Abstract
Colleters are secretory structures involved in the protection of young and developing plant organs. Although the presence of colleters in Gentianales is described as a synapomorphy, studies on the morphofunctionality of colleters and the mechanisms underlying the synthesis and release of colleter secretion in Gentianaceae are scarce. Here, we described the ontogeny and the morphological and functional aspects of colleters of Prepusa montana, revealed the nature of the key compounds present in the secretion, and explored the cellular aspects of the synthesis and release of secretion and senescence of colleters. Samples of the stem apical meristem with leaf primordium and young leaves; adult and senescent leaves were observed using light and electron microscopy. The colleters, located in the axil of the leaf, have a protodermal origin and develop asynchronously. They are digitiform, possessing a short peduncle and a secretory head containing homogeneous cells with dense cytoplasm and abundant endoplasmic reticulum and Golgi bodies. The secretion, composed of polysaccharides and proteins, is accumulated in schizogenous spaces and released through the separation of peripheral secretory cells and loosening of the external periclinal wall. Presumably, senescence is caused by programmed cell death. The morphoanatomical characterization of P. montana leaf colleters described here is the first record for the genus and the peculiar accumulation of colleter secretion in schizogenous spaces expanding our knowledge on the diversity of these secretory structures. Our results also provide insights into programmed cell death as an eminent topic related to secretory structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secretory structures are common in plants. They can be internal or external and serve different functions in the plant body due to their diverse structures, locations, and exudate chemical compositions, in particular (Fahn 1979; Roshchina and Roshchina 1993; Evert 2006). In most cases, the glands develop early—before the complete development of the organ in which they are located. The composition of exudate and the duration and intensity of secretory activity differ across glands. In some cases, these structures undergo senescence after the cessation of their activity, as observed in colleters (Paiva 2009; Fernandes et al. 2016; Ribeiro et al. 2017).
Colleters (from the Greek colla) are multicellular structures that secrete a sticky substance (Thomas 1991). They occur in different parts of the plant, such as stipules (Klein et al. 2004; Pinheiro et al. 2019); at the base of the leaf blade (Appezzato-da-Glória and Estelita 2000; Silva et al. 2019), leaf margin and/or in the leaf teeth (Fernandes et al. 2016; Almeida and Paiva 2019; Rios et al. 2020), in the petiole, bracts (Thomas and Dave 1989), in the sepals (Dalvi et al. 2020), and mainly in the vegetative and/or reproductive shoot apexes (Thomas 1991; Costa et al. 2020).
Colleter secretions serve various functions such as lubricating and protecting the developing meristems and organs by preventing desiccation (Fahn 1979; Thomas 1991); facilitating bacterial symbiosis via leaf nodules in Rubiaceae plants (Lersten 1974a, b, 1975; Miller et al. 1983); activating defense against fungal, bacterial, and herbivore attacks (Miguel et al. 2006; Ribeiro et al. 2017); and keeping the petals together until anthesis (Mayer et al. 2013).
Chemically, the colleter secretions are composed of mucilaginous and lipophilic substances or a mixture of both (Fahn 1979; Thomas 1991). Proteins (Miguel et al. 2006; Coelho et al. 2013; Dalvi et al. 2014), phenolics (Ribeiro et al. 2017), terpenes (Cassola et al. 2019), and alkaloids (Almeida and Paiva 2019) may also be present. In addition to the chemical complexity, the secretion composition may vary at different stages of development (Thomas and Dave 1989; Appezzato-da-Glória and Estelita 2000; Klein et al. 2004), which warrants further research.
The mechanisms of secretion and colleter senescence have been addressed, and dynamic involvement of the cell wall in the release of secretion in the former and programmed cell death in the latter has been proposed (Miguel et al. 2006, 2010, 2016, 2017). These two processes occur simultaneously (Coelho et al. 2013; Miguel et al. 2016). However, there is little literature on the autophagic processes, which are essential for understanding the senescence of colleters and the end of their secretory activity.
Solereder (1908) described colleters as finger-like structures with elongated axial cells and a palisade epidermis, which is the standard form observed in Rubiaceae. By observing colleters in Rubiaceae, Lersten (1974a, 1974b) proposed a classification based on their morphoanatomical characteristics including “standard type,” “reduced standard,” “intermediate,” “dendroid,” and “brush-like” types. Over time, other types have been described, such as “filiform” (Robbrecht 1983) and “winged” (Robbrecht 1987), among others. Standard type colleters are comprised of a secretory palisade epidermis covering a non-secretory central axis, which may or may not be vascularized with xylem and phloem (Lersten 1974a, b) being the most common type of colleter reported in the literature. However, given the increasing records of the occurrence of these structures in different families of angiosperms, including monocots (Leitão and Cortelazzo 2008; Mayer et al. 2011; Cardoso-Gustavson et al. 2014; Ballego-Campos and Paiva 2018), gymnosperms (Sacher 1954, 1955), and ferns (Oliveira et al. 2017), the morphoanatomical types and functional aspects of the colleters do not conform to the existing classification. Some authors even consider function as fundamental aspect for defining a secretory structure as a colleter (Mayer et al. 2011).
In view of the diversity of colleters, especially regarding the distribution, function, and morphoanatomy within the taxa, the colleters are relevant in taxonomic interpretations (Simões et al. 2006; Sheue et al. 2013). The occurrence of colleters in Gentianales, for example, is described as a synapomorphy (Struwe and Albert 2002; Judd et al. 2009). Even so, there are few studies on the morphofunctionality of these secretory structures in Gentianaceae (Nemomissa 1997; Renobales et al. 2001; Delgado et al. 2011; Dalvi et al. 2013, 2014, 2020; Guimarães et al. 2013; Zanotti 2018).
Recent field observations have demonstrated the potential occurrence of colleters in Prepusa montana Mart. (Gentianaceae: Helieae), which is the first described species of this genus (von Martius 1827) and is the only species Prepusa spp. that reaches the size of a shrub or small tree (Fig. 1a). This species still differs from the others species of the genus by the calyx and corolla of greenish-yellow, yellowish-green, or cream (Fig. 1b and c). It is endemic to the state of Bahia, Brazil and shows a restricted distribution in the campos rupestres (Fig. 1d) and Cerrado, occurring on rocky outcrops (Struwe and Albert 2002; Calió et al. 2008; Calió 2009).
To this end, the objectives of the present study were (i) to describe the structure of colleters in P. montana at different stages of leaf development; (ii) to investigate the chemical nature of the major components of the colleter exudate based on histochemical analyses; and (iii) to elucidate the cellular aspects of the synthesis and release of exudates and senescence of colleters.
Material and methods
Collection and sampling
Samples of the stem apical meristem with leaf primordia and young leaves (i) and the base of adult (ii) and senescent (iii) leaves were collected from three P. montana individuals (Fig. 1). The samples were collected from a campo rupestre in Chapada Diamantina, Mucugê, Bahia, Brazil (13° 00′ 35.2″ S, 41° 22′ 34.7″ W) (Fig. 1d). The environment at the study site is characterized by a predominance of herbaceous and shrub vegetation growing on rocks, strong winds, and marked climatic variations. The fertile material was pressed and deposited in the Herbarium of Rio Verde (IFRV), Rio Verde, Goiás, Brazil (IFRV numbers 1042, 1043, and 1187). The collected samples were visualized under a stereomicroscope (Stemi DV4, Carl Zeiss™, Germany) to confirm the presence of colleters and to observe their morphological characteristics.
Light microscopy
The samples were fixed in FAA (formalin, acetic acid, and 70% ethanol 1:1:18 by volume) for approximately 48 h and preserved in 70% ethanol (Johansen 1940) for structural characterization. Subsequently, the samples were dehydrated in an increasing ethanol series, subjected to pre-infiltration, and embedded in methacrylate resin (Historesin Leica, Leica Microsystems, Heidelberger, Germany). Transverse and longitudinal sections (thickness, 5 µm) were obtained using a rotary microtome (1508R; Logen Scientific) with low-profile disposable steel blades (Leica 819, Leica Biosystems, Buffalo Grove, Illinois, USA). The sections on glass slides were stained with toluidine blue (pH 4.6) (O'Brien et al. 1964) and mounted in synthetic resin (Permount; Fisher Scientific, NJ, USA).
Histochemistry
Different fixatives were used for histochemical tests: FAA (formalin, acetic acid, and 50% ethanol; 1:1:18 by volume) (Johansen 1940) for polysaccharides, proteins, and water-soluble phenolics; neutral-buffered formaldehyde solution (phosphate buffer:formalin, 9:1 v/v) (Lillie 1965) for lipids and lipid-soluble phenolics; and formalin–ferrous sulfate solution (9:1 v/v) (Johansen 1940) for general phenolics.
The samples were dehydrated in an increasing ethanol series, embedded in methacrylate resin (Historesin Leica, Leica Microsystems, Heidelberger, Germany), and sectioned (thickness, 5 µm) using a rotary microtome (1508R; Logen Scientific). The transverse and longitudinal sections were treated with periodic acid–Schiff (PAS) stain to detect total polysaccharides (McManus 1948); Lugol’s solution (Johansen 1940) to detect starch; potassium dichromate (Gabe 1968) to detect phenols; Ponceau Xylidine (O'Brien and McCully 1981) to detect total proteins; ruthenium red (Johansen 1940) to detect pectin/mucilage; and Sudan III to detect total lipids (Pearse 1985). The respective controls and samples not subjected to the reagent (blank) were observed under a light microscope.
Electron microscopy
For scanning electron microscopy (SEM), samples were fixed in FAA (formalin, acetic acid, and 50% ethanol; 1:1:18 by volume) for approximately 48 h, dehydrated in an increasing ethanol series (80%, 90%, and 100%), and critical point-dried with CO2 (Autosamdri®-815; Series A, MD, USA). The samples were placed onto stubs using double-sided tape, sputter-coated with gold (25 nm) (Desk V, Denton Vacuum, NJ, USA), and observed under a scanning electron microscope (JSM 6610; Jeol, Tokyo, Japan). For transmission electron microscopy (TEM), the material was fixed in Karnovsky solution (Karnovsky 1965) for 48 h, post-fixed in 1% osmium tetroxide (0.1 M phosphate buffer, pH 7.2) for 2 h, dehydrated in an increasing acetone series, and embedded in EPON. Ultrathin Sects. (70 nm) were contrasted with 2% uranyl acetate and 0.2% lead citrate (Reynolds 1963) and observed under a transmission electron microscope (JEM 2100; Jeol, Tokyo, Japan) equipped with an energy-dispersive detector (at 80 kV).
To understand the dynamics of the external periclinal cell wall during secretion releases, we have used the different layers of it followed the classification proposed by Miguel et al. (2016).
Results
Distribution, morphoanatomy, and ontogeny
P. montana colleters were located at the base of the leaf blade (Fig. 2a). Several colleters were present at the early stages of leaf development. In the shoot apex, in leaf primordium and young leaves, they surrounded the shoot apical meristem (Fig. 2b).
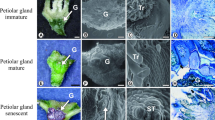
Distribution and characterization of Prepusa montana foliar colleters. a. Stereomicroscopic. b,c,e Cross sections stained with toluidine blue. d Scanning electron microscopy (SEM). a Colleters at the base of the leaf blade (arrows). b Colleters distributed around the shoot apical meristem (AM), at the base of the leaf primordium (LP) and young leaves. c Asynchronous development of colleters through the following three phases: (1) undifferentiated, (2) mature-active, and (3) senescent colleters. d Digitiform colleters (arrows) with secretion (*) around the leaf primordium (LP). e Homogeneous cells of the secretory head and peduncle. Bars: a = 5 mm; b = 500 µm; c and e = 100 µm, d = 200 µm
Colleters developed asynchronously (Fig. 2c and d), and three distinct development stages could be observed: undifferentiated or pre-secreting colleters (phase 1), mature-active colleters (phase 2), and senescent colleters, whose secretory activity had ceased (phase 3) (Fig. 2c). The mature-active colleters are digitiform (Fig. 2d and e), possessing a secreting apical head and a short non-secreting peduncle (Fig. 2e). These colleters, together with senescent ones, predominated the adult leaves.
P. montana colleters have a protodermal origin. At the early stages of development, the volume of two or more protodermal cells increased (Fig. 3a). Elongation and anticlinal division formed a bulge (Fig. 3b), and periclinal divisions formed a globular structure (Fig. 3c). These divisions continued in different planes resulting in the growth of the structure body, comprising round cells, without significant differentiation (Fig. 3d). Subsequently, the internal or central cells were elongated, while the cells lining the structure, called peripheral cells, remained round (Fig. 3e).
Colleter development. a Beginning of colleter formation: dividing protodermal cells. b Elongation and anticlinal division of the protodermal cells. c Division in different planes forming a globular structure. d Colleter body composed of undifferentiated cells. e Elongation of the internal secretory cells. f A mature colleter, with a clear distinction between the peduncle and the secretory head. g Cell retraction or lysis starts at the apex and promotes the formation of large intercellular spaces. h Points of the middle lamella (black arrows) indicate the separation of the internal secretory cells. i Cells with irregular contours lacking the cytoplasmic content (arrowhead). j Degradation along the entire structure. k Lignification of the cells walls (white arrows). l Total colleter collapse. Bars: j and k = 100 µm; e–g, i, and l = 50 µm; a, c, d, and h = 20 µm; b = 10 µm
Colleters are described as mature or active (phase 2) following cellular differentiation, with a distinction between the basal and the secretory portions (Fig. 3f and g). The basal portion constitutes the peduncle, which possesses two to three layers of vacuolated hyaline cells with visible nuclei (Fig. 3f). In contrast, the secretory portion is composed of elongated cells with large nuclei and dense cytoplasm and was intensely stained with toluidine blue (Fig. 3f and g). Anatomical sections indicated that the colleters are avascularized (Fig. 3f–i).
During the secretory phase, large intercellular spaces beginning in the apical region of the secretory portion toward the base (Fig. 3g) were formed, characterizing the beginning of the senescent phase. These intercellular spaces were formed by the displacement or separation of internal cells from the periphery of the structure (Fig. 3h). Cell displacement was characterized by decreased cell volume and middle lamella rupture (Fig. 3h), while cell death was characterized by cytological changes such as retraction of the protoplast, partial or total loss of nuclear and cytoplasmic contents, and change in cell contour (Fig. 3i). Notably, the activity of the internal secretory cells ceased before that of the peripheral ones, which remain adhered to the cuticle and intact (Fig. 3g–i).
Cell death continued and throughout the secretory portion, with only a small set of central and peripheral cells remaining (Fig. 3j). Following this, the colleters were flattened, and an abscission region, indicated by the cell wall lignification of the peduncle cells, was formed in some of them (Fig. 3k). All these stages were observed in the leaf primordium and young leaves. Finally, the colleters persisting in the senescent leaves showed a total cell collapse and could no longer be distinguished (Fig. 3l).
Secretion composition
The colleters of P. montana were slightly brown initially (Fig. 2a) and tended to darken as the leaves developed. The cellular cytoplasm was also slightly brown in the secretory portion but not in the peduncle (Fig. 4a).
Staining and histochemical characterization of colleters. a Natural coloring of colleters. b Proteins detected in the cytoplasm of the cells located in the secretory portion of the active colleters. c–h Section subjected to periodic acid–Schiff staining. c Colleters in young leaves showing evidence of extravasated exudate (black asterisk) and accumulation in intercellular spaces (inclusion). d Forming colleter (negative for polysaccharides). e Mature colleter. f–g Accumulation of polysaccharide in intercellular spaces. h Collapsed colleter with accumulated secretion. i–l Sections subjected to the red ruthenium staining. i Middle lamella (white arrows) showing cell displacement. j Possible passage of the exudate to the outside (black arrows). k Formation of large intercellular spaces, with accumulation of pectic exudate and cell death, as evidenced by the retraction of the protoplast (arrowhead). l Intense staining for pectin in senescent colleters. Bars: a = 100 µm; b and f = 200 µm; c = 500 µm; d and e = 50 µm; g–l = 10 µm
Histochemical tests showed the presence of proteins in the cytoplasm of the cells in the secretory portion of the colleters (Table 1; Fig. 4b) only at the initial stages of leaf development. Conversely, the presence of polysaccharides was confirmed in the secretion at all stages of leaf development. Extravasated polysaccharide was observed around the stem apical meristem and leaf primordium (Fig. 4c) and accumulated in the intercellular spaces (Fig. 4c). Polysaccharides were not detected at the early stages of colleter development (phase 1) (Fig. 4d). These compounds were detected only after cell differentiation (Table 1; Fig. 4e–h) and were accumulated in the intercellular spaces in phases 2 and 3 of colleter development (Fig. 4f–h). The senescent stage of colleters was characterized by complete degradation of the structure, but the remaining secretion was retained (Fig. 4h).
Ruthenium red staining distinguished the internal secretory cells from the peripheral ones (Fig. 4i and j). Spaces between the peripheral cells may serve as the pathways of secretion release to the external environment (Fig. 4j). Large intercellular spaces were observed in which pectic secretion were accumulated (Fig. 4k), with the adjacent cells showing protoplast retraction (Fig. 4k). Pectic exudates became abundant at the final stage of colleter development (Fig. 4l) and were retained in the collapsed structure. The results of other histochemical tests were negative (Table 1).
Ultrastructure
Internal and peripheral secretory cells showed the same cytological characteristics (Fig. 5a) at the early stages of colleter development. Both types of cells contained dense cytoplasm with a conspicuous nucleus as well as abundant Golgi bodies associated with small vesicles, endoplasmic reticulum, small vacuoles (Fig. 5b), and plasmodesma (Fig. 5c). Well-developed periplasmatic spaces were filled with exudate (Fig. 5d), and in some colleters, exudate was also accumulated in the intercellular spaces among the peripheral secretory cells (Fig. 5e).
Ultrastructural aspects during secretory activity. a Undifferentiated peripheral and internal secretory cells. b Vacuoles, endoplasmic reticulum, and Golgi apparatus secreting small vesicles. c Plasmodesma (black arrows). d Accumulation of exudate in the periplasmatic space and swelling of the anticlinal cell wall. e Accumulated exudate (asterisk) in the intercellular space and between peripheral cells. f Degradation of the middle lamella (arrowheads). g Vesicles (arrows) adjacent to the anticlinal wall. h Formation of an intercellular space (dotted arrow) between the adjacent two-cell anticlinal wall. cw, cell wall; Ga, Golgi apparatus; ml, middle lamella; ps, periplasmatic space; er, endoplasmic reticulum; va, vacuole. Bars: a, e = 5 µm; b, d, f–h = 1 µm; c = 500 nm
These spaces were formed due to the degradation of the middle lamella (Fig. 5f–h). The middle lamella of the peripheral cells became loose, reticulated, and disassociated from the cell wall (Fig. 5f). Dense circular aggregates were formed, and the middle lamella was detached from the cell wall (Fig. 5f). Small vesicles emerging from the plasma membrane adjacent to the anticlinal walls of the two neighboring cells (Fig. 5g) were observed. Finally, an intercellular space resulting from the dissolution of the middle lamella, which connected two anticlinal walls of the peripheral cells (Fig. 5h), emerged.
The external periclinal wall was composed of three structurally distinct layers: a basal polysaccharide layer (pol), cuticular membrane (cm), and thin cuticular layer (cut) (Fig. 6a). However, differences in the structure and thickness of cell wall were observed during secretory activity (Fig. 6b). The wall was more disorganized in regions where the exudate accumulated in the periplasmatic spaces (Fig. 6b). Interestingly, vesicles were observed close to these regions of exudate accumulation (Fig. 6b). The cuticle was reticulated, with dense projections throughout the structure (Fig. 6c) but without pores or rupture in the layers constituting the external periclinal wall of the colleters in the secretory phase.
Ultrastructural aspects during secretory activity and senescence. a–c Different aspects of the external periclinal wall. a Dense and defined wall with three noticeable layers. b Disorganization of the cuticular membrane with electrodense projections. A vesicle (black circle) close to the periplasmatic space. c Reticulated matrix in the cuticular membrane. d Beginning of protoplast retraction and exudate in the intercellular space. e Electrodense bodies (arrow) associated with the tonoplast. f A large prominent vacuole containing fibrillar material. g Cell collapse. cm, cuticular membrane; cut, thin cuticular layer; Ga, Golgi apparatus; pol, polysaccharide layer; ps, periplasmatic space. Bars: d, f, and g = 5 µm; e = 1 µm; a–c = 500 µm
As the colleters developed, the protoplasm was retracted, and the number and size of the vacuoles in the cells were apparently increased (Fig. 6d). Unidentified materials of different electron densities were detected inside these organelles. These vacuoles showed small electrodense bodies associated with the tonoplast (Fig. 6e). They coalesced with one another, and a large vacuole with fibrillar material was observed (Fig. 6f). These events continued and finally resulted in a partial or complete loss of cytoplasmic content, protoplast retraction reflected by irregular contours, and loss of turgor, making it difficult to distinguish the subcellular aspects (Fig. 6g), characterizing the end of the secretory activity of colleters.
Discussion
Anatomy and development of colleters
The morphoanatomy of P. montana colleters differs from the types described by Lersten (1974a). In the present study, we observed colleters comprising a secretory head formed of homogeneous cells, similar to the colleters described in other Gentianaceae plants (Nemomissa 1997; Renobales et al. 2001; Delgado et al. 2011; Dalvi et al. 2013, 2014, 2020; Zanotti 2018). The same pattern of cell organization was observed in Chamaecrista (Fabaceae) colleters (Coutinho et al. 2015), and the name “homogeneous colleters” was proposed based on the cellular composition, coining a novel terminology for colleter classification. These colleters usually originate exclusively from the protoderm, suggesting that the pattern or shape of cells remains the same (Paiva 2009), and they can be distinguished from the commonly reported colleters of mixed origin (protoderm and ground meristem) (Appezzato-da-Glória and Estelita 2000; Coelho et al. 2013; Mercadante-Simões and Paiva 2013).
Studies to date have indicated a predominance of homogeneous colleters in Gentianaceae species, except in Macrocarpaea obtusifolia (Griseb.) Gilg (Dalvi et al. 2014). P. montana and M. obtusifolia belong to the Helieae tribe—a problematic clade from a taxonomic and phylogenetic point of view (Molina and Struwe 2009; Struwe et al. 2009). The most recent phylogenetic study of Helieae (Calió et al. 2017) has described Prepusa as a basal group and sister to the rest of the members of the tribe but has maintained Macrocarpaea as a distinct clade. Considering the use of anatomy in such studies, the structural variation in the colleters may be a characteristic to support the phylogeny of the group. Moreover, Macrocarpaea is one of the few genera of the family with a shrub or an arboreal habit. However, the association between the type of colleters and the habit of the species has not been evaluated to date. Studies in other Gentianaceae species are ongoing, and their findings may contribute to the phylogeny of the group and highlight the taxonomic potential of this characteristic at the family level.
In addition to cellular homogeneity in the secretory portion, P. montana colleters possess evident intercellular spaces. Such spaces arise through schizogenesis as also described for the colleters of Calolisianthus speciosus (Cham. & Schltdl.) Gilg. (Zanotti 2018). Although few studies have reported the schizogenous origin of the intercellular spaces in colleters, these spaces filled with exudate are recurrent in colleters with secretory heads composed of homogeneous cells (Paiva and Machado 2006; Paiva 2009; Delgado et al. 2011; da Silva et al. 2012, 2019; Zanotti 2018). The arrangement of such cells may explain the formation of the intercellular spaces. For instance, homogeneous colleters are generally smaller than the standard, reduced, or dendroid ones, which may be the reason all cells in the head exhibit secretory activity to maximize exudate production.
Secretion composition
Protecting the developing meristems and organs is the key function of colleters (Fahn 1979; Thomas 1991). We observed the predominance of polysaccharide secretion in the colleters of P. montana, although proteins were also detected at the early stages of leaf development. The same composition has been reported for the leaf colleters of other Gentianaceae species (Delgado et al. 2011; Dalvi et al. 2013, 2014; Zanotti 2018), albeit with minor variations for calycinal colleters, which also possess phenolic compounds (Zanotti 2018).
The mucilaginous nature of colleter secretions is mainly aimed at preventing desiccation (Demarco 2008; da Silva et al. 2012; Mayer et al. 2013; Zanotii 2018). The presence of pectin also contributes to this function, as its hygroscopic properties facilitate water absorption (Célino et al. 2014). Such protection is consistent with the xeric habitat of P. montana—the campos rupestres characterized by constant winds and intense solar radiation. According to Miguel et al. (2006), the presence of proteins might be associated with the inhibition of microorganism growth.
Recent studies have suggested an association between the environmental parameters and secretion composition (Tresmondi et al. 2015, 2017; Costa et al. 2020). Plants producing hydrophilic exudates tend to predominate the forests, whereas lipophilic or mixed exudates tend to predominate the savannas. On the other hand, Santos de Faria (2019) did not find differences in the secretion composition of Casearia species collected from forests and Cerrado. Nonetheless, the predominance of polysaccharide secretion in the colleters combined with the location of colleters in the leaves of P. montana confirms the traditional functions of these structures.
Ultrastructure
The apical cells of the colleters show characteristics of secretory cells, such as dense cytoplasm; conspicuous nucleus; and abundant endoplasmic reticulum, Golgi bodies, mitochondria, and small vacuoles, indicating high metabolic activity of the cells (Klein et al. 2004; Paiva and Machado 2006; Miguel et al. 2006, 2010; Machado et al. 2012; Coelho et al. 2013; Mercadante-Simões and Paiva 2013; Tulli et al. 2013; Machado et al. 2015; Miguel et al. 2016). The endoplasmic reticulum and Golgi apparatus are typically associated with the synthesis, modification, and transport of proteins and polysaccharides (Mollenhauer and Morré 1966; Dexheimer and Guenin 1981; Dupree and Sherrier 1998; Fahn 2000; Nebenführ and Staehelin 2001). However, the endoplasmic reticulum and Golgi apparatus were also observed during senescence of P. montana colleters, and they might be involved in the enzymatic activity (Zer and Fahn 1992) and emergence of lytic compartments (Matile and Moor 1968; Marty 1978; 1999; Viotti et al. 2013; Viotti 2014). According to Viotti et al. (2013), the endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole. However, further analyses are necessary to confirm the presence of lytic compartments during the senescence of P. montana leaf colleters.
The vesicles observed in the periplasmatic spaces of P. montana colleters were similar to paramural bodies involved in the collapse of the cell wall by the transport of hydrolytic enzymes during the senescence of Iris and Dendrobium flowers (Kamdee et al. 2015), and these structures were interpreted to play similar roles in the present study. The compounds present in the vesicles of P. montana could dissolve the lamella, contributing both to the increase in intercellular spaces and the passage of exudate among peripheral cells. This hypothesis corroborates the anatomical data indicating the separation of cells and formation of large intercellular spaces as well as the results of ruthenium red staining demonstrating the dissolution of the middle lamella as colleter development progressed in P. montana.
The disorganization of the external periclinal cell wall layers indicates the involvement of the external periclinal wall in exudate release (Miguel et al. 2010; Canaveze and Machado 2015; Miguel et al. 2016; 2017). Our results indicate a possible association between the accumulation of exudate in the periplasmatic space and the loosening of the cell wall layers.
According to Miguel et al. (2017), the outer wall layers of Bathysa nicholsonii K. Schum. colleters were altered due to the development of an accumulation region in the polysaccharide layer, which collapsed upon reaching the cuticular membrane. Interestingly, the dense projections observed in the cuticular layer are described as microchannels (Coelho et al. 2013; Canaveze and Machado 2015), which likely contribute to the passage of exudate through the cuticular membrane. In fact, the absence of pores or rupture in the cuticle implies that the passage of exudate in P. montana occurs via diffusion, as already reported in Bathysa (Miguel et al. 2006; Coelho et al. 2013) and Simira (Klein et al. 2004). Furthermore, turgor facilitates the passage of exudate from the central vacuole, which expels the exudate stored in the periplasmatic space (Coelho et al. 2013) and promotes the continuous synthesis of exudate, thus maintaining the cycle (Paiva 2016).
As the secretory activity of P. montana colleters is rapid and cell death is imminent, intercellular spaces increase in size, and consequently, there is not enough turgor to expel all exudate, which is thus partially retained. The persistence of peripheral secretory cells may reflect the maximization of the secretory activity. Finally, the permanence of dying cells and the cell wall lignification observed in the peduncle cells of the colleters protect the plant from pathogens by preventing their entry (Dalvi et al. 2014).
The senescence of P. montana leaf colleters was characterized by the presence of small vacuoles with unidentified materials inside these organelles, which might be a result of cytoplasm portions that protrude into the vacuoles. The occurrence of fibrillar material inside the vacuolar membrane may be detrimental to cell degradation and indicates autophagy (van Doorn and Woltering 2005; van Doorn and Papini 2013). Autophagy, including programmed cell death, can occur at several stages of plant development (Khan and Hemalatha 2015). Here, we observed an overlap between the secretory and the senescent phases, with the latter appearing early. This overlap between the secretory and the senescent phases corroborates the reports of Coelho et al. (2013) and Miguel et al. (2016) that it is incoherent to delimitate the phases, as colleters show secretory activity throughout their useful life and this activity ceases only with cell collapse via programmed cell death (Coelho et al. 2013; Tulli et al. 2013; Miguel et al. 2010, 2016). Nonetheless, autophagic processes in colleters are not comprehensively described in the literature, and further research is warranted to characterize the mechanisms involved in the programmed cell death of these structures.
Based on these ultrastructural observations, we conclude that P. montana colleters have a short shelf life, as evidenced by the early secretory activity as well as recurrent and premature characteristics of senescence. The structure of P. montana colleters can be a useful attribute for the taxonomy of Helieae and future studies on the evolution of colleters within Gentianaceae. We also implicate the role of the cell wall in the formation of intercellular spaces as well as the accumulation and release of exudate. Finally, we suggest that programmed cell death is involved in the differentiation and senescence of P. montana colleters. Taken together, these findings advance our understanding of the development and morphofunctionality of secretory structures in plants.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Almeida AL, Paiva EAS (2019) Colleters in Mabea fistulifera Mart. (Euphorbiaceae): anatomy and biology of the secretory process. Flora 258:1–9. https://doi.org/10.1016/j.flora.2019.151439
Appezzato-da-Glória B, Estelita MEM (2000) Development, structure and distribution of colleters in Mandevilla illustris and M. velutina (Apocynaceae). Brazil J Bot 23(2):113–120
Ballego-Campos I, Paiva EAS (2018) Colleters in the vegetative axis of Aechmea blanchetiana (Bromeliaceae): anatomical, ultrastructural and functional aspects. Austral J Bot 66(5):379–387. https://doi.org/10.1071/BT18095
Calió MF, Lepis KB, Pirani JR, Struwe L (2017) Phylogeny of Helieae (Gentianaceae): resolving taxonomic chaos in a Neotropical clade. Molec Phylogen Evol 106:192–208. https://doi.org/10.1016/j.ympev.2016.09.013
Calió MF, Pirani JR, Struwe L (2008) Morphology-based phylogeny and revision of Prepusa and Senaea (Gentianaceae: Helieae) - rare endemics from eastern Brazil. Kew Bull 63(2):169–191
Calió MFA (2009) Sistemática de Helieae Gilg (Gentianaceae). Dissertation, University of the São Paulo, São Paulo, Brazil.
Canaveze Y, Machado SR (2015) Leaf colleters in Tabernaemontana catharinensis (Apocynaceae, Rauvolfioideae): structure, ontogenesis, and cellular secretion. Botany 93(5):287–296
Cardoso-Gustavson P, Campbell LM, Viveiros-Mazzoni SC, de Barros F (2014) Floral colleters in Pleurothallidinae (Epidendroideae: Orchidaceae). Amer J Bot 101(4):587–597. https://doi.org/10.3732/ajb.1400012
Cassola F, Nunes CEP, Lusa MG, Garcia VL, Mayer JLS (2019) Deep in the jelly: histochemical and functional aspects of mucilage-secreting floral colleters in the orchids Elleanthus brasiliensis and E. crinipes Front Pl Sci 10(518):1–11. https://doi.org/10.3389/fpls.2019.00518
Célino A, Fréour S, Jacquemin F, Casari P (2014) The hygroscopic behavior of plant fibers: a review. Front Chem 43:1–12
Coelho VPM, Leite JPV, Fietto LG, Ventrella MC (2013) Colleters in Bathysa cuspidata (Rubiaceae): development, ultrastructure and chemical composition of the secretion. Flora, Morphol Distrib Funct Ecol Pl 208(10–12):579–590. https://doi.org/10.1016/j.flora.2012.08.005
Costa ISC, Lucena EMP, Bonilla OH, IR Guesdon Coutinho ÍAC (2020) Seasonal variation in colleter exudates in Myrcia splendens (Myrtaceae). Austral J Bot 68:403–412. https://doi.org/10.1071/BT20020
Coutinho ÍAC, Francino DMT, Meira RMSA (2015) New records of colleters in Chamaecrista (Leguminosae, Caesalpinioideae s.l.): structural diversity, secretion, functional role, and taxonomic importance. Int J Pl Sci 176(1):72–85. https://doi.org/10.1086/679016
da Silva CJ, Barbosa LCA, Marques AE, Baracat-Pereira MC, Pinheiro AL, Meira RMSA (2012) Anatomical characterisation of the foliar colleters in Myrtoideae (Myrtaceae). Austral J Bot 60:707–717. https://doi.org/10.1071/bt12149
da Silva CJ, Ribeiro JPO, Meira RMSA (2019) New registers of colleters in species of Myrtaceae from Brazilian Cerrado. Rodriguésia 70:1–9. https://doi.org/10.1590/2175-7860201970055
Dalvi VC, Cardinelli LS, Meira RMSA, Azevedo AA (2014) Foliar colleters in Macrocarpaea obtusifolia (Gentianaceae): anatomy, ontogeny, and secretion. Botany 92(1):59–67. https://doi.org/10.1139/cjb-2013-0203
Dalvi VC, de Faria GS, Azevedo AA (2020) Calycinal secretory structures in Calolisianthus pedunculatus (Cham. & Schltdl) Gilg (Gentianaceae): anatomy, histochemistry, and functional aspects. Protoplasma 257:275–284. https://doi.org/10.1007/s00709-019-01436-5
Dalvi VC, Meira RMSA, Francino DMT, Silva LC, Azevedo AA (2013) Anatomical characteristics as taxonomic tools for the species of Curtia and Hockinia (Saccifolieae-GentianaceaeJuss.). Pl Sys Evol 300(1):99–112. https://doi.org/10.1007/s00606-013-0863-1
Delgado MN, Azevedo AA, Silva LC, Valente GE, Kasuya MCM (2011) Comparative anatomy of Calolisianthus species (Gentianaceae – Helieae) from brazil: taxonomic aspects. Edinburgh J Bot 68(1):139–155. https://doi.org/10.1017/s0960428610000284
Demarco D (2008) Glândulas de órgãos vegetativos aéreos e florais de espécies de Asclepiadeae (R. Br) Duby (Asclepiadoideae, Apocynaceae) de Mata Atlântica do estado de São Paulo. Ph.D. Thesis, University of Campinas, Campinas, Brazil.
Dexheimer J, Guenin F (1981) Étude de la sécrétion de mucilage par le trichomes stipulaires de Psychotria bacteriophyla (Rubiaceae). Cytologia 46:731–747
Dupree P, Sherrier DJ (1998) The plant Golgi apparatus. Biochim Biophys Acta 1404:259–270
Evert RF (2006) Esau’s Plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development, 3rd edn. John Wiley & Sons Inc, Hoboken
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Fahn A (2000) Structure and function of secretory cells. Advances Bot Res 31:37–75. https://doi.org/10.1016/s0065-2296(00)31006-0
Fernandes VF, Thadeo M, Dalvi VC, Marquete R, Meira RMSA (2016) Colleters in Casearia (Salicaceae): a new interpretation for the theoid teeth. Bot J Linn Soc 181(4):682–691. https://doi.org/10.1111/boj.12432
Gabe M (1968) Techniques histologiques. Masson and Cie, Paris
Guimarães EF, Dalvi VC, Azevedo AA (2013) Morphoanatomy of Schultesia pachyphylla (Gentianaceae): a discordant pattern in the genus. Botany 91(12):830–839. https://doi.org/10.1139/cjb-2013-0077
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2009) Sistemática Vegetal um enfoque filogenético, 3rd edn. Porto Alegre, Artmed
Kamdee C, Kirasak K, Ketsa S, van Doorn WG (2015) Vesicles between plasma membrane and cell wall prior to visible senescence of Iris and Dendrobium flowers. J Pl Physiol 188:37–43. https://doi.org/10.1016/j.jplph.2015.02.013
Karnovsky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Khan MS, Hemalatha S (2015) Autophagy: molecular insight and role in plant programmed cell death and defense mechanism. Int Res J Biological Sci 4(2):78–83
Klein DE, Gomes VM, Silva-Neto SJ, Cunha M (2004) The structure of colleters in several species of Simira (Rubiaceae). Ann Bot 94(5):733–740
Leitão CAE, Cortelazzo AL (2008) Structural and histochemical characterisation of the colleters of Rodriguezia venusta (Orchidaceae). Austral J Bot 56:161–165
Lersten NR (1974a) Colleter morphology in Pavetta, Neorosea and Tricalysia (Rubiaceae) and its relationship to the bacterial leaf nodule symbiosis. Bot J Linn Soc 69(2):125–136. https://doi.org/10.1111/j.1095-8339.1974.tb01620.x
Lersten NR (1974b) Morphology and distribution of colleters and crystals in relation to the taxonomy and bacterial leaf nodule symbiosis of Psychotria (Rubiaceae). Amer J Bot 61(9):973–981. https://doi.org/10.1002/j.1537-2197.1974.tb14037.x
Lersten NR (1975) Colleter types in Rubiaceae, especially in relation to the bacterial leaf nodule symbiosis. Bot J Linn Soc 71(4):311–319. https://doi.org/10.1111/j.1095-8339.1975.tb01207.x
Lillie RD (1965) Histopathologic technic and practical histochemistry. McGraw Hill, New York
Machado SR, Barreiro DP, Rocha JF, Rodrigues TM (2012) Dendroid colleters on vegetative and reproductive apices in Alibertia sessilis (Rubiaceae) differ in ultrastructure and secretion. Flora, Morphol Distrib Funct Ecol Pl 207(12):868–877. https://doi.org/10.1016/j.flora.2012.09.013
Machado SR, Paleari LM, Paiva ÉAS, Rodrigues TM (2015) Colleters on the inflorescence axis of Croton glandulosus (Euphorbiaceae): structural and functional characterization. Int J Pl Sci 176(1):86–93. https://doi.org/10.1086/678469
Martius CFP von. (1826) 1827. Nova genera et species plantarum quas in itinere per Brasiliam annis 1817–1820. Vol. 2. Wolf, München 148 pp.
Marty F (1978) Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cells of Euphorbia. Proc Natl Acad Sci 75(2):852–856. https://doi.org/10.1073/pnas.75.2.852
Marty F (1999) Plant vacuoles. Pl. Cell 11(4):587–599. https://doi.org/10.1105/tpc.11.4.587
Matile P, Moor H (1968) Vacuolation: origin and development of the lysosomal apparatus in root-tip cells. Planta (Berl. 80: 159–175. https://doi.org/https://doi.org/10.1007/bf00385592.
Mayer JLS, Cardoso-Gustavson P, Appezzato-da-Glória B (2011) Colleters in monocots: new record for Orchidaceae. Flora, Morphol Distrib Funct Ecol Pl 206:185–190. https://doi.org/10.1016/j.flora.2010.09.003
Mayer JLS, Carmello-Guerreiro SM, Mazzarefa P (2013) A functional role for the colleters of coffee flowers. AoB Plants 5:1–13. https://doi.org/10.1093/aobpla/plt029
McManus JFA (1948) Histological and histochemical uses of periodic acid. Stain Technol 23:99–108
Mercadante-Simões MO, Paiva EAS (2013) Leaf colleters in Tontelea micrantha (Celastraceae, Salacioideae): Ecological, morphological and structural aspects. C R Biol 336:400–406. https://doi.org/10.1016/j.crvi.2013.06.007
Miguel EC, da Cunha M, Miguel TBAR, Barros CF (2016) Ontogenesis secretion and senescence of Tocoyena bullata (Vell.) Mart. (Rubiacaeae) colleters. Pl Biol 18(5):851–858. https://doi.org/10.1111/plb.12473
Miguel EC, Gomes VM, de Oliveira MA, da Cunha M (2006) Colleters in Bathysa nicholsonii K. Schum. (Rubiaceae): ultrastructure, secretion protein composition, and antifungal activity. Pl Biol 8:15–722
Miguel EC, Klein DE, de Oliveira MA, da Cunha M (2010) Ultrastructure of secretory and senescence phase in colleters of Bathysa gymnocarpa and B. stipulata (Rubiaceae). Brazil J Bot 33(3):425–436. https://doi.org/10.1590/s0100-84042010000300006
Miguel EC, Pireda S, Barros CF, Zottich U, Gomes VM, Miguens FC, da Cunha M (2017) Outer cell wall structure and the secretion mechanism of colleters of Bathysa nicholsonii K. Schum. (Rubiaceae). Acta Bot Brasil 31(3):411–419
Miller IM, Scott A, Gardner IC (1983) The development, structure and function of dendroid colleters in Psychotria kirkii Hiern (Rubiaceae). Annals Bot 51(5):621–630. https://doi.org/10.1093/oxfordjournals.aob.a086509
Molina J, Struwe L (2009) Utility of secondary structure in phylogenetic reconstructions using nrDNA ITS sequences—an example from Potalieae (Gentianaceae: Asteridae). Syst Bot 34:414–428
Mollenhauer HH, Morre DJ (1966) Golgi apparatus and plant secretion. Annual RPl Physiol 17(1):27–46. https://doi.org/10.1146/annurev.pp.17.060166.000331
Nebenführ A, Staehelin LA (2001) Mobile factories: Golgi dynamics in plant cells. Trends Plant Sci 6(4):160–167. https://doi.org/10.1016/s1360-1385(01)01891-x
Nemomissa S (1997) Floral character states of the Northeast and Tropical East African Swertia species (Gentianaceae). Nordic J Bot 17(2):145–156. https://doi.org/10.1111/j.1756-1051.1997.tb00301.x
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
O’Brien TP, Mccully ME (1981) The study of plant structure principles and selected methods. Termarcarphi Ptey. Ltd., Melbourne
Oliveira CS, Salino A, Paiva EAS (2017) Colleters in Thelypteridaceae: unveiling mucilage secretion and its probable role in ferns. Flora 228:65–70. https://doi.org/10.1016/j.flora.2017.01.009
Paiva EAS (2009) Occurrence, structure and functional aspects of the colleters of Copaifera langsdorffii Desf. (Fabaceae, Caesalpinioideae). C R Bio 332(12):1078–1084. https://doi.org/10.1016/j.crvi.2009.08.003
Paiva EAS (2016) How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Annals Bot 117(4):533–540. https://doi.org/10.1093/aob/mcw012
Paiva EAS, Machado SR (2006) Ontogenesis, structure and ultrastructure of Hymenaea stigonocarpa (Fabaceae: Caesalpinioideae) colleters. Revista Biol Trop 54(3):943–950
Pearse AGE (1985) Histochemistry theoretical and applied: preparative and optical technology. Churchill Livingston, Edinburgh
Pinheiro SKP, Teófilo FBS, Lima AKM, Cordoba BV, Migeul TBAR, Miguel EC (2019) Ontogegenesis and secretion mecanismo of Morinda citrifolia L. (Rubiaceae) colleters. S African J Bot 21:26–33
Renobales G, Diego E, Urcelay B, López-Quintana A (2001) Secretory hairs in Gentiana and allied genera (Gentianaceae, subtribe Gentianinae) from the Iberian Peninsula. Bot J Linn Soci 136(1):119–129. https://doi.org/10.1111/j.1095-8339.2001.tb00560.x
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1):208–212
Ribeiro JC, Ferreira MJP, Demarco D (2017) Colleters in Asclepiadoideae (Apocynaceae): protection of meristems against desiccation and new functions assigned. Int J Pl Scis 178(6):465–477. https://doi.org/10.1086/692295
Rios ABM, Menino GCdeO, Dalvi VC, (2020) Leaf teeth in eudicots: what can anatomy elucidate? Bot J Lin Soc 193(4):504–522. https://doi.org/10.1093/botlinnean/boaa028
Robbrecht E (1983) The African genus Tricalysia A. Rich. (Rubiaceae) 3. Probletostemon revived as a section of subgenus Tricalysia. Bulletin van Natomale Plantentuin van Belgie 53:299–320
Robbrecht E (1987) The African genus Tricalysia A. Rich. (Rubiaceae) 4. A revision of the species of section Tricalysia. Bulletin van Natomale Plantentuin van Belgie 57:39–208
Roshchina VV, Roshchina VD (1993) The excretory function of higher plants. Springer-Verlang, Berlin
Sacher JA (1954) Structure and seasonal activity of the shoot apices of Pinus lambertiana and Pinus ponderosa. Amer J Bot 41(9):749–759. https://doi.org/10.2307/2438961
Sacher JA (1955) Cataphyll ontogeny in Pinus lambertiana. Amer J Bot 42(1):82–91. https://doi.org/10.2307/2438596
Santos de Faria DN, Fernandes VF, Marquete R, Meira RMSA (2019) Morphology, anatomy, and exudates of stipularcolleters in Casearia Jacq (Salicaceae) across two tropical plant communities. Int J Pl Sci 180:141–152. https://doi.org/10.1086/700637
Sheue C-R, Chesson P, Chen Y-J, Wu S-Y, Wu Y-H, Yong JWH, Guu T-Y et al (2013) Comparative systematic study of colleters and stipules of Rhizophoraceae with implications for adaptation to challenging environments. Bot J Linn Soc 172(4):449–464. https://doi.org/10.1111/boj.12058
Simões AO, Castro MM, Kinoshita LS (2006) Calycine colleters of seven species of Apocynaceae (Apocynoideae) from Brazil. Bot J Linn Soc 152:387–398
Solereder H (1908) Systematic anatomy of the dicotyledons, vol 1. Clarendon Press, Oxford
Struwe L, Albert VA (2002) Gentianaceae: systematic and natural history. University Press, Cambridge
Struwe L, Albert VA, Calió FM, Frasier C, Lepis KB, Mathews KG, Grant JR (2009) Evolutionary patterns in neotropical Helieae (Gentianaceae): evidence from morphology, chloroplast and nuclear DNA sequences. Taxon 58(2):479–499. https://doi.org/10.1002/tax.582013
Thomas V (1991) Review article. Structural, functional and phylogenetic aspects of the colleter. Annals Bot 68(4): 287–305. https://doi.org/https://doi.org/10.2307/42758461.
Thomas V, Dave Y (1989) Histochemistry and senescence of colleters of Allamanda cathartica (Apocynaceae). Annals Bot 64(2):201–203
Tresmondi F, Nogueira A, Guimarães E, Machado SR (2015) Morphology, secretion composition, and ecological aspects of stipular colleters in Rubiaceae species from tropical forest and savanna. Sci Nat 102(11–12):1–15. https://doi.org/10.1007/s00114-015-1320-5
Tresmondi F, Canaveze Y, Guimarães E, Machado SR (2017) Colleters in Rubiaceae from forest and savanna: the link between secretion and environment. Sci Nat 104(3–4):1–12. https://doi.org/10.1007/s00114-017-1444-x
Tullii CF, Miguel EC, Lima NB, Fernandes KVS, Gomes VM, da Cunha M (2013) Characterization of stipular colleters of Alseis pickelii. Botany 91(6):403–413. https://doi.org/10.1139/cjb-2012-0249
van Doorn WG, Papini A (2013) Ultrastructure of autophagy in plant cells - a review. Autophagy 9(12):1922–1936. https://doi.org/10.4161/auto.26275
van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Pl Sci 10(3):117–122. https://doi.org/10.1016/j.tplants.2005.01.006
Viotti C (2014) ER and vacuoles: never been closer Front. Plant Sci 5(1):7. https://doi.org/10.3389/fpls.2014.00020
Viotti C, Kruger F, Krebs M, Neubert C, Fink F, Lupanga U, Scheuring D et al (2013) The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Pl Cell 25(9):3434–3449. https://doi.org/10.1105/tpc.113.114827
Zanotti A (2018) Estruturas secretoras em Calolisianthus speciosus (Cham. & Schltdl.) Gilg. (Gentianaceae): ontogenia e biologia da secreção. Dissertation, Federal University of Viçosa, Viçosa, Brazil.
Zer H, Fahn A (1992) Floral nectaries of Rosmarinus officinalis L. structure, ultrastructure and nectar secretion. Ann Bot 70:391–397
Acknowledgements
We are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting a Scientific Initiation scholarship to Jailma Rodrigues Gonçalves. We thank the Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico (MCT/CNPq; Brasília, Brazil; Grant 406824/2016-9) and the Instituto Federal de Educação, Ciência e Tecnologia Goiano (IF Goiano, campus Rio Verde) for the financial support, the Laboratório Multiusuário de Microscopia de Alta Resolução (LabMic) of the Universidade Federal de Goiás (UFG) and Núcleo de Microscopia e Microanálise of the Universidade Federal de Viçosa (UFV) for the preparation and analysis of electron microscopy samples, and the Instituto Chico Mendes de Conservação da Biodiversidade/Sistema de Autorização e Informação em Biodiversidade (ICMBio/SISBIO) for collection license.
Funding
This study was supported by the Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brasília, Brazil; Grant 406824/2016–9 to Valdnéa Casagrande Dalvi).
Author information
Authors and Affiliations
Contributions
The research project was designed by Valdnéa Casagrande Dalvi. The samples were collected by Valdnéa Casagrande Dalvi; light microscopy and histochemical analyses were performed by Jailma Rodrigues Gonçalves and Luana Silva dos Santos; scanning and transmission microscopy were performed by Valdnéa Casagrande Dalvi, Jailma Rodrigues Gonçalves, and Diego Ismael Rocha. The manuscript was written by Valdnéa Casagrande Dalvi, Jailma Rodrigues Gonçalves, and Diego Ismael Rocha.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Dorota Kwiatkowska.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonçalves, J.R., Rocha, D.I., dos Santos, L.S. et al. The short but useful life of Prepusa montana Mart. (Gentianaceae Juss.) leaf colleters—anatomical, micromorphological, and ultrastructural aspects. Protoplasma 259, 187–201 (2022). https://doi.org/10.1007/s00709-021-01651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01651-z