Abstract
Hippophae rhamnoides L. ssp. turkestanica (Elaeagnaceae) is a predominantly dioecious and wind-pollinated medicinal plant species. The mature fruits of the species possess antioxidative, anti-inflammatory, antimicrobial, anticancerous, and antistimulatory properties that are believed to improve the immune system. The identification of male and female plants in H. rhamnoides ssp. turkestanica is quite difficult until flowering which usually takes 3–4 years or more. A sex-linked marker can be helpful in establishing the orchards through identification of genders at an early stage of development. Therefore, we studied the genetic diversity of populations in Ladakh with the aim to identify a gender-specific marker using ISSR markers. Fifty-eight ISSR primers were used to characterize the genome of H. rhamnoides ssp. turkestanica, of which eight primers generated 12 sex-specific fragments specific to one or more populations. The ISSR primer (P-45) produced a fragment which faithfully segregates all the males from the female plants across all the three valleys surveyed. This male-specific locus was converted into a SCAR. Forward and reverse primers designed from this fragment amplified a 750-bp sequence in males only, thus specifying it as an informative male-specific sex-linked marker. This SCAR marker was further validated for its capability to differentiate gender on an additional collection of plants, representing three geographically isolated valleys (Nubra, Suru, and Indus) from Ladakh region of India. The results confirmed sex-linked specificity of the marker suggesting that this conserved sequence at the Y chromosome is well preserved through the populations in Ladakh region. At present, there are no reliable markers which can differentiate male from female plants across all the three valleys of Ladakh region at an early stage of plant development. It is therefore envisaged that the developed SCAR marker shall provide a reliable molecular tool for early identification of the sex in this commercial crop. The genetic diversity of populations as surveyed by ISSR primers revealed 85.71 % polymorphism at the population level. The dendrogram obtained divided the genotypes into three different clusters, and the distribution of male and female genotypes in all the clusters was random. The Nei’s genetic similarity index was in the range of 0.63–0.96.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiation of sex is one of the major events in the evolutionary history of higher plants (Barrett 2002). Unisexuality has evolved from the hermaphrodite ancestors through complex developmental processes. Formation of unisexual flowers could occur due to the termination of androecium/gynoecium in a cosexual ancestor or the differentiation of sex can occur before initiation of anthers and carpels (Ming et al. 2015). There are reports of sex-determining genes present on allosomes that faithfully segregate into their respective phenotypes without undergoing crossing over (VanBuren et al. 2015). Other factors, which regulate sex differentiation, involve interaction between hormones and genetic and epigenetic modifications (Ming et al. 2015). Among the well-studied model plants like Carica papaya, highly evolved XY chromosomes control sex. The males (Y) and hermaphrodites (Yh) have slight differences in their Y chromosomes (Wang et al. 2012). In Silene latifolia, two different loci are present on the Y chromosomes. The first locus is involved in the suppression of carpel development and the second activates anther development (Zluvova et al. 2006).
Elaeagnaceae is a core eudicot family containing three unisexual genera, i.e., Eleagnus, Hippophae and Shepherdia. This family is sister to Rhamnaceae, Barbeyaceae, Ulmaceae, and Cannabaceae in Rosales (APG III 2009). All these families contain both unisexual and bisexual plant species. H. rhamnoides is a nitrogen-fixing plant with wide distribution in temperate regions of Asia and Europe. In India, the plant shows luxurious growth in cold high altitude regions of Ladakh (Jammu and Kashmir), Himachal Pradesh, and Uttarakhand (Mangla et al. 2015). Several authors have designated H. rhamnoides in Indian Himalayas as H. rhamnoides ssp. turkestanica (Singh et al. 1995). The plant possesses lanceolate leaves with silvery green shine on the dorsal surface. The male inflorescence bears four to six flowers. The female flowers are borne in condensed axillary raceme, represented by a unicarpellary pistil. Natural pollination occurs through wind. The fruits ripen in about 3 months after pollination (Mangla and Tandon 2014; Mangla et al. 2015).
The plant species, especially the fruits and seeds, are used in Oriental traditional system of medicine and Tibetan pharmacopeia; it possesses anti-inflammatory, antimicrobial, and antistimulatory properties. The plant is known to have anticancer activity (Teng et al. 2006), cures cardiovascular diseases, and improves the immune system (Ruan and Li 2000). Survey reports confirm that berries of this plant acts as radioprotectant due to the presence of flavonoids (Suryakumar and Gupta 2011). The fruits contain a large amount of vitamin C and essential oils and, therefore, are being used as important raw material in pharmaceutical and cosmetic industries (Bal et al. 2011; Suryakumar and Gupta 2011).
In a plantation, the number of female plants should be more than the males. According to Gakov (1980), 6–7 % of males are sufficient for pollination, whereas recent reports suggest that 10 % of well-distributed male plants are needed for optimum pollination (Jadhav and Sharma 2014). The identification of the gender in H. rhamnoides ssp. turkestanica is not possible until flowering which usually takes 3–4 years or more in the field conditions. This poses problems for orchards which retain large numbers of superfluous males for several years. In addition, due to increasing demand, the plant species is being overexploited by the rural populations, which in consequence causes adverse impacts to the ecosystem as well as a loss of genetic diversity (Tian et al. 2004). Understanding the gender demography and genetic structure of natural populations of this species may also help in establishing reforestation programs under a system appropriate to control the overexploitation of this resource (Barrett and Kohn 1991). Considering these facts, sex identification in H. rhamnoides ssp. turkestanica at an early stage is a prerequisite. There are reports which confirm early sex identification in H. rhamnoides ssp. turkestanica genotypes with morphological, physiological, and biochemical parameters (Korekar et al. 2012). However, such procedures are tedious and do not present a reliable and simple method for early determination of sex at a young stage (Lebeda 2003). An alternate solution is to develop a sex-linked molecular marker which can identify sex at an early stage of plant development. There have been attempts to identify sex-linked markers but the developed marker failed to identify sex when tested on varied populations especially from different geographical locations (Jadhav and Sharma 2014). In the present study, we undertook a study in different genotypes of H. rhamnoides ssp. turkestanica using inter simple sequence repeat (ISSR) with a specific goal of identifying sex-specific sequence characterized amplified region (SCAR) marker(s) which can be used for differentiating the genders at an early stage of plant development, thereby helping in conserving natural and economic resources. ISSR markers have the advantage over other dominant markers; these markers have high reproducibility than random amplification of polymorphic DNAs (RAPD) (Ganie et al. 2015a, b) and are very easy to handle compared to amplified fragment length polymorphisms (AFLP) where silver staining is required to elute the species-specific fragment from polyacrylamide gels.
Materials and methods
Plant materials
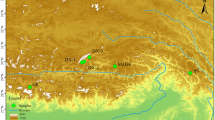
An extensive survey was carried out to collect H. rhamnoides ssp. turkestanica from three different valleys (viz. Indus, Suru, and Nubra valleys) of Ladakh region from Jammu and Kashmir (Fig. 1). An earlier study from our group (Raina et al. 2012) has clearly established that the sea buckthorn available in Ladakh region belongs to H. rhamnoides ssp. turkestanica. Voucher specimens for the material used in the present study have been submitted at Delhi University Herbarium (DUH). Samples from the Indus Valley have been submitted earlier under accession number 14221 (Mangla et al. 2015), and additional samples from the Suru and Nubra valleys have been submitted under accession numbers 14324 and 14325, respectively.
From each valley, both male and female plant leaves were collected and brought to the lab after freezing in liquid nitrogen. The material was stored at −80 °C till used for DNA extraction. A total of 92 collections were made and the distance between any two samples was at least 10 m. Samples from the Indus Valley consisted of 12 male and female plants each. Twenty-five males and 21 females were collected from Nubra Valley. From the Suru Valley, 10 males and 12 females were harvested. The names of collection sites and sample codes are provided in Table 1.
DNA extraction
DNA was isolated from the leaves following the modified protocol of Doyle and Doyle (1990). One gram of leaf material was pulverized to a fine powder in a chilled mortar using liquid nitrogen. The powder was transferred into 50 ml centrifuge tubes containing 10 ml CTAB buffer and 200 mg polyvinylpyrrolidone (PVP). Ten milliliters of chloroform (CHCl3)/isoamyl alcohol (IAA) (24:1) was added and mixed carefully for 15 min. The tubes were centrifuged at 10,000 rpm for 15 min at 15 °C. The upper phase was transferred into an autoclaved centrifuge tube and 0.5 volume of 5 M NaCl was added to it. The precipitation of DNA was carried out with an equal volume of isopropanol at −20 °C for 2 h. The tubes were again centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was discarded and the pellet was washed with 70 % ethanol, air dried, and dissolved in 2 ml high salt TE buffer. The RNA was degraded with 5 μl of RNase A (10 mg/ml). To inactivate the RNase, 2 ml of phenol/chloroform/isoamyl alcohol (25:24:1) solution was added followed by centrifugation at 10,000 rpm for 10 min at 15 °C. The traces of phenol were removed by adding 2 ml chloroform/isoamyl alcohol (24:1) and centrifuged at 10000 rpm for 10 min at 15 °C. The DNA was precipitated using an equal volume of absolute ethanol and 1/10th volume of sodium acetate (pH 5.2) for 2 h at −20 °C. The tubes were recentrifuged at 8000 rpm for 15 min at 4 °C. The supernatant was discarded and the pellet was washed with 70 % ethanol, air dried, and dissolved in 100 μl of autoclaved double distilled water. The DNA obtained through this modified method still contained impurities and was not suitable for polymerase chain reaction (PCR) analysis. Therefore, the DNA was further purified with PEG (20 %) (Paithankar and Prasad 1991) and quantified using a Nanodrop spectrophotometer and diluted to obtain a concentration of 100 ng/μl.
PCR amplification
PCR was carried out in a 25-μl reaction mixture containing 50 ng DNA, 1 U Taq DNA polymerase, 2.0 mM MgCl2, 0.8 μM 10-mer primers, 0.53 mM dNTPs, and 2.5× Taq polymerase buffer. The final volume was adjusted with sterile Milli-Q water. The amplifications were carried out on a DNA thermal cycler (Applied Biosystems, USA). The PCR amplification conditions consisted of an initial step of denaturation at 94 °C for 4 min, 40 cycles of denaturation at 94 °C for 1 min, annealing at suitable temperature (primer dependent) for 1 min, polymerization at 72 °C for 2 min, followed by a final extension at 72 °C for 8 min. The amplified product was separated on 2.0 % agarose gel in 1× TAE buffer containing 5 μl ethidium bromide (5 mg/ml) and photographed using a gel documentation system (UVP, Germany). Fifty-eight ISSR primers (Qiagen, USA) were screened. Data analysis was carried out by scoring well-marked amplified fragments. Only high-intensity fragments were scored for data analysis; the presence or absence of each amplified fragment was coded as 1 or 0, to create a binary data matrix. Genetic similarity (GDxy) was calculated as per Nei and Li (1979) using the equation GDxy = 1 − d xy / d x + d y − d xy, where d xy is the number of common loci in two genotypes, d x is the number of loci in genotype 1, and d y is the number of loci in genotype 2. The distance matrix was used to construct a dendrogram by the neighbor-joining (NJ) method with 1000 bootstrap replicates. The principal coordinate analysis (PCoA) was carried out by using DARwin version 6.0.155 (http://darwin.cirad.fr/darwin; Perrier and Jacquemoud-Collet 2006).
Cloning and characterization of sex-specific fragments
The desired fragment was excised from the gel and DNA was eluted using MiniElute® kit (Qiagen, USA) following the manufacturer’s instructions. The fragment was cloned into the pGEM®-T Easy Vector (Promega, USA), transformed into the Escherichia coli strain DH5α-competent cells, and plated onto LB/ampicillin/IPTG/X-Gal plates. Individual positive colonies were used for plasmid isolation using a plasmid isolation kit (Qiagen, Germany). Vectors having fragments of the desired size were sequenced at Eurofins Biotechnologies Pvt Ltd and in both reverse and forward directions using M-13 universal primers on ABI3700 DNA analyzer (Applied Biosystems, USA). The sequence of SCAR has been deposited in GenBank under the accession number KX347093.
Design of SCAR oligonucleotides
The sex-specific fragment sequence was used to design SCAR primers with the help of Primer Explorer V3 (http://primerexplorer.jp/elamp3.0.0/index.html). The primer set consisted of one forward and one reverse primer (Table 2).
Testing of SCAR primers
The SCAR primer designed was tested on 47 male and 45 female plants of H. rhamnoides ssp. turkestanica. The SCAR reaction was carried out in a 15 μl reaction volume containing 50 ng genomic DNA, 10 mmol each of forward and reverse primer, 1.5× buffer, and 0.5 U Taq DNA polymerase. The amplifications were performed using the following reaction conditions: initial denaturation at 94 °C (5 min) followed by 28 cycles of 94 °C (30 s), 60 °C (1 min) and 72 °C (1 min), and a final extension step at 72 °C (7 min). The PCR amplicon was checked on 1.2 % agarose gel.
Results
Identification of sex-linked marker(s) and development and validation of SCAR
Initially, 17 males and 17 females representing the three different valleys were used to identify primers which could differentiate males and females. These plants were monitored and assigned sex based on flowering in the month of April. Fifty-eight ISSR primers were screened to identify a sex-linked marker among different genotypes of H. rhamnoides ssp. turkestanica collected from different localities of Ladakh region of Jammu and Kashmir, India. Each amplification was repeated twice to ensure the reproducibility of the fragments. The amplified products were in the range of 0.25 to 1.6 kb.
The ISSR primers exhibited variation in amplification patterns of the DNA samples, both within and between male and female genotypes. However, when these primers were tested on individual male and female samples from all the populations, only one ISSR primer, P-45, amplified a male-specific fragment of approximately 0.9 kb consistently, whereas many primers generated putative sex-specific amplicons in some of the genotypes but could not differentiate males from females across all the three valleys. All the male- and female-specific amplicons produced are listed in Table 3. Among these, the amplicons obtained with primer 8 (CT CT CT CT CT CT CT CT A) were unique. This primer amplified male-specific fragments ranging from 0.775 to 2.0 kb in different populations, and the size varied between the populations of same valley and even the individual genotypes produced multiple sex-specific fragments. The male genotypes from LRP and LWP populations from Indus Valley and the male genotype from BA population of Suru Valley generated a common fragment of 1.3 kb. Similarly, the male genotypes from LRP, LWP, and FRL populations of Indus Valley; SU1 and SU3 populations of Nubra Valley; and BA populations of Suru Valley produced a male-specific fragment of 0.775 kb with this primer. Other primers as listed in Table 3 did produce sex-specific fragments but none other than primer 8 generated multiple fragments with different sizes with the same populations. Two primers produced female-specific amplicons in some populations. Female genotypes LRP and LS of Indus Valley generated a unique fragment of 1.4 kb with P-37. In addition, P-45 amplified 1.0 kb amplicon in the genotypes of LC and UP (Indus Valley) and UT genotype of Suru Valley.
As the ISSR primer P-45 (CA CA CA CA CA CA CA CA RG) generated a common male-specific fragment across all the three valleys (Fig. 2), this amplicon was cloned into pGEM-T Easy Vector and sequenced. The exact nucleotide sequence of the male-specific ISSR amplicon was 936 bp (Fig. 3) with a GC content of 32.37 %. A homology search using BLASTn could not identify homology to any known sequence. A forward and a reverse primer designed from the sequence (Table 2) generated the expected amplicon of the length of 750 bp in 17 male genotypes from the three different valleys. The SCAR primer was further validated by testing on an additional collection of 47 male and 45 female genotypes from different populations of the three valleys. These genotypes were marked as male and female in the month of April. All the 47 male genotypes amplified the expected male-specific 750-bp fragment (Fig. 4). Of the 45 females, 42 did not show any amplification; however, as an exception, three female samples from TK and SR (Nubra Valley) populations showed amplification of this fragment.
Genetic diversity
A total of 84 fragments were amplified of which 72 were polymorphic. Two to 11 fragments were generated by each primer with an average of 7 fragments per primer. The mean percentage of polymorphic fragments was 85.71. Of 84 fragments, 12 were detected in all the samples (1, 1, 1, 1, 4, 2, 1, 1, 1, 1 by primers P-7, P-9, P-11, P-17, P-20, P-30, P-35, and P-45, respectively). Primers P-2, P-12, P-23, and P-24 generated absolute polymorphism (100 %), followed by P-45 (90.5 %). Least polymorphism was obtained with P-35 (50 %) (Table 4, Fig. 5). The Nei’s genetic similarity index calculated was in the range of 0.63–0.96 (Suppl. Table 1).
The dendrogram (Fig. 6) constructed using the neighbor-joining method divided the different genotypes into three main clusters with low support. All the major clusters were again split into two subclusters. Grouping of genotypes was not strictly gender based but similar genders tend to group together. For example, the NJcl III contained the female genotypes only. In NJcl II, one subcluster contained all male genotypes, and of the seven genotypes in the other subcluster, four represent females. Similarly, three of the four genotypes in one subcluster of NJcl I constitute males; however, the other subcluster is again divided into two subminor clusters. One subminor cluster was entirely composed of male genotypes, whereas the other represented seven genotypes of which six constitute females.
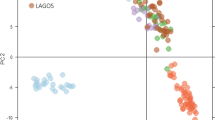
In PCoA (Fig. 7), the genotypes were distributed in all the four quadrants, indicating that the different genotypes of H. rhamnoides ssp. turkestanica harbor substantial polymorphism. Maximum numbers of genotypes were distributed in quadrant (clockwise) IV, followed by quadrant I. Quadrants II and III contained two and six genotypes, respectively.
Discussion
The pathway to the XY system is believed to involve suppression of recombination on the Y chromosome to avoid sterility (Bull 1983). Recombination allows positive mutation to dissociate from the negative mutations, but negative mutations tend to be retained in the populations by hitchhiking with positive mutations on the sex chromosomes (Bachtrog 2013). This suppression over time expands to the rest of the Y chromosome causing degeneration of the rest of the Y chromosome (Bachtrog 2013). Discovery of the sex-linked regions can lead to a better understanding of the sequence divergence between sex-linked alleles that might help in estimating the time at which recombination first stopped among them (Charlesworth 2016).
AFLP-, ISSR-, and RAPD-based gender-specific SCAR markers have been reported in many dioecious plants. Of these, ISSR markers are convenient and cost effective when compared to RAPDs and AFLPs. Several research reports reveal the presence of microsatellites on sex chromosomes. Parasnis et al. (1999) used microsatellite (GATA)4 probe to identify a sex-specific marker in C. papaya. Banded krait minor satellite (Bkm) repeat satellites (GATA and GACA) are also known to accumulate on several male plants (Adhikari et al. 2014). We amplified the DNA of H. rhamnoides ssp. turkestanica with 58 microsatellite (ISSR) markers. Eight primers amplified sex-specific fragments which discriminated gender only in a few populations. However, only one primer amplified a male-specific fragment across all male plants collected from the three valleys of Ladakh.
Lack of homogeneity on the Y chromosome across populations
H. rhamnoides ssp. turkestanica reproduces by sexual reproduction assisted by wind pollination but can also perennate vegetatively through suckers spreading to long distances. A recent report also indicates the presence of apomixes in the species (Mangla et al. 2015). Asexual means of reproduction might allow the unique features of the Y chromosome to be retained in the population longer. Combined with physical isolation of populations due to topographic features of Ladakh region, the Y chromosome might show population-specific features which have not been homogenized across all the populations of the species. In the present study, many primers amplified sex-specific amplicons which were region specific. Earlier studies have also identified sex-linked markers, which worked on individual population(s) but were not conserved across the different populations of H. rhamnoides ssp. turkestanica (Persson and Nybom 1998; Jadhav and Sharma 2014). H. rhamnoides ssp. turkestanica might be representing a young and evolving sex chromosome, which can provide a crucial window into the initial stages of Y chromosome evolution and might be an invaluable source of information regarding ecological and evolutionary studies (Wilkinson et al. 2015). Additionally, H. rhamnoides ssp. turkestanica grows in extreme conditions and is exposed to a variety of selection pressures and environmental changes, imparting the qualities of a system wherein the hypothesis suggesting that sex can accelerate adaptation to complex environments can be tested (Gray and Goddard 2012; Goddard 2016).
Identification and development of a reliable sex-linked SCAR marker
One of the ISSR primers, P-45, amplified a unique male-specific fragment across all the three valleys. Three female samples also produced amplification which could possibly be due to the sampling error during collection. We will check more samples from this region to find out whether it is a sampling error or has a biological relevance.
The present study has provided a robust reliable male-specific marker, which can reliably differentiate the sex at an early stage of plant development, which previous related investigations could not provide (Sharma et al. 2010; Jadhav and Sharma 2014; Korekar et al. 2012; Persson and Nybom 1998). These studies did not have adequate number of genotypes examined from different geographical regions and also failed to mention the subspecies of H. rhamnoides which they have studied. Sharma et al. (2010) used RAPD-based markers to identify gender in H. rhamnoides ssp. turkestanica with only five samples. The same technique was employed by Jadhav and Sharma (2014) in two populations of H. rhamnoides; the results were reliable only for one population. Persson and Nybom (1998) also used RAPD-based markers to obtain a male-specific marker in F1 progenies derived from crossing of the varieties ‘Leikora’ and ‘Pollmix I’. However, the same primer was able to identify only one F1 male when the varieties BHi 102240 and ‘2–240’ were crossed. Korekar et al. (2012) also reported a RAPD-based SCAR which worked as female-specific markers but they did not test them on genotypes from different geographical regions. When these primers were used in our collections, they did not show the desired female specificity.
Genetic diversity
Although the genetic variation is very useful in devising programs for the management of optimal utilization and conservation of medicinal plants, our purpose of studying genetic diversity was to analyze the relationship among the male and female genotypes of H. rhamnoides ssp. turkestanica. The 34 plants representing the three valleys used for initial screening of 58 SSR primers were used for the diversity analysis. After careful screening, the reproducible and reliable data of only 12 primers was used for this analysis. Nearly 86 % variation was observed across all genotypes. Previous studies on the same plant species showed higher polymorphism (Raina et al. 2012; Srihari et al. 2013). The higher polymorphism obtained by them can be attributed to the inclusion of genotypes collected from regions other than Ladakh.
In the present study, Nei’s genetic similarity values had a moderate range varying from 0.63 to 0.96. The highest genetic similarity value (0.96) observed was between two different populations of Sumur-3 and Hundar from Nubra Valley area of Leh, Ladakh. This value matches with Nei’s genetic similarity index value (0.94) calculated from the study of Srihari et al. (2013) in different populations of H. rhamnoides. Many studies have corroborated the correlation between genetic distance and geographical distance (Alpert et al. 1993). In the present study, some populations exhibited high heterogeneity within the same populations (e.g., Sumur region) indicating that the frequency of cross pollination is more between the samples of the nearby region. The cross pollination of H. rhamnoides occurs through anemophily limiting the distance traveled by pollen. However, this trend was not observed across all the three valleys. In some cases, more genetic similarity was observed within the population. This nonuniformity might be due to several reasons. The seeds of this plant species can easily be dispersed to distant regions by birds or human intervention, thereby causing intermixing of populations. Further, H. rhamnoides is grazed by domestic animals; hence, these agents might act as pollen/seed carriers to far-off regions, causing amalgamation of populations.
The 34 genotypes were grouped into three major clusters. Many of the ISSR primers produced gender-specific fragments due to which a gender bias can be seen in these clusters. However, the population-specific clusters were not observed. For example, the first major cluster represented a mixture of genotypes of the three different valleys (Suru, Nubra, and Indus). A similar observation was made in the case of the other two clusters. This might be due to the pollen and/or seed dispersal which contributed to the absence of a genetic structure in a local geographic area (Sarwat et al. 2011). Analysis of the population structure using PCoA supported the data obtained through NJ clustering to a greater extent. Majority of the genotypes of NJcl I are grouped together in quadrant I in PCoA. Similarly, most of the genotypes clustered in the NJcl II are grouped in quadrant IV in PCoA. Further, the genotypes SU2-M, BA-M, SU2-F, SP-F, and UT-F are more genetically distant among their respective clades. The same results were obtained with PCoA.
A number of fragments amplified were monomorphic. H. rhamnoides ssp. turkestanica is widely used in pharmaceuticals, which require monitoring of plant material to avoid adulteration with other unrelated and cheap drugs. The monomorphic bands obtained with different primers can be converted to SCAR and used for molecular identification of this taxon. Molecular markers have been used in medicinal plant authentication (Cheng et al. 2015; Ganie et al. 2015a, b; Moon et al. 2015).
In conclusion, the present study was able to provide a robust sex-linked universal marker which can distinguish male plants from female plants across populations of H. rhamnoides ssp. turkestanica in Ladakh region. It also proposes H. rhamnoides as a new system to study the evolution of the sex chromosome which is even more important since it can grow in a wide range of environments including extreme cold conditions. The study also signifies the applicability of ISSR markers for studying the genetic diversity and genetic relationships in different genotypes of H. rhamnoides ssp. turkestanica. Our study could successfully be utilized to remold the cultivation of H. rhamnoides ssp. turkestanica by maintaining a proper gender ratio of plants at early seedling stage enabling the production of sufficient females that have more economical and pharmaceutical value compared to males. Further, the information gathered in the present study could be used for devising strategies for conservation of H. rhamnoides ssp. turkestanica. The monomorphic fragments generated could distinguish this taxon from possible adulterants. We are continuing with the efforts to increase the number of the sex-specific marker and to increase the length of the amplified product through chromosome walking. The exact location of these sex-specific markers through fluorescent in situ hybridization can provide useful information about the extent of Y chromosome degeneration and evolutionary/ecological compartmentalization of sex chromosomes across different populations.
References
Adhikari S, Saha S, Bandyopadhyay TK, Ghosh P (2014) Identification and validation of a new male sex-specific ISSR marker in pointed gourd (Trichosanthes dioica Roxb.). Sci World J. doi:10.1155/2014/216896
Alpert P, Lumaret RD, Giusto F (1993) Population structure inferred from allozyme analysis in the clone herb Fragaria chiloensis (Rosaceae). Am J Bot 80:1002–1006
APG III (2009) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Bachtrog D (2013) Y chromosome evolution: emerging insights into processes of Y chromosome degeneration. Nat Rev Genet 14:113–124
Bal LM, Meda V, Naik SN, Satya S (2011) Sea buckthornberries: apotential source of valuable nutrients for neutraceuticals and cosmoceuticals. Food Res Int 44:1718–1727
Barrett SCH (2002) The evolution of plant sexual diversity. Nature Rev Genet 3:274–284
Barrett SCH, Kohn JR (1991) Genetics and evolutionary consequences of small population size in plants: implications for conservation. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 3–30
Bull JJ (1983) Evolution of sex determining mechanisms. Benjamin/Cummings, Menlo Park
Charlesworth D (2016) The status of supergenes in the 21st century: recombination suppression in Batesian mimicry and sex chromosomes and other complex adaptations. Evol Appl 9:74–90
Cheng J, Long Y, Khan MA, Wei C, Shelly F, Junjiang F (2015) Development and significance of RAPD-SCAR markers for the identification of Litchi chinensis Sonn. by improved RAPD amplification and molecular cloning. Electron J Biotechnol 18:35–39
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Gakov MA (1980) Prospects for the development of Hippophae rhamnoides in the USSR. Lesn khoz 2:51–52
Ganie SH, Ali Z, Das S, Srivastava PS, Sharma MP (2015a) Molecular characterization and chemical profiling of different populations of Convolvulus pluricaulis (Convolvulaceae); an important herb of Ayurvedic medicine. 3 Biotec 5:295–302
Ganie SH, Upadhyay P, Das S, Sharma MP (2015b) Authentication of medicinal plants by DNA markers. Plant Gene 4:83–99
Goddard MR (2016) Sex accelerates adaptation. Nature 531:176–177
Gray JC, Goddard MR (2012) Gene-flow between niches facilitates local adaptation in sexual populations. Ecol Lett 15:955–962
Jadhav MS, Sharma TR (2014) Identification of gender specific DNA markers in sea buckthorn (Hippophae rhamnoides L.). Ind Res J Genet Biotech 6:464–469
Korekar G, Sharma RK, Kumar R, Meenu, Bisht NC, Srivastava RB, Ahuja PS, Stobdan T (2012) Identification and validation of sex-linked SCAR markers in dioecious Hippophae rhamnoides L. (Elaeagnaceae). Biotechnol Lett 34:973–978
Lebeda A (2003) Danube seabuckthorn populations, valuable for selections. In: Singh V, Khosla PK (eds) Proceedings of international workshop on seabuckthorn. New Delhi, 8–21 Lee T T. 1973. extraction and quantification of plant peroxidase isoenzymes, vol 29, Physiol Plants., pp 198–203
Mangla Y, Tandon R (2014) Pollination ecology of Himalayan sea buckthorn, Hippophae rhamnoides L. (Elaeagnaceae). Curr Sci 106(12):1731
Mangla Y, Chaudhary M, Gupta H, Thakur R, Goel S, Raina SN, Tandon R (2015) Facultative apomixis and development of fruit in a deciduous shrub with medicinal and nutritional uses. AoB Plants 7:plv098. doi:10.1093/aobpla/plv098
Ming GAO, Chen YC, Yang SS et al (2015) Progress on sex differentiation in unisexual flower plants. Acta Prataculturae Sin 2015:206–217
Moon BC, Yunui J, Young ML, Kang YM, Kim HK (2015) Authentication of Akebia quinata DECNE. from its common adulterant medicinal plant species based on the RAPD-derived SCAR markers and multiplex-PCR. Genes Genom 37:23–32
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76:5269–5273
Paithankar KR, Prasad KSN (1991) Precipitation of DNA by polyethylene glycol and ethanol. Nucleic Acids Res 19:1346
Parasnis AS, Ramakrishna W, Chowdari KV, Gupta VS, Ranjekar PK (1999) Microsatellite (GATA)n reveals sex-specific differences in papaya. Theor Appl Genet 99:1047–1052
Perrier X, Jacquemound-Collet JP (2006) Darwin software. Available at http://darwin.cirad.fr/darwin
Persson HA, Nybom H (1998) Genetic sex determination and RAPD marker segregation in the dioecious species sea buckthorn (Hippophae rhamnoides L.). Hereditas 129:45–51
Raina S N, Jain S, Sehgal D, Kumar A, Dar T H, Bhat V, Pandey V, Vaishnavi S, Bhargav A, Singh V, Rani V, Tandon R, Tewari M, Mahmoudi A (2012) Diversity and relationships of multipurpose seabuckthorn (Hippophae L.) germplasm from the Indian Himalayas as assessed by AFLP and SAMPL markers. Gen Resour Crop Evol. doi:10.1007/s10722-011-9742-1
Ruan CJ, Li DQ (2000) Function and benefits of sea buckthorn improving eco-environment of loess plateau. Environ Prot 5:30–31
Sarwat M, Das S, Srivastava PS (2011) AFLP and SAMPL markers for characterization of genetic diversity in Terminalia arjuna: a backbone tree of Tasar silk industry. Plant Syst Evol 293:13–23
Sharma A, Zinta G, Rana S, Shirkot P (2010) Molecular identification of sex in Hippophae rhamnoides L. using isozyme and RAPD markers. For Stud China 12:62–66
Singh V, Singh B, Awasthi CP (1995) Distribution, taxonomy and nutritional values of seabuckthorn (Hippophae L.) growing in dry temperate Himalayas. In: Proceedings of international workshop Seabuckthorn, ICRTS, Beijing, China, pp 52–59
Srihari JM, Verma B, Kumar N, Chahota RK, Singh V, Rathour R, Singh SK, Sharma SK, Sharma TR (2013) Analysis of molecular genetic diversity and population structure in sea buckthorn (Hippophae spp L.) from north-western Himalayan region of India. J Med Plants Res 7:3183–3196
Suryakumar G, Gupta A (2011) Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol 138:268–278
Teng BS, Lu YH, Wang ZT, Tao XY, Wei DZ (2006) In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol Res 54:186–194
Tian CJ, Lei YD, Shi SH, Nan P, Chen JK, Zhong Y (2004) Genetic diversity of sea buckthorn (Hippophae rhamnoides) populations in northeastern and northwestern China as revealed by ISSR markers. New Forest 27:229–237
VanBuren R, Zeng F, Chen C, Zhang J, Wai CM et al (2015) Origin and domestication of papaya Yh chromosome. Genome Res 25:524–533
Wang J, Na JK, Yub Q et al (2012) Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc Natl Acad Sci U S A 34:13710–13715
Wilkinson GS, Breden F, Jmank JE et al (2015) The locus of sexual selection: moving sexual selection studies into the post-genomics era. J Evol Bio. doi:10.1111/jeb.12621.
Zluvova J, Nicolas M, Berger A, Negrutiu I, Monéger F (2006) Premature arrest of the male flower meristem precedes sexual dimorphism in the dioecious plant Silene latifolia. Proc Natl Acad Sci U S A 103:18854–18859
Acknowledgments
This work was supported by the Department of Biotechnology, Govt. of India (Project: BT/PR10800/NDB/51/172/2008); KD extends thanks to UGC and the Ministry of Social Justice and Empowerment for providing fellowship during the course of the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Kamal Das and Showkat Hussain Ganie contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Table 1
Nei’s genetic similarity index based on 12 ISSR primers in different populations of H. rhamnoides ssp. turkestanica. (DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Das, K., Ganie, S.H., Mangla, Y. et al. ISSR markers for gender identification and genetic diagnosis of Hippophae rhamnoides ssp. turkestanica growing at high altitudes in Ladakh region (Jammu and Kashmir). Protoplasma 254, 1063–1077 (2017). https://doi.org/10.1007/s00709-016-1013-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1013-8