lens during accommodation. Understanding the differences in accommodative response of the lens cortex and the lens nucleus could lead to more effective options in restoring accommodation.
Several approaches are in use to predict the potential accommodative response of the aging lens, such as ultrasound biometry [5]. Other studies have focused on static and dynamic open-field autorefractor measurements [6]. Correlations between A‑scan ultrasound and refractive changes were shown to be high [7].
The aim of this study was to measure subjects’ ametropia in two different age groups in an accommodated and unaccommodated state using a sophisticated set-up that allowed measuring of the central axial anterior and posterior cortex, as well as the nucleus thickness of the lens.
Methods
This prospective study included volunteers between 18 and 39 years of age. Inclusion criteria were a best distance-corrected visual acuity of at least 0.0 LogMAR in both eyes. Exclusion criteria were any history of accommodative problems in childhood or adolescence, ocular disease, trauma, or surgery. All the research and measurements followed the tenets of the Helsinki agreement. Informed consent was obtained from all subjects prior to inclusion. The study was approved by the ethics committee of the Medical University of Vienna.
In the present study, six groups of healthy subjects were allocated according to their ametropia and age. Emmetropic (±0.5 dioptres, D), myopic (−3 to −6 D), and hyperopic (+1.5 D or more) groups were divided into two age groups: a group of those about 20 years of age (18–25 years), called the 20s group, and a group of individuals in their 30s (31–39 years), called the 30s group. Only one eye of each volunteer was included in the study. Whether the right or the left eye was determined for investigation was randomly assigned using an envelope technique.
To assess objective refraction, an autorefractometer (Automatic Refractor Model 597, Zeiss Humphrey, Germany) was used. Additionally, subjective refraction and visual acuity were evaluated using trial frames, and the Jackson cross-cylinder method was used with a target at a 6‑m distance.
Axial eye length (AL), defined as the distance from the anterior corneal surface to the retinal pigment epithelium, was measured using the IOLMaster (Carl Zeiss Meditec, Jena, Germany) in myopic and hyperopic eyes. The principle of this technique is based on partial coherence interferometry (PCI) and has been described in detail in previous articles [8, 9]. For measuring AL, at least five successful measurements were taken, and the mean and standard deviation (SD) were calculated automatically.
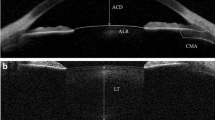
Anterior segment biometry was assessed using the ACMaster (anterior chamber [AC] biometer; Carl Zeiss Meditec, Jena, Germany). This instrument measures corneal thickness (CT), anterior chamber depth (ACD), and lens thickness (LT) in one measurement [8]. Methods of AC biometer measurements are explained elsewhere [9]. In short, each distance between two peaks gives the optical path length between two surfaces with a different refractive index, but not directly anatomical distances.
Therefore, the anatomical distances have to be calculated [10]. The PCI technology has shown an accuracy of 3 µm for measurements of ACD in pseudophakic eyes [11].
All subjects were seated in front of the AC biometer and focused on a defined internal target with the study eye. Corneal thickness, ACD, and LT (Fig. 1) were measured using best-distance correction, representing the unaccommodated eye. Afterwards, AC biometer measurements were performed under maximal accommodation. The unmeasured eye was occluded, and the subject had to focus on the fixation target, which was blurred by negative spherical glasses. Accommodation of up to −5 D was presented within the AC biometer. If a patient was able to accommodate more than 5 D, additional minus glasses in a trial frame were used. The examined eye had to accommodate in order to see the cross clearly. Minus dioptres were added until the subject saw the cross blurred. Measurements were taken at that dioptre step, where the subject was still able to visualise the fixation target clearly. A set of at least ten successful measurements was taken while the subject was continuously motivated to accommodate and to focus on the cross. For further analyses, the median of the consecutive measurements was used.
Data analysis
The results were analysed offline with special software (IOLMaster demo version 1.09). The software calculated the mean and standard deviation of all optical distances of the anterior eye segment of each subject. If the software could not detect a signal peak, the position for the cursor was set manually.
Corneal thickness was defined as the distance from the anterior corneal surface (ACS) to the posterior corneal surface (PCS), the ACD as the distance from the PCS to the anterior lens surface (ALS), and the LT as the distance from the ALS to the posterior lens surface (PLS).
The anterior eye segment (AS) was defined as the distance between the PCS and PLS. The anterior cortex (AC) was defined as the distance from the ALS to the anterior nucleus surface (ANS). The posterior cortex (PC) was defined as the distance between the posterior nucleus surface (PNS) and the PLS, and the nucleus thickness as the distance between the ANS and the PNS.
For detecting nucleus thickness (NT), the ANS and the PNS were evaluated. A graphically averaged A‑scan of all measurements in each eye under the same conditions was used. The scan was enlarged by the software, and a significant signal peak near the ALS was detected by the software. For this purpose, the PLS cursor was set to this signal peak. Then, for evaluating PNS, the same procedure was set to the posterior cortex (PC) interface peak. Additionally, all data were analysed manually to verify the findings of the software.
For statistical analysis, Microsoft Excel 2002 was used. Because data were not normally distributed, the median and range are given for descriptive analysis. The Mann–Whitney U test was used to compare different age groups and differences between emmetropic, myopic, and hyperopic volunteers. To compare changes of one parameter before and after accommodation, the Wilcoxon signed-rank test was used.
Results
Subjects
In total, 104 eyes of 104 subjects were recruited for this study. In the 20s group (range 19–25 years), 60 eyes of 60 volunteers were included. Nine eyes had to be excluded from analysis: 5 eyes due to insufficient accommodation (less than 3 D) and 4 eyes due to unsuccessful measurements with the AC biometer. In the emmetropic, myopic, and hyperopic groups, 26, 18, and 7 eyes were included for analysis, respectively.
In the 30s group (range 33–38 years), 44 eyes of 44 volunteers were included. Five eyes had to be excluded from the analysis: 3 eyes due to insufficient accommodation (less than 3 D) and 2 eyes due to unsuccessful measurements. In the emmetropic, myopic, and hyperopic groups, 25, 9, and 5 eyes were included in the analysis, respectively (Table 1).
Refraction
In the 20s group, refraction of the emmetropic, myopic, and hyperopic groups was −0.25 D (−0.50 to +0.50), −4.50 D (−3.00 to −6.38), and +2.00 D (+1.50 to +3.88), respectively.
In the 30s group, refraction of the emmetropic, myopic, and hyperopic groups was −0.13 D (−0.50 to +0.25), −4.25 D (−3.38 to −5.88), and +2.88 D (+1.50 to +3.50), respectively.
Axial eye length
In the 20s group, axial eye length of the myopic and hyperopic groups was 24.87 mm (24.43–26.63) and 22.32 mm (21.80–23.12), respectively.
In the 30s group, axial eye length of the myopic and hyperopic groups was 25.49 mm (24.44–26.50) and 22.36 mm (21.37–22.85), respectively.
Anterior segment biometry
The standard deviation of three consecutive AC biometer measurements was ±5 µm for CT, ±20 µm for ACD, and ±20 µm for LT in this study.
Baseline
Twenties group
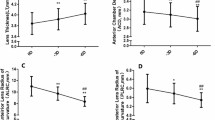
The anterior chamber depth (Table 2; Fig. 2) was found to be significantly different between the emmetropic and the hyperopic groups (p = 0.012) and between the emmetropic and the myopic groups (p < 0.001). Lens thickness (Table 2; Fig. 2) was significantly different between emmetropic and myopic eyes (p = 0.047), but not between emmetropic and hyperopic eyes (p = 0.139). There was no significant difference between groups concerning NT, AC, and PC, respectively (Table 2; Fig. 3).
A comparison of the anterior chamber depth (acd, blue ring), anterior nuclear surface (acdac, green ring), posterior nuclear surface (acdacnt, yellow ring), and anterior segment (as, purple ring) between the age groups. The ordinate describes the distance in mm; the x-axis shows the ametropia and age groups (E20 = emmetropic 20s, E30 = emmetropic 30s, M20 = myopic 20s, M30 = myopic 30s, H20 = hyperopic 20s, H30 = hyperopic 30s)
Median of the anterior cortex (AC), nucleus thickness (NT), and posterior cortex (PC) in baseline for the 20s and 30s groups in mm. Different ametropia are represented by different colours: green = emmetropic, red = myopic, blue hyperopic group. The dotted lines below and above the coloured lines represent the minimum and maximum values within the group
Thirties group
Anterior chamber depth (Table 3; Fig. 2), LT (Table 3; Fig. 2), and NT were significantly different between emmetropic and myopic eyes (p = 0.001, 0.047, and 0.030) but not significantly different between emmetropic and hyperopic eyes (p = 0.774, 0.503, and 0.370). Concerning AC and PC, no significant differences were found (Table 3; Fig. 3).
Differences in baseline values between age groups
A comparison between the two emmetropic groups showed that ACD was significantly more shallow (p < 0.001) and LT (p = 0.04) and NT (p < 0.001) were significantly larger in the 30s group (Fig. 2). In the myopic 30s group, ACD was significantly more shallow (p = 0.027) and LT (p < 0.001) and NT (p = 0.002) were significantly higher in the 30s group. In the hyperopic 30s group, ACD was deeper than in the 20s group, and LT was significantly higher (p = 0.012). In the myopic and hyperopic 30s groups, AC was larger than in volunteers in their 20s (p < 0.007), and PC was larger in all 30s groups (p < 0.006) (Fig. 3).
In the emmetropic group, NT increased by 5.8%, and the cortex increased almost 4 times more, by 21.1% (AC for 18.9% and PC for 24.4%).
In the myopic group, NT increased by 9.7% and the cortex by 15.2% (AC for 14.7% and PC for 15.9%). In this group, the thickness of the cortex increased 1.6 times more than the nucleus.
Changes with accommodation
Twenties group
Anterior chamber depth decreased significantly in the emmetropic, myopic, and hyperopic groups (p < 0.005): by −0.334 mm (−0.064 to −0.430), −0.334 mm (−0.161 to −0.682), and −0.312 mm (−0.151 to −0.387), respectively. Lens thickness increased significantly during accommodation in the emmetropic, myopic, and hyperopic groups (p < 0.005): by +0.432 mm (+0.073 to +0.526), +0.427 mm (+0.157 to +0.786), and +0.407 mm (+0.167 to +0.494), respectively. Changes within the lens are shown in Table 4 and Fig. 4.
Median of the change during accommodation for the anterior cortex (ac), nucleus thickness (nt), and posterior cortex (pc) 20s and 30s groups in mm. Different ametropia are represented by different colours: green = emmetropic, red = myopic, blue hyperopic group. The dotted lines below and above the coloured lines represent the minimum and maximum values within the group
Thirties group
Anterior chamber depth in the emmetropic, myopic, and hyperopic groups decreased by −0.213 mm (−0.434 to −0.038), −0.199 mm (−0.322 to −0.067), and −0.125 mm, (−0.166 to −0.032 (E/M:p < 0.001, E/H: p = 0.211, M/H: p = 0.121), respectively.
Lens thickness in the emmetropic, myopic, and hyperopic groups increased by 0.261 mm (0.041–0.413), 0.248 mm (0.08–0.362), and 0.109 mm (0.40–0.305), respectively (E/M: p = 0.128, E/H: p = 0.732, M/H: p = 0.312). Changes within the lens are shown in Table 5 and Fig. 4.
Differences between age groups
The decrease of ACD during accommodation was lower in all 30s groups than in the 20s groups (p = 0.008). The increase of LT in the emmetropic group during accommodation was significantly lower in the 30s groups than in the 20s groups (p = 0.002).
Change of AS and LT during accommodation
Concerning the 20s group, correlation between AS changes and LT changes during accommodation for the emmetropic, myopic, and hyperopic groups was r2 = 0.39 (gradient = 0.37), r2 = 0.22 (gradient = 0.22), and r2 = 0.44 (gradient = 0.48), respectively.
Concerning the 30s group, correlation between AS changes and LT changes during accommodation for the emmetropic, myopic, and hyperopic groups was r2 = 0.30 (gradient = 0.24), r2 = 0.41 (gradient = 0.14), and r2 = 0.71 (gradient = 0.48), respectively.
Discussion
There is a variety of published papers on crystalline lens changes during accommodation, but there is a lack of knowledge concerning changes of different parts of the lens. In the past, several different techniques were used to measure lens changes during accommodation, such as Scheimpflug imaging [12], spectral domain optical coherence tomography (OCT), swept-source OCT [13], extended-scan-depth OCT [14, 15], and, in some studies, magnetic resonance imaging [16,17,18]. All of these techniques have their advantages, but the AC biometer used in this study allows measurements of the field axial biometric characteristics within the lens with high precision and reproducibility, especially the two-dimensional imaging of the lens [11].
There are some limitations of the study. Because it was not a longitudinal study, it is possible that there were differences between the 20s and 30s groups that were not entirely age dependent. Furthermore, the measurement technique that was used (PCI technology) allows very precise measurements, but because the target was internal, there could be slight differences compared with a real target shown in the distance.
Changes with age
In this study, ACD decreased in the emmetropic and myopic groups with age, but increased in the hyperopic group. Hyperopic eyes need to be accommodated during normal conditions in order to see clearly without glasses at each distance. With increasing age, accommodation power decreases. Although an increase in LT was observed in hyperopic volunteers, the deeper ACD could be explained by a more relaxed ciliary body, resulting in a backward movement of the lens equator. However, the number of subjects in the hyperopic group was small, and the results of this subgroup need to be interpreted with caution. Tsorbatzoglou et al. [19] showed that the ACD also decreased significantly with age and during accommodation in three age groups of emmetropic eyes. Koretz et al. [20] showed that the ACD decreases with age at a rate equal to the increase in thickness of the lens. In our study, LT increased two times more than the ACD decreased in the emmetropic groups with age. In the myopic groups, the ACD decreased with age at a rate equal to the increase in thickness of the lens. In our hyperopic group, LT and ACD increased with age.
The anterior eye segment was slightly influenced by age in the emmetropic and myopic groups and increased slightly in the hyperopic group. The results of the hyperopic group need to be interpreted with caution due to the small number of subjects measured. Similar to our study, Dubbelman et al. [12] and Koretz [22] showed that the anterior segment (distance from the posterior corneal surface to the posterior lens surface) remains unchanged with age.
In the present study we found that the change of the lens cortex thickness (AC + PC) increased four times that of the nucleus thickness in the emmetropic eyes. In the myopic eyes, the thickness of the cortex increased 1.6 times more than the nucleus. Therefore, the increase in cortex thickness in comparison to the nucleus was not as high as that found in the study of Dubbelman el al. [12] using the Scheimpflug photographic principle and finding a seven-fold increase. The reason could be that the Dubbelman study included patients with a larger age range, from 16 to 65 years. Differing from the results of Dubbelman et al. [12], the posterior cortex increased slightly more than the anterior cortex in the emmetropic and myopic groups in our study. Brown [21] found that the growth of the cortex was three times the growth of the nucleus, which is similar to our measurements. In contrast to the findings of Cook et al. [22], we found a small increase in nucleus thickness with age in our study.
Changes with accommodation
In all groups, ACD decreased and LT and NT increased during accommodation, as expected. In 1855, Helmholtz [23] suggested that accommodation is a result of lens deformation and movement of the ciliary muscle. More recently, it was shown that presbyopia is mainly the result of the lens hardening during aging [24]. Richdale et al. [25] showed that the per-dioptre ciliary muscle contraction is age dependent. Wolffsohn et al. [26] investigated the accommodative response in pre-presbyopic patients, observing that the mean proportion of accommodation was relatively high (80.6%).
The AS increased slightly during accommodation, which indicates that the posterior lens pole moves backwards with accommodation. In accordance with Dubbelman et al. [12], we found that the increase in lens thickness is mainly due to an increase in the thickness of the nucleus, not the cortex.
Twenties group
In the emmetropic group, the nucleus increased in thickness, but the thickness of the cortex remained relatively unchanged. In the myopic group, the nucleus and the cortex became thicker, whereas the AC was unchanged.
Thirties group
In the emmetropic group, the nucleus increased in thickness, but the thickness of the cortex remained relatively unchanged. In the myopic group, the nucleus and the PC became thicker, whereas AC decreased. In the hyperopic group, NT and AC increased, whereas PC was unchanged. However, some of these changes were minor and potentially due to noise of the measurement.
In summary, during accommodation the thickening of the lens is primarily explained by the thickening of the lens nucleus. The increase of the human crystalline lens with age is characterised by a higher increase of cortex than of nucleus thickness.
References
Ong HS, Evans JR, Allan BD. Accommodative intraocular lens versus standard monofocal intraocular lens implantation in cataract surgery. Cochrane Database Syst Rev. 2014; https://doi.org/10.1002/14651858.CD009667.pub2.
Nishi Y, Mireskandari K, Khaw P, et al. Lens refilling to restore accommodation. J Cataract Refract Surg. 2009;35(2):374–82.
Gwon A. Lens regeneration in mammals: a review. Surv Ophthalmol. 2006;51(1):51–62.
Schachar RA. The mechanism of accommodation and presbyopia. Int Ophthalmol Clin. 2006;46(3):39–61.
Ramasubramanian V, Glasser A. Prediction of accommodative optical response in prepresbyopic subjects using ultrasound biomicroscopy. J Cataract Refract Surg. 2015;41(5):964–80.
Win-Hall DM, Houser J, Glasser A. Static and dynamic accommodation measured using the WAM-5500 Autorefractor. Optom Vis Sci. 2010;87(11):873–82.
Ostrin L, Kasthurirangan S, Win-Hall D, et al. Simultaneous measurements of refraction and A‑scan biometry during accommodation in humans. Optom Vis Sci. 2006;83(9):657–65.
Drexler W, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126(4):524–34.
Findl O, Drexler W, Menapace R, et al. Accurate determination of effective lens position and lens-capsule distance with 4 intraocular lenses. J Cataract Refract Surg. 1998;24(8):1094–8.
Drexler W, Hitzenberger CK, Baumgartner A, et al. Investigation of dispersion effects in ocular media by multiple wavelength partial coherence interferometry. Exp Eye Res. 1998;66(1):25–33.
Findl O, Drexler W, Menapace R, et al. High precision biometry of pseudophakic eyes using partial coherence interferometry. J Cataract Refract Surg. 1998;24(8):1087–93.
Dubbelman M, Van der Heijde GL, Weeber HA, et al. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res. 2003;43(22):2363–75.
Neri A, Ruggeri M, Protti A, et al. Dynamic imaging of accommodation by swept-source anterior segment optical coherence tomography. J Cataract Refract Surg. 2015;41(3):501–10.
Leng L, Yuan Y, Chen Q, et al. Biometry of anterior segment of human eye on both horizontal and vertical meridians during accommodation imaged with extended scan depth optical coherence tomography. Plos One. 2014;9(8):e104775.
Zhong J, Tao A, Xu Z, et al. Whole eye axial biometry during accommodation using ultra-long scan depth optical coherence tomography. Am J Ophthalmol. 2014;157(5):1064–9.
Kasthurirangan S, Markwell EL, Atchison DA, et al. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis. 2011;11(3):19.
Sheppard AL, Evans CJ, Singh KD, et al. Three-dimensional magnetic resonance imaging of the phakic crystalline lens during accommodation. Investig Ophthalmol Vis Sci. 2011;52(6):3689–97.
Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of the anteroposterior position and thickness of the aging, accommodating, phakic, and pseudophakic ciliary muscle. J Cataract Refract Surg. 2010;36(2):235–41.
Tsorbatzoglou A, Nemeth G, Szell N, et al. Anterior segment changes with age and during accommodation measured with partial coherence interferometry. J Cataract Refract Surg. 2007;33(9):1597–601.
Koretz JF, Kaufman PL, Neider MW, et al. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Res. 1989;29(12):1685–92.
Brown N. Dating the onset of cataract. Trans Ophthalmol Soc U K. 1976;96(1):18–23.
Cook CA, Koretz JF, Pfahnl A, et al. Aging of the human crystalline lens and anterior segment. Vision Res. 1994;34(22):2945–54.
Helmholtz. Über die Akkommodation. Graefe’s Arch. Ophthalmologie. 1855;1/2:1–74.
Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39(11):1991–2015.
Richdale K, Sinnott LT, Bullimore MA, et al. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Investig Ophthalmol Vis Sci. 2013;54(2):1095–105.
Wolffsohn JS, Sheppard AL, Vakani S, et al. Accommodative amplitude required for sustained near work. Ophthalmic Physiol Opt. 2011;31(5):480–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a financial interest in this study. N. Hirnschall is a scientifc advisor (Carl Zeiss Meditec AG and Hoya Surgical). O. Findl is a scientific advisor (Alcon, Carl Zeiss Meditec AG, Croma Pharma, Johnson & Johnson Vision Care, Merck). B. Kiss and A. Guer declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirnschall, N., Kiss, B., Guer, A. et al. Biometric changes of the crystalline lens during accommodation. Spektrum Augenheilkd. 35, 221–228 (2021). https://doi.org/10.1007/s00717-020-00464-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00717-020-00464-4