Abstract
Copper (Cu) interferes with numerous biological functions in plants, including plant growth, which is partly governed by plant hormones. In the present study, Cu stress effect on the roots of pre-emerging maize seedlings in terms of growth, nutrient composition, protein modifications, and root hormone homeostasis was investigated, focusing on possible metabolic differences between the root apex and the rest of the root tissues. Significant decreases in root length and root biomass after 72 h of Cu exposure (50 and 100 μM CuCl2), accompanied by reductions in Ca, Mg, and P root contents, were found. Cu also generated cell redox imbalance in both root tissues and revealed by altered enzymatic and non-enzymatic antioxidant defenses. Oxidative stress was evidenced by an increased protein carbonylation level in both tissues. Copper also induced protein ubiquitylation and SUMOylation and affected 20S proteasome peptidase activities in both tissues. Drastic reductions in ABA, IAA, JA (both free and conjugated), GA3, and GA4 levels in the root apex were detected under Cu stress. Our results show that Cu exposure generated oxidative damage and altered root hormonal homeostasis, mainly at the root apex, leading to a strong root growth inhibition. Severe protein post-translational modifications upon Cu exposure occurred in both tissues, suggesting that even when hormonal adjustments to cope with Cu stress occurred mainly at the root apex, the entire root is compromised in the protein turnover that seems to be necessary to trigger and/or to sustain defense mechanisms against Cu toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is an essential element for normal plant growth and development; however, excess of Cu interferes with numerous biological functions in plants (Burkhead et al. 2009). Like other metals, Cu may be released to the environment by human activities and accumulated in the soil, from where it can be transferred to growing plants. According to several publications, Cu is gradually accumulating in agricultural soils due to different sustained practices, mainly to the extended use of copper as a fungicide for the treatment of mildew and other fungal diseases (Vavoulidou et al. 2005), as well as the long-term use of irrigation water containing Cu (Shiyab 2018).
Metallic ions can be absorbed through the entire root by means of different metal transporters; however, the lack of a Caspary band at the root tip allows direct xylem load of metallic ions in this portion (Piñeros et al. 1998). Therefore, the root tip is considered the major site for the perception of metal ions.
Copper is a redox-active metal that can lead to the production of reactive oxygen species (ROS) through the Fenton and Haber-Weiss reactions (Ninh Pham et al. 2013). To counteract ROS excess, the plant cell has developed an efficient antioxidant defense system known as the Foyer-Halliwell-Asada pathway; this system includes a set of enzymes and antioxidant compounds (Foyer and Noctor 2011). Nonetheless, if the cellular antioxidant capacity is exceeded, ROS accumulation results in the oxidative damage of macromolecules such as lipids and proteins, which typically undergo peroxidation and carbonylation, respectively. Oxidative-damaged proteins, in turn, can compromise several cellular functions; therefore, they have to be rapidly degraded, mainly by the 20S proteasome, which is specifically responsible for carbonylated protein degradation (Pena et al. 2007; Polge et al. 2009).
Plants control their root growth through the interaction of the redox signaling hub with hormonal plant growth regulators (Bartoli et al. 2013; De Tullio et al. 2010). Phytohormones are involved in the regulation of plant growth and development, and they are also essential for adaptive responses to abiotic stress (Santner and Estelle 2009). Besides, a complex net of interactions exists between plant hormones and protein metabolism. For example, the ubiquitylation of jasmonates’ repressors was found to be necessary to derepress the transcription of several JA-responsive genes (Wasternack and Song 2017).
Under adverse environmental conditions, the ability to redirect resources from growth to enhance defenses is a crucial step to cope with the stress factor. Some authors reported that the exogenous application of hormones reduced the toxic effects of metals, partly due to an improvement in the cell antioxidant potential (Ben Massoud et al. 2018; Singh et al. 2016). However, little is known about the adjustments in the endogenous hormone levels during metal stress and its impact on the early root growth.
Maize (Zea mays L.) is one of the most important crops in the world, and it is cultivated in several agricultural areas under copper pollution risk (Antoniadis et al. 2019; Gu et al. 2019; Tóth et al. 2016). It has been reported that maize suppressed stem growth under copper excess and plants had a stunted appearance (Kumar et al. 2008); also, yield reductions were reported (Farahat et al. 2017). At the cellular level, copper excess resulted in decreased photosynthetic pigment contents and induced oxidative damage (AbdElgawad et al. 2020; Liu et al. 2018; Madejón et al. 2009; Sharma and Uttam 2018).
Previous data indicate that maize seedlings tend to accumulate the excess of metallic ions in their roots (Florijn and Van Beusichem 1993; Nannoni et al. 2016; Vatehová et al. 2016). To get a deeper insight into the mechanisms involved in plant early responses to copper excess, an integrative metabolic study focused on redox balance, hormonal homeostasis, and protein modifications was performed in early maize root tissues subjected to Cu stress.
Materials and methods
Plant material and growth conditions
Our study focused on the effects of Cu excess on maize before the onset of the phototrophic lifestyle during the imbibition–germination–pre-emergence stages. According to previous literature, the emergence of maize coleoptile from the soil surface takes place at 5 to 7 days after planting under favorable natural conditions (Abendroth et al. 2011). To mimic such conditions, seeds of maize (Zea mays L., cv 2741MGRR2, Don Mario Semillas, Buenos Aires, Argentina) were first submitted to a 72-h imbibition period. Uniformly germinated seeds with roots of 1–2 cm length were subsequently transferred to a hydroponic system containing 250 mL of diluted (1/4) Hoagland’s nutrient solution (Hoagland and Arnon 1950) without (control, C) or with 50 and 100 μM CuCl2 (30 seedlings per pot) and kept in the darkness in a controlled climate room (24 ± 2 °C). After 72 h of treatment, root length, fresh weight (FW), and dry weight (DW) were measured. Dry weight was determined after drying the roots at 80 °C until constant weight. For tissue analyses including elemental composition, roots were gently washed with distilled water before processing. Determinations were performed in parallel using apical root segments (first 5 mm from the tip) (Ap) and all the remaining root tissue (Rt).
Root elemental analysis
For Cu, Ca, Fe, K, Mg, Mn, P, S, and Zn determination, dried root samples were submitted to the Spectrometry Core Facility INQUISAL, Universidad Nacional de San Luis (UNSL-CONICET). Briefly, samples (50 mg) were disrupted in 1 mL of 65% (v/v) HNO3 during 30 s in an ultrasonic bath. Then, 0.5 mL of H2O2 was added, and the reaction was incubated for 1 h at 60 °C in a thermostatic bath. After dilutions with ultrapure water, inductively coupled plasma mass spectrometry (ICP-MS) (Perkin Elmer Elan DRC) was used to estimate nutrient accumulation.
Peroxidase activities determination
Protein extracts for determination of catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX) activities were prepared from 0.1 g of fresh tissue homogenized in 1 mL of 50 mM phosphate buffer (pH 7.4) containing 1 mM EDTA and 0.5% (v/v) Triton X-100, at 4 °C. The homogenates obtained were centrifuged at 13,000×g for 30 min at 4 °C, and the supernatant fractions were used for the assays. CAT activity was determined by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2) and 2 mM H2O2. The pseudo-first-order reaction constant (k’ = k × [CAT]) of the decrease in H2O2 absorption was determined, and the catalase content in pmol mg−1 protein was calculated using k = 4.7 × 10−7 M−1 s−1 (Aebi 1984). APX activity was measured as described by Nakano and Asada (1981), using a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM H2O2, 0.5 mM ascorbate, and 0.1 mM EDTA. The hydrogen peroxide‑dependent oxidation of ascorbate was followed by the decrease in the absorbance at 290 nm (ε = 2.8 mM−1 cm−1). GPX activity was determined by measuring the increase in absorption at 470 nm due to the formation of tetraguaiacol (ε = 26.6 mM−1 cm−1) in a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 10 mM guaiacol, and 10 mM H2O2. Total protein concentration was determined (Bradford 1976), using bovine serum albumin as a standard.
Analytical determinations
Root tissue (0.1 g) was homogenized in 1 mL of 0.1 N HCl and centrifuged at 13,000×g for 30 min (4 °C); the supernatants were used to quantify reduced glutathione (GSH), ascorbate (ASC), dehydroascorbate (DHAs), and proline.
To determine GSH, 20-μL samples were derivatized with 180 μL of 1.3 mM o-phthaldialdehyde (OPA) in borate buffer (0.4 M, pH 9.7) at room temperature (Robyt and White 1990), and the fluorescence measured at 455 nm (λ excitation, 340 nm). A standard curve of GSH was prepared and measured.
Ascorbate (ASC) and dehydroascorbate (DHAs) were determined as described by Law et al. (1983), using a standard curve of ASC for calibration.
Proline content was determined according to Bates et al. (1973). Following acid extraction, 100-μL samples were incubated with 100 μL of acidic ninhydrin reagent (2.5% (w/v) in 60% (v/v) glacial acetic acid and 40% (v/v) phosphoric acid 6 M at 100 °C for 60 min, and the absorbance was measured at 520 nm. A standard curve was prepared using commercial proline.
Total carbohydrates were quantified in 0.1-g samples homogenized in 1 mL of distilled water. The phenol-sulfuric acid method was used (Robyt and White 1990). Glucose was used to prepare the standard curve.
Ammonia content was determined by the reaction with phenol and hypochlorite in an alkaline medium. Reagents A and B from a commercial uremia kit (Wiener Lab) were used for the assay. A standard curve with NH4Cl was prepared.
Quantitative dot blot analysis
Protein extracts were prepared by homogenizing 0.1 g of root tissue in 0.5 mL of the loading buffer, composed of 60 mM Tris-ClH (pH 6.8) to which 5% (v/v) of β-mercaptoethanol was added. Determinations were performed in the supernatants after centrifugation at 26,000×g for 15 min at 4 °C.
Quantification of oxidized proteins was performed by dot blot after its derivatization with 2,4-dinitrophenylhydrazine (2,4-DNPH) (Wehr and Levine 2012). Equal amounts of total protein (7.5 μg) from each sample were blotted onto a polyvinylidene fluoride (PVDF) membrane by vacuum filtration using a hybri.dot manifold (Life Technologies, Inc.). Then, the membrane was blocked in 5% (w/v) non-fat dried milk in PBS for 1 h. After blocking, the membrane was washed three times with PBS and incubated at room temperature for 1 h with anti-DNP (Sigma, St Louis) as the primary antibody. Dots were subsequently visualized using a secondary rabbit antibody conjugated with horseradish peroxidase (HRP) and stained using 3,3′-diaminobenzidine (DAB) as substrate. Ponceau S staining was used as a loading control. Membranes were photographed, and then images were analyzed with Gel-Pro Analyzer software (Media Cybernetics, L.P). The amount of oxidized proteins was expressed as arbitrary units (assuming control value equal to 100 units), based on the absolute integrated optical density of each dot. Global changes in Ub- and SUMO1-conjugated proteins were also immunochemically detected using dot blot (Todgham et al. 2007; Častorálová et al. 2012). For this purpose, membranes were soaked in the primary antibodies anti Ub (Sigma, St. Luis) and anti AtSUMO1 (Agrisera, Sweden) and visualized as described above.
Proteasome activities determination
The 20S core complex of the 26S proteasome contains three different peptidase activities: chymotrypsin-like, trypsin-like, and caspase-like (peptidylglutamyl-peptide hydrolyzing, PGPH) peptidases (Ingvardsen and Veierskov 2001; Orlowski and Wilk 2000). Tissue (0.1 g) was homogenized in 135 mM Tris-acetate buffer (0.5 mL, pH 7.5) containing 12.5 mM KCl, 80 lM EGTA, 6.25 mM 2-mercaptoethanol, and 0.17% (w/v) octyl-b-d-glucopyranoside. Extracts were centrifuged, and the supernatants used to determine chymotrypsin-like, trypsin-like, and peptidylglutamylpeptide hydrolase (PGPH) proteasome peptidase activities. The cleavage of three different fluorogenic peptide substrates linked to the fluorescence reporters Ala-Ala-Phe-7-amido-4-methyl coumarin (AAF-AMC), Boc-Leu-Ser-Thr-Arg-7-amido-4-methyl coumarin (Boc-LSYR-AMC), and Clz-Leu-Leu-Glu-β-naphthylamide (Clz-LLE-βNA) was monitored in the absence or presence of the proteasome inhibitor carbobenzoxy-Leu-Leu-leucinal (MG132). Results were expressed as the differences between both measurements (Pena et al. 2007). Enzymatic activities were normalized for protein concentrations previously estimated as described by Lowry et al. (1951) and expressed as percentages of activity present in control extracts.
Endogenous phytohormone contents
Hormone extraction and analysis were carried out as described in Durgbanshi et al. (2005), with few modifications. In brief, for gibberellins (GAs), abscisic acid (ABA), jasmonic acid (JA), JA-isoleucine conjugate (JA-Ile), indole-3-acetic acid (IAA), and salicylic acid (SA) extraction, 0.1 g of ground frozen root tissue was extracted in 2 mL of ultrapure water, after spiking with 25 μL of a solution containing 1 mg L−1 of [2H]-GA1, [2H]-GA7, [2H6]-ABA, DHJA, and [13C6]-SA and 0.1 mg L−1 of [2H2]-IAA in a ball mill (MillMix20, Domel, Železniki, Slovenija). After centrifugation at 4700×g for 10 min (4 °C), the supernatants were recovered, and the pH was adjusted to 3 with 30% acetic acid. All extracts were partitioned twice against 2 mL of diethyl ether, and then the organic layer was recovered and evaporated under vacuum in a centrifuge concentrator (Speed Vac, Jouan, Saint Herblain Cedex, France). Once dried, the residue was resuspended in 500 μL of a 10:90 methanol:water solution by gentle sonication. The resulting solution was filtered through 0.22-μm polytetrafluoroethylene membrane syringe filters (Albet S.A., Barcelona, Spain) and directly injected into an ultra-performance liquid chromatography system (Acquity SDS, Waters Corp., Milford, MA, USA, or Waters Alliance 2695, Waters Corp.). Chromatographic separations were carried out on a reversed-phase C18 column (Gravity, 50 × 2.1 mm 1.8-μm particle size, Macherey-Nagel GmbH, Germany) using a methanol:water (both supplemented with 0.1% acetic acid) gradient at a flow rate of 300 μL min−1. Compounds were quantified with a triple quadrupole mass spectrometer (Micromass, Manchester, UK) connected online to the output of the column through an orthogonal Z-spray electrospray ion source. The spectrometer was operated in negative ionization electrospray mode, and plant hormones were detected according to their specific transitions using a multi-residue mass spectrometric method. Metabolites were monitored at m/z: SA2 137 > 93, 13C6-SA 143 > 99, IAA 174 > 130, IAA-d2 176 > 132, JA 209 > 59, DHJA 211 > 59, ABA-d6 269 > 159, ABA 263 > 153, JA-Ile 322 > 130, GA1-d2 349 > 231, GA3 345 > 143, GA4 331 > 213, GA7-d2 331 > 225, GA7 329 > 223, GA20 331 > 287. All data were acquired and processed using MassLynx v4.1 software. Relative quantification was achieved by comparing the areas of the different samples.
Statistical analysis
Each container had 30 seeds from which 0.1 g of tissue was collected and considered as a biological replicate. Tables and figures show means ± SEM of three or five independent experiments, with three biological replicates per treatment. Differences among treatments were analyzed by 1-way ANOVA, taking P < 0.05 as significant, followed by Tukey’s multiple comparisons test.
Results
Copper reduced root maize growth and affected root nutritional status
After 72 h of Cu treatment, the root elongation of maize seedlings was significantly reduced (Table 1 and Supplemental Figure 1). Cu addition in the nutrient solution also reduced maize root biomass compared with control plants (Table 1), with no significant differences between the metal concentrations tested.
As expected, Cu addition resulted in enhanced internal copper concentrations (Table 2). Additionally, a significant decrease in Mg content was detected in the roots of Cu-treated seedlings. Also, Ca and P contents were significantly diminished at the highest Cu dose tested.
Copper alters redox cell balance and generates oxidative stress
Cu effects on redox balance during the early stage of the root growth were evaluated in terms of peroxidase activities (CAT, APX, and GPX), ASC and DHAs contents, and their ratio, as well as GSH, carbohydrates, and proline contents.
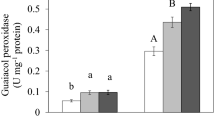
Catalase activity was mainly localized in the root apex (Ap), and it was drastically reduced by copper treatment in both regions of the root (Fig. 1A). On the contrary, GPX specific activity was mainly localized in the remaining root tissue (Rt) and was significantly induced by the metal in both parts (Fig. 1B). APX had similar specific activity in Ap and Rt and was significantly induced only by the higher metal concentration (Fig. 1C).
A–C Effect of copper on antioxidant enzyme-specific activities. Maize seedlings were subjected to hydroponic culture without (control, C) or containing 50 and 100 μM of CuCl2 for 72 h. Determinations were performed on extracts obtained from the root apex (solid line, Ap) and the remaining root tissue (dotted line, Rt). Values represent means ± SEM of five independent experiments, with three biological replicates per treatment. Different lowercase letters indicate significant differences for the same tissue (P < 0.05), according to Tukey’s multiple range test
A different distribution pattern for ASC and DHAs was detected in control roots, with an ASC/DHAs ratio < 1 in Ap and > 1 in Rt. Cu applied at the highest dose significantly incremented DHAs levels in both parts of the root, resulting in drastically reduced ratios (Table 3).
Both Cu doses significantly incremented GSH content in Rt, and the highest dose of the copper treatment reduced GSH in Ap (Fig. 2A). Carbohydrate and proline contents significantly decreased under metal treatment, being these reductions more pronounced in Ap than in Rt (Fig. 2B, C). NH4+ content was decreased only under 100 μM Cu in Ap (Fig. 2D).
Effect of copper on GSH (A), carbohydrates (B), proline (C), and ammonium content (D). Maize seedlings were subjected to hydroponic culture without (control, C) or containing 50 and 100 μM of CuCl2 for 72 h. Determinations were performed on extracts obtained from the root apex (solid line, Ap) and the remaining root tissue (dotted line, Rt). Values represent means ± SEM of five independent experiments, with three biological replicates per treatment. Different lowercase letters indicate significant differences for the same tissue (P < 0.05), according to Tukey’s multiple range test
Copper induced post-translational protein modifications and affected proteasome peptidase activities
As shown in Fig. 3A and Supplemental Figure 2, Cu treatment resulted in a strong degree of protein carbonylation, mainly at Ap with the lower Cu concentration tested (50 μM) and along the whole primary root under 100 μM Cu. In addition, copper treatment significantly increased the levels of proteins conjugated with SUMO1 and Ub both in Ap and in Rt (Fig. 3B, C).
Effect of copper on root carbonylated and SUMO1- and Ub-conjugated proteins. Maize seedlings were subjected to hydroponic culture without (control, C) or containing 50 and 100 μM of CuCl2 for 72 h. Root protein extracts obtained from the root apex (white, Ap) and the remaining root tissue (gray, Rt) were used for the determination of DNPH-derivatized proteins by dot blot. Membranes were photographed and then analyzed with Gel-Pro software. Quantification of oxidized (A), SUMO1- (B), and Ub-conjugated (C) proteins are expressed in arbitrary units (assuming control value equal to 100), based on absolute integrated optical density (IOD) of each dot. Bars represent mean ± SEM. Different lowercase letters indicate significant differences (P < 0.05) for the same tissue, according to Tukey’s multiple range test
The trypsin-like activity of the 20S core complex of the 26S proteasome was clearly reduced under Cu treatment in both tissues in a dose-dependent manner (Fig. 4B), while chymotrypsin- and PGPH-like activities increased (Fig. 4A, C). At the highest Cu dose, Rt was the tissue that most contributed to the enhanced activity of the latter, being notorious the negative effect of 100 μM Cu on PGPH activity in Ap (Fig. 4C).
Effect of copper on proteasome peptidase activities. Maize seedlings were subjected to hydroponic culture without (control, C) or containing 50 and 100 μM of CuCl2 for 72 h. Root protein extracts obtained from the root apex (white, Ap) and the remaining root tissue (gray, Rt) were used for determination. Trypsin-like (B), chymotrypsin-like (A), and peptidyl glutamyl peptide hydrolase (PGPH) (C) proteasome 20S activities were measured in the extracts using three peptide substrates (Boc-LSYRAMC, AAF-AMC, and Clz-LLE-βNA, respectively) in the absence or presence of the proteasome inhibitor MG132. Enzymatic activities were normalized for protein concentrations and expressed as percentages of the activity present in the controls. Bars represent means ± SEM of five independent experiments, with three biological replicates per treatment. Different lowercase letters indicate significant differences (P < 0.05) for the same tissue, according to Tukey’s multiple range test
Copper modified root hormonal balance
Among the various gibberellins measured, GA4 was found to be the most affected by Cu stress: the concentration of this gibberellin strongly decreased after Cu treatment in both portions of the root (Fig. 5B). The active GA3 was also strongly decreased in Cu-treated seedlings in Ap, but not in Rt (Fig. 5D). The rest of the gibberellins determined (GA7 and GA20) showed some changes only in Ap (Fig. 5A, C).
Effect of copper on gibberellins (GA) accumulation. GA7 (A); GA4 (B); GA20 (C); GA3 (D). Determinations were performed in extracts obtained from the root apex (solid line, Ap) and the remaining root tissue (dotted line, Rt) of maize seedlings subjected to hydroponic culture without (control, C) or with 50 and 100 μM of CuCl2 for 72 h. Values represent means ± SEM of three independent experiments, with three biological replicates per treatment. Different lowercase letters indicate significant differences (P < 0.05) for the same tissue, according to Tukey’s multiple range test
IAA levels increased on considering the entire root (Ap plus Rt) of seedlings exposed to Cu in a dose-dependent manner, but the distribution was not uniform: diminished IAA levels in Ap and increased IAA levels in Rt were detected (Fig. 6A). Even when ABA levels in Ap decreased with respect to the control, in Rt, they remained stable, with no difference between doses (Fig. 6B). A sharp increase in SA content, mainly due to the increment observed in Rt, was observed under 50 μM of Cu (Fig. 6C). Finally, we also observed a strong decrease in JA accumulation after Cu exposure, particularly in Ap (Fig. 7A). The active JA-Ile conjugated was also strongly diminished but to a similar extent in both tissues (Fig. 7B).
Effect of copper on the accumulation of indole-3-acetic acid (IAA) (A); abscisic acid (ABA) (B); salicylic acid (SA) (C). Determinations were performed in extracts obtained from the root apex (solid line, Ap) and the remaining root tissue (dotted line, Rt) of maize seedlings subjected to hydroponic culture without (control, C) or with 50 and 100 μM of CuCl2 for 72 h. Values represent means ± SEM of three independent experiments, with three biological replicates per treatment. Different lowercase letters indicate significant differences (P < 0.05) for the same tissue, according to Tukey’s multiple range test
Effect of copper and cadmium on the accumulation of jasmonic acid (JA) (A), JA-isoleucine conjugate (JA-Ile) (B). Determinations were performed on extracts obtained from the root apex (solid line, Ap) and the remaining root tissue (dotted line, Rt) of maize seedlings subjected to hydroponic culture without (control, C) or with 50 and 100 μM of CuCl2 for 72 h. Values represent means ± SEM of three independent experiments, with three biological replicates per treatment. Different lowercase letters indicate significant differences (P < 0.05) for the same tissue, according to Tukey’s multiple range test
Discussion
Maize root system comprises distinct embryonic and postembryonic root types that are formed during different stages of development. While the adult root system is determined by an extensive shoot-borne rootstock, the embryonically preformed roots dominate during the first weeks after germination. This early root system, which is made up of the primary root and a variable number of seminal roots (Hochholdinger 2009), is vital for the vigor of young maize plants and is the primary organ that eventually deals with pollutants present in soils (Tai et al. 2016). This research was designed to evaluate the effects of Cu excess on maize seedlings before the onset of the phototrophic lifestyle to let aside from our analysis copper effects on photosynthesis and photoassimilates’ transport.
Though being Cu an essential micronutrient, when copper ions (Cu2+) are taken up by roots in excess and reach internal concentrations above a certain threshold, it becomes a stress factor that negatively impacts root growth. Nevertheless, it should be taken into account that hydroponic cultures are oversimplified study models in which the complex physicochemical soil interactions remain unconsidered. Many variables modify the bioavailability of plant nutrients under natural conditions. In this sense, Gu et al. (2019) recently reported that total organic carbon, Mn, pH, and CaO affected uptake and bioaccumulation of copper by maize plants.
Cu toxicity typically disturbs the ion balance in plant tissues (Marastoni et al. 2019; Li et al. 2019; Zeng et al. 2019). Mechanisms by which copper alters ion homeostasis include uptake competition, regulation of transporters’ gene expression, and nonspecific metal-induced impairment of root functions (Zeng et al. 2019). Under our experimental conditions, the excess of Cu2+ in the hydroponic solution altered the root nutritional status, mainly with decreases in Ca2+ and Mg2+ levels. Guzel and Terzi (2013) found that cell osmotic adjustments in two maize cultivars subjected to Cu stress involved modifications in Ca2+ and Mg2+ levels. Likewise, Ouzounidou et al. (1995) reported a progressive reduction of Ca concentration in maize plants growing in a nutrient solution supplied with up to 80 μM of Cu2+. More recently, it has been indicated that the decrease in Ca and Mg ions and also the imbalance between them can affect optimal plant growth and development (Tang and Luan 2017), and Ca2+ was considered essential for orchestrating the responses to plant abiotic stress through the crosstalk between H2O2 and phytohormones (Černý et al. 2018).
We detected that copper affected the H2O2-scavenging system in both parts of the root (Ap and Rt), being particularly adverse for CAT activity. CAT activity decrease is a common response described for plants under copper stress (Zeng et al. 2019; Mostofa et al. 2015; Pena et al. 2011). Enzyme inactivation by the metal has been associated with oxidation of the protein structure (Pena et al. 2011) as well as suppression of CAT gene expression (Ye et al. 2014). Thus, the inability to trap H2O2 by CAT together with an excess of Cu ions could have led to the uncontrolled production of the highly harmful hydroxyl radical (HO.) by the Haber-Weiss reactions, as previously reported (Mostofa et al. 2015). In this context, GPX seems to be an intrinsic defense tool to overcome Cu-imposed oxidative stress (Zeng et al. 2019; Mostofa et al. 2015; Thounaojam et al. 2012). On the other hand, though APX activity increased notoriously under the highest Cu concentration tested, and total ASC (ASC plus DHAs) and GSH contents also increased, suggesting an adequate functioning of the ASC-GSH cycle, these adjustments were not enough to maintain the ASC/DHAs ratio and prevent cell redox imbalance, as previously observed in rice roots (Mostofa et al. 2015). Likewise, proline and carbohydrate decreases could be indicating a metabolic shift to defense compounds involving ammonium demand. In accordance, at the highest Cu dose, the concentration of ammonium—a central component of nitrogen metabolism—in the root apex was significantly diminished. On the other hand, it was reported that proline and carbohydrate metabolism generates the reducing equivalents needed for the maintenance of many antioxidant molecules in their reduced state (Hossain et al. 2014)
A strong degree of protein oxidation was detected in maize roots apexes under mild Cu stress, while at the highest Cu concentration, this protein modification was verified in the entire root tissue. Recognition and removal of mildly oxidatively damaged proteins from the cytoplasm and nucleus depend on the 20S proteasome activity; however, excessively oxidized proteins tend to accumulate (Jung et al. 2014; Raynes et al. 2016). The effect of other metals on proteolytic abilities was already assessed. In this sense, Djebali et al. (2008) reported the up-regulation of chymotrypsin activity in vitro in tomato plants subjected to Cd stress. In addition, it was suggested that direct oxidative modifications of 20S protein subunits could contribute to modulate peptidase activities (Pena et al. 2007), and trypsin-like activity was previously found as the most sensitive to inactivation under oxidative stress (Bulteau et al. 2001). Under our experimental conditions, the partial inactivation of the proteasome peptidic activities could also have contributed to carbonylated protein accumulation.
On the other hand, it was stated that SUMOylation could be triggered by reactive oxygen species as a cellular protective response (Augustine et al. 2016). A growing number of reports indicate the importance of protein conjugation with Ub and SUMO, and the interplay between them in the integration of environmental cues and the responses of plants to environmental challenges (Augustine et al. 2016; Catala et al. 2007; Conti et al. 2008; Kurepa et al. 2003; Miura et al. 2011; Yoo et al. 2006). Changes in the overall ubiquitylation and SUMOylation degree in maize roots in response to Cu treatment could be part of a post-translational protein modification program directed to regulate plant growth under heavy metal stress. In this sense, it was proposed that the combined control of transcriptional regulators involved in hormonal perception by conjugation with Ub and SUMO may contribute to plant adaptation to stressful conditions (Skelly et al. 2016). Chen et al. (2011) described that an increase in the abundance of SUMOylated species was involved in Cu tolerance and homeostasis in Arabidopsis.
Copper altered root phytohormone balance, mainly by downregulating several of these signaling molecules in the root apex, confirming that the root meristem is the “first line” in the sensing of external soluble stressors. The notorious decrease in GA3 and GA4 levels in the root tip upon Cu treatment may be involved in the reduction of the root elongation under stress. Cu treatment could have interfered in GA3/4 biosynthesis, but also it could have led to subsequent gibberellin transformations, including deactivation. For instance, upregulation of two genes encoding GA2-oxidase, a major enzyme for deactivating bioactive gibberellins, was previously detected in response to Cd stress (Liu et al. 2015). Also, growth impairments due to the stimulated expression of many GA2ox genes were demonstrated in Arabidopsis thaliana during salt and cold stress (Achard et al. 2008; Magome et al. 2008).
It is well-known that auxins can either stimulate or inhibit root growth depending on their concentrations. In maize roots, it has been confirmed an inverse relationship between endogenous IAA level and growth rate (Pilet and Saugy 1987). In line with this previous observation, we found decreased growth and increased IAA concentrations in Cu-treated seedlings on considering the entire root, but root apexes showed significantly decreased IAA contents. It has been described that Cu influences the transport of auxin within root tissues in Arabidopsis, partly by modulating PIN1, one of the main carrier proteins described for this hormone (Yuan et al. 2013). Coincidently with this observation, we corroborated altered auxin distribution in seminal maize roots under Cu stress. It has been well established that auxin signaling requires the ubiquitin-proteasome degradation system (Kelley and Estelle 2012). In our study, copper stress led to the accumulation of ubiquitin-conjugated proteins suggesting that the metal had no effects on the ubiquitin-conjugating system. Nevertheless, Cu could alter IAA function through the modifications of the proteasome activity. A recent study demonstrated that exogenous supply of IAA and GA3 exerted protective effects under Cu stress by the recovery of redox homeostasis, resulting in alleviation of protein damage in roots (Ben Massoud et al. 2018).
While ABA levels in the remaining root tissue were not affected by Cu, in root apex, this hormone was strongly decreased under our experimental conditions. The cross-talk between Cu homeostasis and ABA responses was previously explored, and it was found that optimal Cu availability is necessary to keep normal endogenous ABA levels in Arabidopsis seedlings (Carrió-Seguí et al. 2016).
SA was recognized as a very important endogenous plant signal mediating in plant defense against biotic and abiotic stresses (Hayat et al. 2010). Several reports document that exogenous application of SA improved plant tolerance to certain heavy metals by reducing metal uptake and/or promoting redox balance (Hayat et al. 2010; Mostofa and Fujita 2013; Popova et al. 2009; Shakirova et al. 2016), but data on endogenous SA levels under metal stress are scarce. We detected strong increases in SA endogenous levels at both the root apex and the remaining root tissue at the lower Cu dose tested (50 μM), whereas at 100 μM only in the remaining root tissue SA content was increased. Recent studies showed that SA inhibited Cu translocation in maize and alleviated oxidative stress in roots; however, it did not mitigate the growth inhibition exerted by this metal (Moravcová et al. 2018).
Jasmonic acid and methyl jasmonates are derivatives of the fatty acid plant metabolism (Wasternack and Song 2017) and display multiple biological roles. Other jasmonate conjugates, including jasmonoyl isoleucine (JA-Ile), were also found to have important biological functions (Ahmad et al. 2016); among these roles, upregulation of antioxidant enzymes and oxidative stress tolerance was communicated (Soares et al. 2010; Wasternack and Hause 2013). Jasmonates are critical in the coordination of defense responses through their involvement in the interactions with salicylic acid, ethylene, and abscisic acid pathways, but they also regulate growth and development by interacting with auxins and gibberellins (Wasternack and Hause 2013). In this work, we observed in maize root apexes sharp decreases in JA and also in JA-Ile, which is considered the most active form among jasmonates to trigger defense responses (Fonseca et al. 2009). It would be of special interest to assess the activity/expression of jasmonoyl-isoleucine synthetase (JAR1) in the context of Cu stress in future studies.
Conclusion
The effects of Cu excess before the emergence of the coleoptile and the onset of the phototrophic lifestyle were specifically assessed in maize, focusing on an eventual distinctive role of the root apex compared with the rest of the primary root. Besides the strong reduction of the root length and biomass, changes in the elemental composition, redox balance, and hormonal homeostasis were corroborated in these early root tissues. One of the most notorious effects of Cu exposure was the intense degree of protein post-translational modification, including protein oxidation. The entire primary root of maize seedlings was found to be compromised in the protein turnover that seems to be necessary to trigger and/or sustain defense mechanisms against Cu toxicity.
References
AbdElgawad H, Zinta G, Hamed BA, Selim S, Beemster G, Hozzein WN, Wadaan MAM, Asard H, Abuelsoud W (2020) Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ Pollut 258:113705. https://doi.org/10.1016/j.envpol.2019.113705
Abendroth LJ, Elmore RW, Boyer MJ, Marlay SK (2011) Corn growth and development. PMR 1009. Iowa State University Extension, Ames, Iowa
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20:2117–2129. https://doi.org/10.1105/tpc.108.058941
Aebi H (1984) Catalase in vitro. Oxygen radicals in biological systems. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M, Kazi AM, Gucel S (2016) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813. https://doi.org/10.3389/fpls.2016.00813
Antoniadis V, Golia EE, Liu YT, Wang SL, Shaheen SM, Rinkleb J (2019) Soil and maize contamination by trace elements and associated health risk assessment in the industrial area of Volos, Greece. Environ Int 124:79–88. https://doi.org/10.1016/j.envint.2018.12.053
Augustine RC, York SL, Rytz TC, Vierstra RD (2016) Defining the SUMO system in maize: SUMOylation is up-regulated during endosperm development and rapidly induced by stress. Plant Physiol 171(3):2191–2210. https://doi.org/10.1104/pp.16.00353
Bartoli CG, Casalongué CA, Simontacchi M, Márquez-García B, Foyer CH (2013) Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ Exp Bot 94:73–88. https://doi.org/10.1016/j.envexpbot.2012.05.003
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Ben Massoud M, Sakouhi L, Karmous I, Zhu Y, El Ferjani E, Sheehan D, Chaoui A (2018) Protective role of exogenous phytohormones on redox status in pea seedlings under copper stress. J Plant Physiol 221:51–61. https://doi.org/10.1016/j.jplph.2017.11.014
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bulteau AN, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI (2001) Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem 276:30057–30063. https://doi.org/10.1074/jbc.M100142200
Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182(4):799–816. https://doi.org/10.1111/j.1469-8137.2009.02846.x
Carrió-Seguí A, Romero P, Sanz A, Peñarrubia L (2016) Interaction between ABA signaling and copper homeostasis in Arabidopsis thaliana. Plant Cell Physiol 57(7):1568–1582. https://doi.org/10.1093/pcp/pcw087
Častorálová M, Ruml T, Knejzlík Z (2012) Using dot blot with immunochemical detection to evaluate global changes in SUMO-2/3 conjugation. Biotechniques 53(3). https://doi.org/10.2144/000113925
Catala R, Ouyang J, Abreu IA, Hu Y, Seo H, Zhang X, Chua NH (2007) The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell 19:2952–2966. https://doi.org/10.1105/tpc.106.049981
Černý M, Habánová H, Berka M, Luklová M, Brzobohatý B (2018) Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. Int J Mol Sci 19:2812. https://doi.org/10.3390/ijms19092812
Chen CC, Chen YY, Tang IC, Liang HM, Lai CC, Chiou JM, Yeh KC (2011) Arabidopsis SUMO E3 ligase SIZ1 is involved in excess copper tolerance. Plant Physiol 156(4):2225–2234. https://doi.org/10.1104/pp.111.178996
Conti L, Price G, O’Donnell E, Schwessinger B, Dominy P, Sadanandom A (2008) Small ubiquitin-like modifier proteases OVERLY TOLERANT TO SALT1 and −2 regulate SALT stress responses in Arabidopsis. Plant Cell 20:2894–2908. https://doi.org/10.1105/tpc.108.058669
De Tullio MC, Jiang K, Feldman LJ (2010) Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiol Biochem 48:328–336. https://doi.org/10.1016/j.plaphy.2009.11.005
Djebali W, Gallusci P, Polge C, Boulila L, Galtier N, Raymond P, Chaibi W, Brouquisse R (2008) Modifications in endopeptidase and 20S proteasome expression and activities in cadmium treated tomato (Solanum lycopersicum L.) plants. Planta 227:625–639. https://doi.org/10.1007/s00425-007-0644-6
Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gómez-Cadenas A (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J Agric Food Chem 53:8437–8442. https://doi.org/10.1021/jf050884b
Farahat EA, Galal TM, Elawa OE, Hassan LM (2017) Health risk assessment and growth characteristics of wheat and maize crops irrigated with contaminated wastewater. Environ Monit Assess 189:535. https://doi.org/10.1007/s10661-017-6259-x
Florijn PJ, Van Beusichem ML (1993) Uptake and distribution of cadmium in maize inbred lines. Plant Soil 150:25–32. https://doi.org/10.1007/BF00779172
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5:344–350. https://doi.org/10.1038/nchembio.161
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. https://doi.org/10.1104/pp.110.167569
Gu Q, Yu T, Yang Z, Ji J, Hou Q, Wang L, Wei X, Zhang Q (2019) Prediction and risk assessment of five heavy metals in maize and peanut: a case study of Guangxi, China. Environ Toxicol Pharmacol 70:103199. https://doi.org/10.1016/j.etap.2019.103199
Guzel S, Terzi R (2013) Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot Stud 54:26. https://doi.org/10.1186/1999-3110-54-26
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiments Station, Circular 347
Hochholdinger F (2009) The maize root system: morphology, anatomy, and genetics. In: Bennetzen JL, Hake SC (eds) Handbook of maize: its biology. Springer, New York. https://doi.org/10.1007/978-0-387-79418-1_8
Hossain MA, Hoque MA, Burritt DJ, Fujita M (2014) Chapter 16 - proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In: Oxidative damage to plants antioxidant networks and signaling. Academic Press, Cambridge, pp 477–522. https://doi.org/10.1016/B978-0-12-799963-0.00016-2
Ingvardsen C, Veierskov B (2001) Ubiquitin- and proteasome-dependent proteolysis in plants. Physiol Plant 112:451–459. https://doi.org/10.1034/j.1399-3054.2001.1120401.x
Jung T, Höhn A, Grune T (2014) The proteasome and the degradation of oxidized proteins: part II – protein oxidation and proteasomal degradation. Redox Biol 2:99–104. https://doi.org/10.1016/j.redox.2013.12.008
Kelley DR, Estelle M (2012) Ubiquitin-mediated control of plant hormone signaling. Plant Physiol 160:47–55. https://doi.org/10.1104/pp.112.200527
Kumar P, Tewari RK, Sharma PN (2008) Modulation of copper toxicity-induced oxidative damage by excess supply of iron in maize plants. Plant Cell Rep 27:399–409. https://doi.org/10.1007/s00299-007-0453-1
Kurepa J, Walker JM, Smalle J, Gosink MM, Davis SJ, Durham TL, Sung DY, Vierstra RD (2003) The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and −2 conjugates is increased by stress. J Biol Chem 278(9):6862–6872. https://doi.org/10.1074/jbc.M209694200
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplast. The effect of hydrogen peroxide and paraquat. Biochem J 210:899–903. https://doi.org/10.1042/bj2100899
Li Q, Chen HH, Qi YP, Ye X, Yang LT, Huang ZR, Chen LS (2019) Excess copper effects on growth, uptake of water and nutrients, carbohydrates, and PSII photochemistry revealed by OJIP transients in Citrus seedlings. Environ Sci Pollut Res 26:30188–30205. https://doi.org/10.1007/s11356-019-06170-2
Liu T, Zhu S, Tang Q, Tang S (2015) Genome-wide transcriptomic profiling of ramie (Boehmeria nivea L. Gaud) in response to cadmium stress. Gene 558:131–137. https://doi.org/10.1016/j.gene.2014.12.057
Liu J, Wang J, Lee S, Wen R (2018) Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS One 13(9):e0203612. https://doi.org/10.1371/journal.pone.0203612
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Madejón P, Ramírez-Benítez JE, Corrales I, Barceló J, Poschenrieder C (2009) Copper-induced oxidative damage and enhanced antioxidant defenses in the root apex of maize cultivars differing in Cu tolerance. Environ Exp Bot 67:415–420. https://doi.org/10.1016/j.envexpbot.2009.08.006
Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K (2008) The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56:613–626. https://doi.org/10.1111/j.1365-313X.2008.03627.x
Marastoni L, Tauber P, Pii Y, Valentinuzzi F, Astolfi S, Simoni A, Brunetto G, Cesco S, Mimmo T (2019) The potential of two different Avena sativa L. cultivars to alleviate cu toxicity. Ecotoxicol Environ Saf 182:109430. https://doi.org/10.1016/j.ecoenv.2019.109430
Miura K, Lee J, Gong Q, Ma S, Jin JB, Yoo CY, Miura T, Sato A, Bohnert HJ, Hasegawa PM (2011) SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiol 155:1000–1012. https://doi.org/10.1105/tpc.114.133090
Moravcová Š, Tůma J, Dučaiová ZK, Waligórski P, Kula M, Saja D, Słomka A, Bąba W, Libik-Konieczny M (2018) Influence of salicylic acid pretreatment on seeds germination and some defence mechanisms of Zea mays plants under copper stress. Plant Physiol Biochem 122:19–30. https://doi.org/10.1016/j.plaphy.2017.11.007
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicol 22:959–973. https://doi.org/10.1007/s10646-013-1073-x
Mostofa MG, Hossain MA, Fujita M, Tran LS (2015) Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci Rep 5:11433. https://doi.org/10.1038/srep11433
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nannoni F, Rossi S, Protano G (2016) Potentially toxic element contamination in soil and accumulation in maize plants in a smelter area in Kosovo. Environ Sci Pollut Res 23:11937–11946. https://doi.org/10.1007/s11356-016-6411-0
Ninh Pham A, Xing G, Miller CJ, David Waite T (2013) Fenton-like copper redox chemistry revisited: hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J Catal 301:54–64. https://doi.org/10.1016/j.jcat.2013.01.025
Orlowski M, Wilk S (2000) Catalytic activities of the 20S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys 383:1–16. https://doi.org/10.1006/abbi.2000.2036
Ouzounidou G, Čiamporová M, Moustakas M, Karataglis S (1995) Responses of maize (Zea mays L.) plants to copper stress-I. growth, mineral content and ultrastructure of roots. Environ Exp Bot 35:167–176. https://doi.org/10.1016/0098-8472(94)00049-B
Pena LB, Pasquini LA, Tomaro ML, Gallego SM (2007) 20S proteasome and accumulation of oxidized and ubiquitinated proteins in maize leaves subjected to cadmium stress. Phytochemistry 68:1139–1146. https://doi.org/10.1016/j.phytochem.2007.02.022
Pena LB, Azpilicueta CE, Gallego SM (2011) Sunflower cotyledons cope with copper stress by inducing catalase subunits less sensitive to oxidation. J Trace Elem Med Biol 25(3):125–129. https://doi.org/10.1016/j.jtemb.2011.05.001
Pilet PE, Saugy M (1987) Effect on root growth of endogenous and applied IAA and ABA: a critical re-examination. Plant Physiol 83:33–38. https://doi.org/10.1104/pp.83.1.33
Piñeros MA, Shaff JE, Kochian V (1998) Development, characterization, and application of a cadmium-selective microelectrode for the measurement of cadmium fluxes in roots of Thlaspi species and wheat. Plant Physiol 116:1393–1401. https://doi.org/10.1104/pp.116.4.1393
Polge C, Jaquinod M, Holzer F, Bourguignon J, Walling L, Brouquisse R (2009) Evidence for the existence in Arabidopsis thaliana of the proteasome proteolytic pathway: activation in response to cadmium. J Biol Chem 284(51):35412–35424. https://doi.org/10.1074/jbc.M109.035394
Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Kranteva AP, Szalai G, Janda T (2009) Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem 47(3):224–231. https://doi.org/10.1016/j.plaphy.2008.11.007
Raynes R, Pomatto LCD, Davies KJA (2016) Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol Asp Med 50:41–55. https://doi.org/10.1016/j.mam.2016.05.001
Robyt JF, White BJ (1990) Biochemical techniques: theory and practice. Waveland Press, Inc., Prospect Heights
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signalling. Nature 459:1071–1078. https://doi.org/10.1038/nature08122
Shakirova FM, Allagulova CR, Maslennikova DR, Klyuchnikova EO, Avalbaev AM, Bezrukova MV (2016) Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ Exp Bot 122:19–28. https://doi.org/10.1016/j.envexpbot.2015.08.002
Sharma S, Uttam KN (2018) Early stage detection of stress due to copper on maize (Zea mays L.) by laser-induced fluorescence and infrared spectroscopy. J Appl Spectrosc 85:771. https://doi.org/10.1007/s10812-018-0717-2
Shiyab S (2018) Phytoaccumulation of copper from irrigation water and its effect on the internal structure of lettuce. Agriculture 8:29. https://doi.org/10.3390/agriculture8020029
Singh S, Singh A, Bashri G, Prasad SM (2016) Impact of cd stress on cellular functioning and its amelioration by phytohormones: an overview on regulatory network. Plant Growth Regul 80:253–263. https://doi.org/10.1007/s10725-016-0170-2
Skelly MJ, Frungillo L, Spoel SH (2016) Transcriptional regulation by complex interplay between post-translational modifications. Curr Opin Plant Biol 33:126–132. https://doi.org/10.1016/j.pbi.2016.07.004
Soares AM, Souza TF, Jacinto T, Machado OLT (2010) Effect of methyl jasmonate on antioxidative enzyme activities and on the contents of ROS and H2O2 in Ricinus communis leaves. Braz J Plant Physiol 22:151–158. https://doi.org/10.1590/S1677-04202010000300001
Tai H, Lu X, Opitz N, Marcon C, Paschold A, Lithio A, Nettleton D, Hochholdinger F (2016) Transcriptomic and anatomical complexity of primary, seminal, and crown roots highlight root type-specific functional diversity in maize (Zea mays L.). J Exp Bot 67(4):1123–1135. https://doi.org/10.1093/jxb/erv513
Tang RJ, Luan S (2017) Regulation of calcium and magnesium homeostasis in plants: from transporters to signaling network. Curr Opin Plant Biol 39:97–105. https://doi.org/10.1016/j.pbi.2017.06.009
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39. https://doi.org/10.1016/j.plaphy.2012.01.006
Todgham A, Hoaglund E, Hofmann G (2007) Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of Antarctic fish as evidence for cold-denaturation of proteins in vivo. J Comp Physiol B 177(8):857–866. https://doi.org/10.1007/s00360-007-0183-2
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Vatehová Z, Malovíková A, Kollárová K, Kučerová D, Lišková D (2016) Impact of cadmium stress on two maize hybrids. Plant Physiol Biochem 108:90–98. https://doi.org/10.1016/j.plaphy.2016.06.035
Vavoulidou E, Avramides EJ, Papadopoulos P, Dimirkou A, Charoulis A, Konstantinidou-Doltsinis S (2005) Copper content in agricultural soils related to cropping systems in different regions of Greece. Commun Soil Sci Plant Anal 36:759–773. https://doi.org/10.1081/CSS-2000433677/s10812-018-0717-2
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058. https://doi.org/10.1093/aob/mct067
Wasternack C, Song S (2017) Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot 68:1303–1321. https://doi.org/10.1093/jxb/erw443
Wehr NB, Levine RL (2012) Quantitation of protein carbonylation by dot blot. Anal Biochem 423:241–245. https://doi.org/10.1016/j.ab.2012.01.031
Ye N, Li H, Zhu G, Liu Y, Liu R, Xu W, Jing Y, Peng X, Zhang J (2014) Copper suppresses abscisic acid catabolism and catalase activity, and inhibits seed germination of rice. Plant Cell Physiol 55:2008–2016. https://doi.org/10.1093/pcp/pcu136
Yoo CY, Miura K, Jin JB, Lee J, Park HC, Salt DE, Yun DJ, Bressan RA, Hasegawa PM (2006) SIZ1 small ubiquitin-like modifier E3 ligase facilitates basal thermotolerance in Arabidopsis independent of salicylic acid. Plant Physiol 142:1548–1558. https://doi.org/10.1104/pp.106.088831
Yuan HM, Xu HH, Liu WC, Lu YT (2013) Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol 54(5):766–778. https://doi.org/10.1093/pcp/pct030
Zeng Q, Ling Q, Wu J, Yang Z, Liu R, Qi Y (2019) Excess copper-induced changes in antioxidative enzyme activity, mineral nutrient uptake and translocation in sugarcane seedlings. Bull Environ Contam Toxicol 103:834–840. https://doi.org/10.1007/s00128-019-02735-6
Acknowledgments
We thank Dr. Myriam S. Zawoznik for her helpful criticism and for improving the English. CLM is a Research Fellow at the UBA (Argentina). LBP and SMG are Career Investigators from CONICET (Argentina). Hormone measurements were performed at Servei Central d’Instrumentació Científica (SCIC) of Universitat Jaume I (Spain).
Funding
This work was supported by the Universidad de Buenos Aires (20020170100331BA UBACYT), and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0266).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig. 1
Hydroponic culture and maize seedlings. The left panel is a top view of the hydroponic system. The right panels show representative images of control maize seedlings and Cu-treated (100 μM of CuCl2) maize seedlings at 72 h of metal treatment (BMP 1349 kb).
Supplemental Fig. 2
Representative image of the dot blots obtained to determine carbonylated proteins. Root protein extracts obtained from the root apex (Ap) and the remaining root tissue (Rt) of control (C) and Cu-treated seedlings (50 and 100 μM of CuCl2) were derivatized with 2,4-DNPH. Equal amounts of total protein (7.5 μg) were blotted onto a PVDF, blocked in 5% (w/v) non-fat dried milk in PBS, and incubated at room temperature for 1 h with anti-DNP (Sigma, St Luis) as the primary antibody. Dots were subsequently visualized using a secondary rabbit antibody conjugated with horseradish peroxidase (HRP) and stained using 3,3′-diaminobenzidine (DAB) as substrate. The dot blots shown are representative of four replicates prepared in five independent experiments (PPT 136 kb).
Rights and permissions
About this article
Cite this article
Matayoshi, C.L., Pena, L.B., Arbona, V. et al. Early responses of maize seedlings to Cu stress include sharp decreases in gibberellins and jasmonates in the root apex. Protoplasma 257, 1243–1256 (2020). https://doi.org/10.1007/s00709-020-01504-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01504-1