Abstract
New challenges posed by the development of resistance against artemisinin-based combination therapies (ACTs) as well as previous first-line therapies, and the continuing absence of vaccine, have given impetus to research in all areas of malaria control. This review portrays the ongoing progress in several directions of malaria research. The variants of RTS,S and apical membrane antigen 1 (AMA1) are being developed and test adapted as multicomponent and multistage malaria control vaccines, while many other vaccine candidates and methodologies to produce antigens are under experimentation. To track and prevent the spread of artemisinin resistance from Southeast Asia to other parts of the world, rolling circle-enhanced enzyme activity detection (REEAD), a time- and cost-effective malaria diagnosis in field conditions, and a DNA marker associated with artemisinin resistance have become available. Novel mosquito repellents and mosquito trapping and killing techniques much more effective than the prevalent ones are undergoing field testing. Mosquito lines stably infected with their symbiotic wild-type or genetically engineered bacteria that kill sympatric malaria parasites are being constructed and field tested for stopping malaria transmission. A complementary approach being pursued is the addition of ivermectin-like drug molecules to ACTs to cure malaria and kill mosquitoes. Experiments are in progress to eradicate malaria mosquito by making it genetically male sterile. High-throughput screening procedures are being developed and used to discover molecules that possess long in vivo half life and are active against liver and blood stages for the fast cure of malaria symptoms caused by simple or relapsing and drug-sensitive and drug-resistant types of varied malaria parasites, can stop gametocytogenesis and sporogony and could be given in one dose. Target-based antimalarial drug designing has begun. Some of the putative next-generation antimalarials that possess in their scaffold structure several of the desired properties of malaria cure and control are exemplified by OZ439, NITD609, ELQ300 and tafenoquine that are already undergoing clinical trials, and decoquinate, usnic acid, torin-2, ferroquine, WEHI-916, MMV396749 and benzothiophene-type N-myristoyltransferase (NMT) inhibitors, which are candidates for future clinical usage. Among these, NITD609, ELQ300, decoquinate, usnic acid, torin-2 and NMT inhibitors not only cure simple malaria and are prophylactic against simple malaria, but they also cure relapsing malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria continues to persist in 97 countries inhabited by 3.4 billion people. It is reported that in the year 2013, 219 million persons became sick with malaria disease, and of them, 627,000 died including 483,000 children (WHO; World Malaria Report 2012, 2013a). Malaria mortality was more in the countries of Africa than in Asia or South America. There were 60.7 million new infections of malaria in India in 2013 (Murray et al. 2014). In India, ≤0.2 million have been dying of malaria each year, largely in the states of Chattisgarh, Orissa and Jharkhand (Dhingra et al. 2010; Murray et al. 2014). Climate change is allowing malaria to reach new densely populated areas, such as in the highlands (Siraj et al. 2014). Malaria is caused by the parasites of five species of the genus Plasmodium of the phylum Apicomplexa. Malaria disease caused by Plasmodium knowlesi, Plasmodium malariae, Plasmodium ovale and Plasmodium vivax is milder than that caused by Plasmodium falciparum which is often lethal. P. vivax and P. falciparum are respectively the principal agents of morbidity and mortality causing malaria (Garcia et al. 2008). Besides causing disease in vertebrates, Plasmodium parasites infect mosquitoes of the genus Anopheles. Thirty to 40 species of Anopheles are sensitive to Plasmodium. The main vectors of malaria transmission to humans are Anopheles gambiae and Anopheles stephensi (Hay et al. 2010). Females of these mosquito species acquire malarial parasite infection by biting and taking a blood meal from infected humans. About a week or more than a week later, infected mosquitoes transmit infection to humans while taking their next blood meal. The life cycle of Plasmodium is complex (Fig. 1). It comprises of a gametophytic (male and female gamete forming, haploid) phase followed by a sporophytic (arising from zygote/embryo via mitotic divisions, diploid) phase in a female mosquito and a sporophytic phase each in liver and red blood cells and a gametophytic phase in blood in human host (Rosenberg 2008).
Life cycle of the protozoan parasite Plasmodium whose five species induce malaria in the infected humans: P. falciparum, P. malariae, P. ovale, P. vivax and P. knowlesi. P. falciparum is the cause of maximum mortality (nine out of ten malaria deaths), and P. vivax causes maximum morbidity. The genome sequence of P. falciparum is known (Gardner et al. 2002). Malaria parasites are transmitted person to person by Anopheles mosquitoes. There are 430 species of Anopheles of which up to 40 species serve as malaria vectors. Among them, A. gambiae and A. stephensi are prominent vectors in Africa and India, respectively. Genome sequence of A. gambiae is known (Holt et al. 2002). Both mosquitoes have African origin (Liu et al. 2014). The symptoms of malaria in humans are headache, back pain, muscle ache, fatigue, sweats, fever, chills, vomiting and enlarged spleen. The symptoms appear 7 to 40 days after infection. The parasite life cycle is divisible into three parts: a, liver and red blood cell stages in the human host wherein parasite multiplies asexually; b, sexual stage in human bloodstream where some pre-merozoites undergo gametocytogenesis; and c, sexual stages in female mosquito after it has acquired the gametocytes as a part of blood meal from infected human. The antimalarial compounds that interfere with the progression of different stages are shown as numbers against horizontal bars. The antimalarials 1–15 are as follows: 1, dihydroartemisinin; 2, artesunate; 3, artemether; 4, lumefantrine; 5, piperaquine; 6, sulfadoxine; 7, pyrimethamine; 8, chloroquine; 9, primaquine; 10, atovaquone; 11, proguanil; 12, mefloquine; 13, amodiaquine; 14, pyronadrine; and 15, quinine. The antimalarials shown to act in the b and c parts of Plasmodium life cycle negatively control the transmission of infection and those that act in the a part control the progression of malaria and some of them also control sexual gametogenesis and thereby malarial transmission. The blood meal obtained by a female mosquito by biting to an infected person under treatment also contains the drugs which continue to act on the sexual stages undergoing in the mosquito. A developmental stage of parasite in the liver called hypnozoite is not shown in this figure. Hypnozoites are P. vivax or P. ovale parasites that hibernate in hepatocytes for many months and become the cause of relapsed malaria. The only clinical antimalarial that kills them is primaquine. Besides the bite of an infected mosquito, malaria transmission can also occur via mother to newborn, blood transfusion or sharing of contaminated needle or syringe and organ transplanting
As shown in Fig. 1, Plasmodium’s asexual liver stage in human host (exo-erythrocytic schizogony) begins with inoculation of its eight to ten sporozoites, during the meal bite by an infected female mosquito. The sporozoites injected into the bloodstream travel to the liver and invade hepatocytes. In the invaded hepatocyte, the sporozoite differentiates and grows, within 6–8 days into a schizont (mother cell), by dividing mitotically to produce 1 × 104–3 × 104 merozoites (daughter cells). The liver merozoites, released into the bloodstream upon rupturing of liver schizont(s), invade erythrocytes, thus, begins Plasmodium’s human blood stage life cycle (erythrocytic schizogony). Invading merozoite grows into ring stage trophozoite of large size, at the expense of rich nutrition available in the host erythrocyte. Further progression of trophozoite into schizont involves several rounds of mitotic divisions to produce up to 36 merozoites. Erythrocytic schizonts rupture to release merozoites into the bloodstream. The erythrocytic schizogony is completed in about 48 h. Erythrocytic merozoites invade fresh erythrocytes and merozoites get multiplied. This process leads to the presence of 108 to 1012 circulating merozoites in the bloodstream. At this stage of 12–14 days from infection, the inoculation period ends and symptoms of parasitaemia get manifested by the infected human. After parasitaemia has set in, merozoites of some erythrocytic schizonts invade erythrocytes and differentiate into male gametocytes. Similarly, merozoites of relatively more schizonts invade erythrocytes to produce female gametocytes. Gametocytes circulate in the blood of parasitaemic human. Gametocytes are the only forms of Plasmodium that survive in female mosquito when ingested from malarial human during its blood meal. The Plasmodium life cycle starts in mosquito, when the ingested erythrocytes burst and the released gametocytes produce gametes. The male gametocyte undergoes divisions to produce eight male gametes that are exflagellated. The female gametocyte transforms into a female gamete. Fusion of a male gamete and female gamete results in a zygote, which develops into a ookinete. The motile ookinete migrates to the midgut epithelium to initiate an oocyst. Multiple divisions in oocyst result in the production of thousands of sporozoites. Upon release from oocysts, the sporozoites migrate to salivary glands. Sporozoites from salivary glands are injected into a fresh human host during the next blood meal of the Anopheles after 8–10 days of the previous meal (Hall et al. 2005; Eckhoff 2011). The life cycles of P. vivax and P. ovale, which cause relapsing malaria, somewhat differ at the liver stage from that of P. falciparum. The parasites in the hepatocytes invaded by the P. vivax and P. ovale sporozoites often enter into hibernation for a period up to more than a year. Resumption of liver stage in the hibernated hypnozoites becomes the cause of relapsed malaria. Several aspects of malaria, including parasite biology, clinical features in patients, diagnosis of the infected, protection from the disease and treatment of the diseased, have been broadly reviewed recently by White et al. (2014). Epidemiology and research priorities of malaria and strategies to eliminate malaria have been discussed in some detail in the reviews of Feachem et al. (2010), Enayati and Hemingway (2010), Alonso et al. (2011), Cohen et al. (2012), Burrows et al. (2013), Cotter et al. (2013), Whittaker et al. (2014) and White (2014). The reviews reveal that in the history of malaria, evolution of artemisinin resistance in plasmodia in recent years is a landmark. This development has led to revision of research agenda on diagnosis and treatment of malaria and, therefore, significant changes in plans for malaria control and eradication.
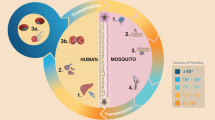
Eradication of malaria from a region requires detection and effective chemotherapeutic treatment of all parasitaemic persons and prevention by blocking of transmission from infected to other persons by a variety of means (Fig. 2). The objective of this review is to discuss new insights into human-Plasmodium-mosquito interaction and its control with reference to eradication of malaria, in the background of the origin of artemisinin resistance in malarial parasites.
Diagramme of a scheme for prevention of malaria in a malaria burdened geographical region. Implementation of such a programme in different regions can roll back by reducing the burden of malaria such that WHO’s goal of eradicating malaria worldwide by 2030 is achieved. Statistical analysis has shown that if transmission of parasites is prevented by the use of bed nets by 75 % of population, malaria will be stopped in the concerned geographical region (Agusto et al. 2013). Once transmission is stopped, the disease is unlikely to reappear (Chiyaka et al. 2013)
Origin of artemisinin-resistant malaria
Table 1 gives a list of 15 antimalarials of seven chemical classes that have been used to treat malarial disease, singly or in combination, at different times in different geographical areas (Biamonte et al. 2013). Since 1996, no new class of antimalarial has been added to clinical practice against malaria. On the other hand, malaria disease treatment has suffered gravely, for more than 60 years, from acquirement of genetic drug resistance property by malarial parasites (Sibley and Ringwald 2006). Malarial parasites developed chloroquine resistance as early as 1950. Subsequently, resistance was noted against other drugs such as pyrimethamine, sulfadoxine, atovaquone, amodiaquine and mefloquine. Occurrence of drug resistance mutations was rare and sporadic, but they subsequently spread worldwide. The main reason for their first appearance and selection has been deduced to be drug underdosing of malaria patients. The dominant nature of certain drug resistance alleles like crt, mdr1, mrp1 and dhfrts was perhaps responsible for the rapid spread of drug resistance in the areas of their origin and subsequently in other areas where migration introduced the resistance alleles (Johnson et al. 2004; Cooper et al. 2007; Plowe 2009; Petersen et al. 2011; Hrycyna et al. 2013; Nwakanma et al. 2013).
In response to the prevalence of resistance to antimalarials commonly used in clinical practice, the artemisinin (ART)-based combination therapy (ACT) was adopted in 1978 as the first line of treatment of malaria (Banek et al. 2014). In ACTs, ART or one of its derivatives—dihydroartemisinin, artesunate, artemether/arteether—is combined with antimalarial(s) such as piperaquine, mefloquine, amodiaquine, sulfadoxine + pyrimethamine, atovaquone + proguanil, pyronaridine and lumefantrine. ARTs via a spectrum of their inhibitory effects, that are not fully understood (Kumar and Srivastava 2005), block the development of parasite’s trophozoites and schizonts in red blood cells and also eliminate, at least partially, the parasite’s gametocytogenesis in the blood of the treated humans (see Table 1). Thus, ARTs not only remediate malaria disease by killing the asexual parasites, but they also block malaria transmission to some extent. The ARTs are fast acting. They kill the malarial parasites present in the blood of patients in 2 days. Their in vivo half life is short. The accompanying drug in ACTs overcomes this deficiency of ARTs. The presence of two drugs in ACTs also serves to safeguard against development of resistance against either of the antimalarials. ACTs are being used in an area-specific manner, such that resistance to ARTs’ partner drug does not exist in the area of usage of an ACT. The ACT comprising of dihydroartemisinin and piperaquine was found to cure 97 % of the P. falciparum-caused malaria patients in Asia, Africa and South America; thus, ACTs have saved millions of lives (WHO 2013b).
The presence of resistance to ARTs of ACTs has been consistently observed in western Cambodia over about the last 10 years (Noedl et al. 2008; Dondorp et al. 2009; Phyo et al. 2012; Satimai et al. 2012; Miotto et al. 2013). As evidence of resistance development, it was noted that clearing of parasites following ACT treatment was taking more than 3 days. Survey in Cambodia showed that in many cases of malaria, clearance of parasites by ACT took up to 20 days. More recently, a similar type of resistance to ARTs has been noted in a wider Southeast Asian region comprising Myanmar, Thailand, Cambodia and Vietnam (WHO 2013a, b).
Measures to contain migration of artemisinin-resistant malaria to outside of Southeast Asia
Evolution of artemisinin resistant in Plasmodium parasites has been a grievous setback to the national and international programmes aiming to eradicate malaria. There is imminent danger of migration of ART-resistant malarial parasites to Sri Lanka, Bangladesh, Nepal, India, Pakistan and Africa. There is an urgent need to stop such possibilities. It is urgently required to detect in the affected Southeast Asian region all persons infected with malarial parasites and treat them by administering the ACT antimalarial to which the local parasites are sensitive. Further, transmission requires to be blocked by the control of mosquitoes as well as by administration of primaquine or an equivalent drug as a prophylactic, in mass campaigns, taking care to avoid this treatment of people afflicted with heritable glucose-6-phosphate deficiency. Such people may be treated with new complementary safe experimental drugs such as ELQ300, GNF156, NITD609 and torin-2. However, until very recently such a programme suffered from deficiencies in rapid method(s) to detect parasitaemic persons on the one hand and persons suffering from ART-resistant infection on the other hand. These lacunae have now been remedied. Juul et al. (2012) have given a cost-effective field method to detect the presence of even one parasite in a drop of blood or saliva of a person to be tested. Ariey et al. (2014) have revealed a DNA marker to rapidly detect the presence of any artemisinin-resistant malarial parasites. The essential and novel features of these techniques are discussed in subsequent sections.
Procedures for the rapid detection of malaria infection in single drops of blood
Sensitive techniques are required to identify and manage cases of malaria infection in general as well as ART-resistant malaria, in hospital laboratories and field conditions, to screen populations and for surveillance at ports and borders (Moody 2002; Congpuong et al. 2012; Hsiang et al. 2012; White et al. 2014). Three kinds of diagnostic tests are already available. In the conventional optical microscopic techniques, parasites present in erythrocytes are visualised in films of blood smear made and stained on a glass slide. This technique, developed in the 1900s, does not detect with certainty the Plasmodium parasites present in the blood of an infected person in the pre-parasitaemic stages of infection. The microscopic test is difficult to perform, is time consuming and requires considerable skill. The sensitivity of this test is only 50–500 parasites/μL of blood (Warhurst and Williams 1996). Despite its limitations, it is the only WHO-standardized and quality-assured test. The immunochromatographic monoclonal antibody-based dip-stick rapid diagnostic tests (RDTs) that qualitatively detect the presence of Plasmodium antigens in human blood comprise the second kind of malaria test. It was introduced in the 1990s (Beadle et al. 1994; WHO 2011; Global Fund 2011; FIND 2013; White et al. 2014). One of the RDTs detects the presence of histidine-rich protein 2 (HRP2) of P. falciparum. HRP2 is a Plasmodium protein present in the parasite’s cytoplasm and cell membrane of merozoite-invaded erythrocytes. The HRP2-based RDT detects only the P. falciparum infections. Its other deficiency is that the test gives a positive result even after the parasitaemia has cleared. Another RDT detects lactate dehydrogenase (LDH), the final enzyme of the parasite’s glycolytic pathway. The LDH-based RDT detects malaria infection of P. falciparum, P. vivax, P. ovale and P. malariae. The sensitivity of RDT is ≤90 % in infections of 100–200 parasites/μL of blood. The advantage of RDTs is that the results become available in less than 30 min. The third kind of rapid malaria test is the nucleic acid amplification (NAA) polymerase chain reaction (PCR) technique that is able to detect malaria infection in all stages of parasite development in humans with the sensitivity ranging from ≤1 to 6 parasites/μL of blood. The technique was introduced in the 1990s (Barker et al. 1992; Erdman and Kain 2008; Cordray and Richards-Kortum 2012; WHO 2014). The PCR technique amplifies and detects Plasmodium-specific DNA or RNA present in the blood sample taken from persons asymptomatic or symptomatic of malaria disease. Several versions of PCR techniques are available: reverse transcriptase-based small or large subunit 18S rRNA molecular amplification (QT-NASBA) that detects P. falciparum, P. vivax, P. ovale and P. malariae infections; nested PCR by the successive use of two sets of Plasmodium genome-specific primer; multiplex PCR which allows detection of more than one Plasmodium species (mixed infections); and quantitative PCR that quantifies the targeted Plasmodium gene(s). The NAAs are more sensitive than RDTs and microscopy and are useful for revealing the areas of oncoming malaria epidemics. Because of the requirement of specialized equipment and trained personnel, the PCR technique is proving difficult to apply in field conditions. The more recently developed technique of Juul et al. (2012) is sensitive and affordable and of practical utility for surveillance of malaria infections in large populations.
Field-applicable rolling circle-enhanced enzyme activity detection diagnostic test of malarial parasites in single drops of blood or saliva
The new technique that detects a Plasmodium-specific enzyme in droplets of blood or saliva is considered easy to use in field conditions on a large scale. The technique called rolling circle-enhanced enzyme activity detection (REEAD) system is used in combination with a fluidic lab-on-a-chip microreactor. REEAD is based on the type 1B topoisomerase activity of the enzyme pTOP1 of Plasmodium species (Fig. 3). For carrying out the Plasmodium-specific REEAD, a DNA sequence or a reaction substrate (called S) for pTOP1 has been designed. On reacting with pTOP1, the sequence folds into a hairpin structure. The DNA sequence (S) has a single-stranded loop and a double-stranded stem. The loop contains a primer annealing sequence and a probing sequence. One of the strands of the stem has a site close to 3′-end that pTOP1 can cleave. Formation of covalent enzyme-DNA intermediate in the stem at the site of cleavage allows religation of the new 3′-end with the protruding 5′-end of the stem’s intact strand. Thus, a covalently closed single-stranded circle is formed. The circle serves as template for its extensive (≥103-fold) amplification by the rolling circle mode, using a small primer (P). The hybridizability of the rolling circle product (RCP) to a short red fluorescent probing nucleotide (FP) sequence allows visual reading of the RCP. The method detects ≤1 parasite/μL of blood or saliva.
Design of the rolling circle-enhanced enzyme activity detection (REEAD) system that detects Plasmodium in droplets of crude human blood or saliva, for field screening of malaria symptomatics plus asymptomatics in large populations. A drop of blood/saliva and S DNA are added to a lysing buffer. A 5 μl sample of it is deposited on a slide that already has attached to it primers for S DNA. The Plasmodium enzyme pTOP1 released from infected blood/saliva reacts with S and via cleavage ligation a single-stranded DNA circle is formed. The P site on it anneals to primer (P) and rolling circle DNA replication occurs producing a long (×103 of original) molecule. Its presence is read by annealing it to a DNA product with rhodamine-labelled complementary (FP) sequence; fluorescent signals are counted microscopically
The blood or saliva sample lysed in low salt buffer is added to the DNA substrate and a very small part of it is transferred to a primer-coated slide. The reaction product annealed to the probe is microscopically read for fluorescence. A microfluidic device is used to deposit the reactants on the slide placed on a platform where many reactions can be conducted simultaneously. The REEAD method diagnoses with equal sensitivity the malaria infection caused by the parasites of P. falciparum, P. vivax, P. ovale, P. knowlesi and P. malariae.
In vitro and ex vivo phenotypic tests of artemisinin-resistant malaria
A significant property of the ART-resistant malaria, of the kind that has emerged in western Cambodia, western Thailand, southern Myanmar and southern Vietnam, is its slower than normal rate of parasite clearance in patients under ACT treatment. The conventional method of measurement of parasite clearance half life is by taking parasite density counts on parasitaemic patients every 6 h, by optical microscopic examination of thick blood films, from the time of start of ART administration to the time disappearance of parasitaemia. However, this kind of diagnosis could require hospitalization of patients for several days. Witkowski et al. (2013) have now provided an in vitro and an ex vivo assay for diagnosing ART-resistant malaria that uses blood sampled from patients before they receive treatment, and hospitalization of patients is not required. In the in vitro ring stage survival assay (RSA0–3 h), the parasites isolated from blood are culture adapted. The parasites in culture are then quantified for the time taken by ART to clear their infection in fresh erythrocytes. In the ex vivo assay, parasites in the blood are cultured directly in the presence and absence of ART. The parasite survival rate suggests whether the patient suffered from ART-sensitive or ART-resistant malaria. The ex vivo test is suitable for large-scale surveillance for ART resistance in affected human populations.
Identification of the principal DNA marker of P. falciparum associated with artemisinin-resistant malaria prevalent in Southeast Asia
Artemisinin-resistant malaria in Southeast Asia has been found to be associated with mutations in K13 propeller domain of the PF3D7_134700 gene of P. falciparum. Ariey et al. (2014) found that ART resistance experimentally generated in a line of P. falciparum and ART resistance that naturally evolved in many P. falciparum parasites present in malaria patients in Cambodia carried mutations at the same locus called PF3D7_1343700. They showed that mutations in the kelch K13 propeller domain part of the gene PF3D7_1343700 makes P. falciparum resistant to ARTs (Fig. 4).
Diagramme of the structure of PF3D7_143700 protein of Plasmodium falciparum. Kelch motives located towards the carboxy terminal are found in a large number of eukaryotic proteins. The KLHL2 human proteins involved in ubiquitin-mediated protein degradation and KEAP1 human protein involved in cellular adaptation to oxidative stress have domains homologous to kelch K13 propeller of Plasmodium. It is thought that for its activity, PF3D7_143700 folds into a six-bladed propeller supported by its interaction with C-terminal beta sheet and N-terminal blade. It is thought that PF3D7_143700 protein is involved in protein degradation-led cytoprotection against oxidative stress imposed by artemisinin. This process is disrupted by mutations in K13 propeller (Ariey et al. 2014; Mitsuishi et al. 2012; Prag and Adams 2003). However, the full mechanism by which the absence of intact PF3D7_143700 gene protects the parasites from the killing action of artemisinin remains to be understood
The wild-type ART-sensitive Tanzanian clone (line) F32 of P. falciparum upon in vitro culturing for 125 cycles in the presence of escalating concentrations of artemisinin developed into an ART-resistant line called F32-ART5. Comparison of the whole genome sequences of the parental ART-sensitive F32 line and derived ART-resistant F32-ART5 line detected eight point mutations in seven genes in the F32-ART5 line. Sequencing of 49 parasite lines collected from malaria patients which responded differentially to ART treatment, in the conventional and developed procedures of Witkowski et al., demonstrated polymorphism at the PF3D7_1343700 locus. The ART-resistant Cambodian lines and F32-ART5 carried mutations in the same general region that is kelch K13 propeller region of the PF3D7_1343700 locus. The locus PF3D7_1343700 was wild type in the ART-sensitive Cambodian lines. These observations implied that mutations in the K13 propeller domain of the protein product of PF3D7_1343700 gene rendered P. falciparum resistant to artemisinins. It was possible to rule out the association between ART resistance and mutation in five other genes of F32-ART5 and mutations/polymorphism observed at other loci in previous P. falciparum population genetic studies in areas of ART resistance (Table 2). Among the ≤1,000 Cambodian clinical parasite isolates that had been genotyped, K13 propeller mutations were found at 17 different sites in PF3D7_1343700 gene. Apparently independent mutations in K13 propeller site were responsible for P. falciparum resistance to ART in Cambodia. Involvement of other genes in the determination of ART resistance has not been ruled out. For the present, K13 propeller sequence of PF3D7_1343700 locus can serve as a reliable DNA marker for surveillance of ART resistance in Southeast Asia.
A question has arisen about the origin and selection of 17 independent recessive mutations at a locus in a gene in a small sample of ≤1,000 of P. falciparum prevalent in provinces of western Cambodia. What mechanisms could be responsible? There is a possibility that the oxidative stress created by free radicals generated by breakage of the endoperoxide bridge of artemisinin may be responsible. Since the malarial parasite genome is methylated at cytosines (Gupta et al. 2013; Ponts et al. 2013) and parasite methylome is a known target of the antimalarial drugs, the ART-imposed stress may result in hypomethylation of the parasite genome; a variety of environmental stresses are known to cause genomic hypomethylation in a variety of eukaryotic systems (Kumar et al. 2013). It is also known that demethylated cytosines are mutational hot spots (see Kumar et al. 2013 for review on epigenetics in plants). Although mutations may occur randomly over the genome, the presence of ART may allow selection of resistance mutations against itself in the isolated niches of P. falciparum occurrence in Cambodia. Mosquito gut provides the opportunity for the production of homozygotes for K13 propeller mutation from a population of gametocytes segregating for the mutation received by the mosquito via blood meal, from a patient in whose liver and/or blood cells parasite’s schizonts or merozoites acquired the ART-resistant mutation. This idea will get tested in future studies on the ART-exposed P. falciparum in vitro and on the extent of genetic polymorphism in P. falciparum populations in Southeast Asia.
Advancements towards the prevention of malaria transmission from person to person
Interference with transmission is the means to reduce infection reservoir from areas of occurrence of malaria. Blocking transmission involves several complementary approaches (Fig. 2). These include elimination of the malaria vector mosquitoes, prevention of mosquito bites and killing of malaria parasite during its development in the vector mosquito. The subject areas of mosquitocides, mosquito repellence and mosquito management in general have been respectively reviewed recently by Prato et al. (2012), Maia and Moore (2011), Dickens and Bohbot (2013) and Beier et al. (2008). New results in each of the three approaches that offer promise of their successful contribution in the control of malaria are discussed below.
Killing of Plasmodium in mosquito
Female Anopheles mosquitoes transmit malaria while biting and taking blood meals from humans. When a mosquito bites a malarial human, it obtains along with blood 103 to 104 sexual stage haploid male and female Plasmodium gametocytes. The gametocytes mature into gametes, fertilization occurs and zygotes form in the mosquito midgut. From the zygotes, 102 to 103 motile ookinetes are formed. These diploid asexual ookinetes form oocysts. Thousands of motile sporozoites released from oocysts into haemocoel accumulate in the salivary glands of the mosquito. Each of the Plasmodium’s developmental stages in mosquito is a potential target for controlling malaria transmission. Two approaches are being pursued to make mosquito refractory to Plasmodium. One of these aims to genetically engineer mosquito genome by the use of the clustered regularly interspaced short palindromic repeat (CRISPR) Cas9 associated system. It is aimed on the one hand to disrupt or delete the genes in mosquito that are essential for Plasmodium’s sporogony and, on the other hand, to add heterologous genes that will antogonise Plasmodium’s survival in mosquito (Oye et al. 2014; Esvelt et al. 2014). Upon their release as per regulations, the genetically engineered mosquitoes are expected to spread the Plasmodium-resistant trait into the local population of mosquito (Marshall 2009; Reeves et al. 2012). In the second approach, the wild-type or genetically engineered bacteria that secrete compounds lethal to Plasmodium are made to co-reside with Plasmodium in the gut of the female mosquitoes. Two experimental results of the approach offer hopes for the development of field-applicable technologies to block malaria transmission.
Bian et al. (2013) have isolated a line of A. stephensi mosquito in whose gut the gram-negative bacterium Wolbachia (strain WAlbB) has resided stably for 34 generations. Wolbachia is harmless to mosquitoes and humans. It was already known that Wolbachia, not a normal resident of mosquito gut, is lethal to parasite when present together with it. The mosquitoes that are stably carrying Wolbachia WAlbB have been observed to be stably refractory to Plasmodium. Malarial parasite’s reproductive development is completely suppressed in the Wolbachia-carrying female mosquitoes. In the new mosquito line, female mosquito egg cytoplasm transmits Wolbachia to the progeny upon mating with either infected or uninfected males. An experimental release of Wolbachia-infected females into a noninfected mosquito population led to all mosquitoes acquiring Wolbachia in less than ten generations. Replacement of mosquito population in a malaria-infected area with Wolbachia-infected mosquitoes is expected to reduce/stop malaria transmission.
The bacterium Pantoea agglomerans is a part of mosquito’s microbiome and co-resides with Plasmodium. Wang et al. (2012) genetically engineered P. agglomerans such that its population in mosquito midgut will kill the sympatric Plasmodium population. The genetically modified P. agglomerans secreted the peptide scorpine or other such proteins that inhibited Plasmodium’s developmental pathway for sporozoite formation. Its presence in the mosquito gut inhibited P. falciparum and Plasmodium berghei by up to 98 %. In the treated mosquito population, only 16 % carried live parasites. Having no effect on humans and the mosquito life cycle, the engineered P. agglomerans is highly promising for field release. It could be spread among mosquitoes by placing in malaria-affected areas many clay jars containing cotton balls soaked in sugar and bacteria to serve as baiting stations.
Malaria prevention by keeping mosquitoes away from the human body
Malaria bites on humans are preventable in several ways. Mosquitocides are sprayed on the walls of homes. Insecticide-treated bed nets are used to protect people while sleeping during nights. Poisonous baits are used to attract and kill mosquitoes in homes and in the open near water bodies (Jawara et al. 2009; Okumu et al. 2010; Smithuis et al. 2013). People are protected by repelling mosquitoes away from them by spraying chemicals that cause hypnosia in mosquitoes, on walls, curtains and other suitable stations in homes (Agusto et al. 2013). Mosquito-repellent-laden bands and patches are used on human clothing and skin; repellents are also added to soaps, shampoos and lotions. There have been developments in each of these strategies to prevent malaria transmission.
Identification of new mosquito repellents
Mosquitoes land on surfaces attracted by certain odours. Their chemoreceptors for odours are borne on sensilla that are attached to the organs antennae, maxillary palp and proboscis (De Moraes et al. 2014; Touhara and Vosshall 2009). The chemoreceptors are non-selective ion channels, each consisting of two proteins, an odour receptor (OR) and a co-receptor called Or83b (orco) which is sensitive to both agonist and antagonist odours (Dickens and Bohbot 2013). Carbon dioxide, lactic acid and octenol present in breath and sweat attract mosquitoes to humans. Malarial parasite-infected female mosquitoes possess 3-fold more attraction to humans than the uninfected mosquitoes (Smallegange et al. 2013). The olfactory sensitivity of mosquitoes is extra high at night (Rund et al. 2013). Mosquito repellents act by shutting the receptors that function in tandem with Or83b. The orco gets overactivated leading to scrabbling of the sense of smell in mosquito such that humans become invisible to them. N,N-diethyl-meta-toluamide (DEET; Fig. 5a) has been a much used mosquito repellent for the last several decades. DeGannarro et al. (2013) by comparing the effect of DEET on wild-type and mutant orco mosquitoes have shown that DEET action is dual. On one hand, it is a strong repellent in the absence CO2, and on the other hand, it deters biting when present on human skin, irrespective of CO2. The search for new repellents, in view of the strong unfavourable smell of DEET, epigenetic changes that it causes and development of tolerance towards DEET in mosquitoes (Bernier 2013; Stanezyk et al. 2013), has led to the discovery of safer and more efficacious compounds than DEET.
Bernier (2013) has developed a formulation, containing homopiperazine (Fig. 5b), 1-methylhomopiperazine (Fig. 5c) and several other human skin secretion compounds, which is more effective than DEET. Chen and Leutje (2013) have identified several orco antagonists of phenylthiophenecarboxamide class of compounds which have the potential to serve as mosquito repellents (Fig. 5d). Kain et al. (2013) have identified several anthranilates [methyl N,N-dimethyl anthranilate, ethyl anthranilate and butyl anthranilate (Fig. 5e–g)] compounds present in fruits which could be used as pleasant smelling, safe and effective mosquito repellents much superior than DEET. Jones et al. (2011) and Pask et al. (2013) have reported about a group of Vanderbilt University Allosteric Agonists (VUAA) such as VUAA1 (Fig. 5h) and amiloride derivatives which are up to hundred thousand fold more potent in activating orco than DEET. Besides, a variety of essential oils rich in geraniol, linalool and/or α-pinene have been shown to be effective mosquito repellents. Some of the fatty oils such as those of castor, Jatropha and catnip have also been observed to be effective mosquito repellents. Many such preparations are listed in en.wikipedia.org/wiki/insect_repellent.
Causing reduction in the indoor mosquito population
Placement of aromatic plants such as of Lantana species, and likewise those of Lippia and Geranium, that spontaneously release essential oils into the air, has proven to be an inexpensive means of reducing mosquito population indoors (Mng’ong’o et al. 2011). Lantana camara adult plants kept in homes were observed to reduce mosquito population by 50 %. Similarly, cultures of the skin bacterium Staphylococcus epidermidis strain DSMZ 11047 can be used to inactivate mosquitoes indoors. The bacterial population produces volatile organic compounds that disrupt the behaviour of mosquitoes (A. gambiae). The stations of bacterial culture can also be used to trap mosquitoes or kill them by combining an insecticide with bacterial culture (European patent application EP2 140764A1/08159618.1, 2010).
Killing of mosquitoes by attracting them to toxic baits
A simple and widely affordable method called attractive toxic sugar bait (ATSB) that controls mosquitoes in the countryside has been found successful when tested at several field locations. In the ATSB procedure, the sweet bait material is produced locally and sprayed on plants growing near the water bodies. The principal component of ATSB is fruit juices from such diverse plants, locally growing in African locations, as of nectarine, guava, honeymelon and cactus. ATSB contains 60–70 % v/v fruit juice, 5–25 % v/v wine and 10–20 % w/v brown sugar. This pulpy material is kept for fermentation for a few days. Finally, 1 % w/v Biostab (a mixture of antibacterial and antifungal substances standardized in Israel that serves as a preservative) and 1 % w/v boric acid (the killing agent) are added. Mosquitoes get attracted to the sprayed plants and intake by them of the sweet mix kills them. The procedure has proven effective in curtailing the local mosquito populations in several different African geographical locations where it was field tested (Muller et al. 2010; Beier et al. 2012; Marshall et al. 2013).
New mosquitocidal formulations
Although costly, spraying of insecticides in homes and the use of insecticide-treated bed nets have contributed immensely in reducing malaria morbidity and mortality. Insecticides of organochlorine, organophosphate, carbamate and pyrethroid classes have been used at various times and locations. Mosquitoes are known to have developed resistance to one or more insecticides of all classes of insecticide compounds at different locations. A single mutation in the upregulated gene for glutathione S-transferase (GSTe2) made a local population of A. funestus malaria vector resistant to DDT as well as pyrethroids in Benin, West Africa (Riveron et al. 2014). Mutations allowing overexpression of P450 family genes CYP6M2 and CYP6P3 and duplication of the genes ACE-1 (allele G119S) encoding acetylcholine esterase have made A. gambiae population of Tiassale in West Africa resistant to all four kinds of insecticides: carbamates, DDT, organophosphates and pyrethroids (Edi et al. 2014).
Pyrethroids are safe, affordable and effective and have long-lasting effects. Therefore, they have been the first choice in malaria control. Since new types of insecticides are yet to arrive, the alternatives to pyrethroids continue to be organophosphates and carbamates. To avoid resistance development, the use of insecticides in rotation is apparent. Whereas one chemical class of insecticide is used for spraying of walls of homes, another kind of insecticide must be used for preparing insecticide-laden bed nets. To control pyrethroid resistance in the environment of sub-Saharan Africa, Rowland et al. (2013) have recommended a long-lasting encapsulated formulation for use in spraying of walls. It consists of the organophosphate insecticide primi-phosmethyl (Fig. 5i). The effect of formulation lasted for about 1 year, whereas the effects of other sprayings lasted for only a few months. Thus far, only the DDT sprays had long-lasting antimosquito effect but DDT is now banned. The new formulation of organophosphate has doubled the lasting effect of DDT which was observed to be 6 months. Several formulations similar to that of Rowland et al. are on the horizon. The use of methoprene on infested soil, mud or puddles and oil film on sedentary water as larvicides and pyrethroid sprays on walls or fogging of environment with malathion in homes as adulticides has proven effective in the control of local mosquito populations (Meister 1992).
Drugs that kill mosquitoes and thereby block sporozoite transmission
The use of the endectocide ivermectin to reduce malaria transmission as well as to kill mosquitoes has been proposed, and experiments on the effectivity and safety of this tool have been initiated (Chaccour et al. 2013). Ivermectin (Fig. 6) is semisynthesized from a Streptomyces avermectinius fermentation precursor. It has been in use for the last 25 years for the control of onchoceriasis and lymphatic filariasis (Omura 2008). Ivermectin is known to agonize glutamate-gated chloride channels of invertebrates which lead to their flaccid paralysis and death. Anopheles mosquitoes have proven to be highly sensitive to ivermectin (Tesh and Guzman 1990). Presently, to control onchoceriasis and filariasis, ivermectin is administered to all residents of a village on a single day. In such villages, mosquitoes that bite humans die because they acquire ivermectin with blood meal. Repetition of ivermectin administration in malaria-burdened villages has been identified as a means to kill mosquitoes and thereby reduce transmission. Another approach considered is to add ivermectin to ACT drugs. The new combination containing three drugs, including ivermectin, will kill malaria parasites in the human body and also kill mosquitoes that may bite the treated patients. The transmission of malaria parasite will be checked because the Plasmodium-infected mosquitoes will be eliminated.
Protecting humans during their sleep
Malaria vector female mosquitoes mostly bite people at nighttime when the latter are asleep. Two inter-country population studies have revealed that malaria transmission can be eliminated altogether by precautionary measures taken at the human population level. Agusto et al. (2013) concluded from their survey that the use of bed nets by 75 % of the population is sufficient for eradication of malaria from any region. Hulden et al. (2013) reached to the conclusion that segmentation of sleeping quarters in households can be a means of stopping malaria transmission. They observed that malaria transmission was negligible where the number of persons sleeping in a bed room was less than four.
Eradication of malaria mosquito by the use of male sterile genetic technique
Experiments are in progress to adapt, in malaria mosquitoes for their control, the conventional and CRISPR-associated genetic engineering techniques to cause heritable male sterility that has been successfully used for eradication of several agricultural pests (Knipling 1959; Whitten and Mahon 2005; Alphey 2014; Esvelt et al. 2014; Oye et al. 2014). In this method, male sterile mosquitoes will be released in cycles until the area becomes free of mosquitoes. The field-released male sterile spermless mosquitoes will mate with females but copulated females will not produce any offspring. Thus, each time male sterile mosquitoes will be released, the size of the mosquito population will get reduced. The technique has been successful in field test conditions for the control of Aedes aegypti, the dengue-spreading mosquito (Harris et al. 2012; Lacroix et al. 2012). Thailayil et al. (2011) have developed a male sterile line of malarial parasite transmitting A. gambiae mosquito, by RNAi silencing of the germ cell differentiation gene ZERO POPULATION GROWTH (ZPG). The genetically modified mosquitoes were found to copulate with wild-type females normally. They switched off in the females the receptivity for further copulation. Subsequent responses of the females were also normal. The spermless mosquitoes are expected to be field tested for mosquito control. The use of the GM mosquitoes will require extensive pre-release safety trials.
Vaccine for malaria prevention
Presently, millions of lives are being saved from malaria morbidity and mortality by the use of drugs that either block malaria transmission or treat malaria disease. Vaccine against malaria could be a cost-effective means to eradicate malaria by reducing the transmission of parasite. Several different approaches to develop efficacious vaccine(s) to stop malaria are in progress. The subject has been recently reviewed by Schwartz (2012) and Riley and Stewart (2013). Although 27 malaria vaccines are under clinical trials, no effective vaccine is as yet available (Moorthy et al. 2013; Cowan et al. 2014). The ongoing programmes are using one or more of the proteins present on the surface of sporozoites and/or merozoites of Plasmodium as antigens. The genetic polymorphism present in the parasite population of an area and among populations of different areas is proving to be a challenge in vaccine development. The RTS,S has been the first-generation malaria vaccine candidate now undergoing phase 3 trials. Besides, there are a few second-generation promising malaria vaccine candidates on which experiments are continuing. Greater understanding of Plasmodium parasite-human host interaction, aided by second-generation genome sequencing and functional genomics of both host and pathogen, will be valuable in improving the vaccines under development and conceptualization of new vaccines.
RTS,S vaccine candidate
The antigen in RTS,S is a hybrid protein, in which R and T domains of the circumsporozoite protein, the major coat protein of the sporozoites of P. falciparum, are covalently linked to the hepatitis B virus antigen (HBs). The vaccine is formulated in the form of virus particles in a liposomal adjuvant (A5P1). The vaccine antagonizes the invasion by sporozoites of host liver hepatocytes; thus, the formation of merozoites in the liver is prevented. RTS,S induces high antibody titres against P. falciparum circumsporozoite protein (CSP) and a moderate CD4+ T cell response. The children vaccinated with RTS,S got protected against uncomplicated as well as severe malarias (Riley and Stewart 2013).
RTS,S is now in the final stages of efficacy trials that started in 1984 and is expected to be released for use in 2015, perhaps in selected geographical areas of high malaria burden. A year after vaccination with RTS,S, about half of young children subjects (aged 5–17 months at the time of vaccination) and one third of infants (aged 6–12 weeks at the time of vaccination) were found malaria protected. However, protection did not last long. The incidence of malaria among the vaccinated children and infants increased progressively over time. Four years from the time of vaccination, protection against malaria in the vaccinated subjects was zero. Recently summarized results of a phase 3 randomized control trial on 8,923 children and 6,537 infants in Africa have led to the estimate that during 18 months after three dose standard vaccination with RTS,S/ASO 1 malaria vaccine, the vaccinated children and infants respectively averted 83 and 45% of clinical cases of malaria (The RTS,S Clinical Trials Partnership 2014).
It is believed that booster dosing of the safe RTS,S vaccine may provide sustained immunity against malaria (Agnandji et al. 2012; Olutu et al. 2013; Riley and Stewart 2013; Fouquet et al. 2014). GlaxoSmithKline have filed an application with the European Medicine Agency for the licensing of RTS,S vaccine.
Whole parasite vaccine of sporozoites attenuated by ionizing radiation(s)
Seder et al. (2013) have developed the PfSPZ vaccine which gave 100 % protection in the phase 1 safety trial. The vaccine consists of metabolically active but replicatively inactive sporozoites that are attenuated by exposure to ionizing radiations (irrspz). The irrspz sporozoites are isolated from the salivary glands of irradiated mosquitoes which are raised under sterile conditions. To generate sporozoites, mosquitoes are fed with malarial parasite-infected human blood. The purified cryopreserved irrspz are used as intravenously administered vaccine. In the phase 1 trial, six volunteers who had been injected with five doses of vaccine did not develop malaria when they were subjected to bites by malaria-infected female mosquitoes. However, among the nine volunteers who were injected with four doses of vaccine, only four demonstrated complete protection from malaria. In all of the 16 subjects, there was a correlation between vaccine dose and antibody level plus immune response. The RTS,S safe vaccine now requires regulatory standardization. New techniques need to be developed for efficient mass production and genetic attenuation of sporozoites to make the vaccine affordable.
Vaccine of genetically attenuated sporozoites
As an alternative to the irradiation-attenuated sporozoites serving as vaccine, Mikolajczak et al. (2014) have developed a line of P. falciparum in which three of the genes involved in the development of malaria disease in humans are deleted. The deleted genes are P36 and P52 whose protein products function in the formation of parasitophorous vacuole in human cells where the parasite invades, grows and multiplies and SAP1 which specifies a protein that regulates RNA stability and thereby the expression of parasites genome in general. The triple mutant has normal gametocytogenesis, mosquito infectivity and sporozoite production. In the humanized mouse model system that harbors human hepatocytes and human erythrocytes, the sporozoites do not progress to produce merozoites and liver stage does not transition into the blood stage. The p36 − p52 − sap1 − vaccine now awaits assessment of safety, effective induction of immune responses and efficacy against infectious wild-type sporozoites.
Novel vaccine candidates, synthetic MSP-1 and SEA-1
Two new antigens identified are as follows: SEA-1, a protein required for egress of schizonts from erythrocytes; and MSP-1, a merozoite surface protein. Synthetic antigen consisting only of the N- and C-terminal regions of MSP-1 expressed in the ciliate Tetrahymena thermophila as a recombinant protein elicits antibodies in mice which provided protection against lethal malaria (Cowan et al. 2014). rPf SEA-1 vaccinated Tanzanian children did not experience severe malaria. Similarly, Kenyan adolescents and adults possessing antibodies against rPf SEA-1 had lower parasite densities than people who did not produce these antibodies (Raj et al. 2014). The two antigens may be used together with others such as RTS,S and p36p52sap1 vaccines.
AMA1-based vaccines
Apical membrane antigen 1 (AMA1) is a microneme protein of P. falciparum present in both sporozoites and merozoites of the parasite. It is essential for the invasion of hepatocytes by sporozoites and of red blood cells by merozoites. Antibodies against AMA1 block the multiplication of parasites in both the liver and blood stages of the parasite’s life cycle in human host (Dutta et al. 2013). One of the vaccines based on AMA1 which is undergoing phase 2 trials in Mali is FMP 2.1/A502A. In this vaccine, AMA1 has been resourced from the corresponding gene of the field strain 3D7 of the P. falciparum parasite. The recombinant form of AMA1 protein called FMP2.1 has been formulated in the A502A adjuvant system. Children of the age group 1–6 years were administered the vaccine at 0, 1 and 2 months and followed for 1 year. The vaccine proved to be safe and well tolerated; it induced and sustained high levels of antibodies against AMA1 in malaria-exposed children. FMP2.1/A502A, is now undergoing phase 2 trial in Mali. If it is found successful in phase 2, FMP2.1/A502A may be used in combination with RTS,S vaccine to achieve additive or synergistic effects (Thera et al. 2012).
AMA1 demonstrates considerable variability among P. falciparum field isolates. Therefore, there is doubt on its success to stop malaria caused by P. falciparum bearing AMA1 alleles genetically diverse from 3D7 type of parasites. Dutta et al. (2013) have developed AMA1-based Quadvax or QV vaccine, a mixed allele vaccine. Here, AMA1 proteins from four strains of P. falciparum namely 3D7, FVO, HB3 and W2mef have been combined to serve as antigen. Antibodies formed against Quadvax were inhibitive to a total of 26 parasite strains which perhaps represented global AMA1 diversity. In an early trial, Quadvax provided 100 % protection. Now, to further improve the efficacy of the AMA1 vaccine, to cover all of the polymorphisms in its structure in natural populations of the parasite, the region of AMA1 that interacts with RON2 protein of the parasite, which is perhaps conserved, has been used in vaccine construction (Dutta et al. 2013; Srinivasan et al. 2014). The AMA1 and RON2 complex injected into mice protected the vaccinated mice from lethal malaria (Srinivasan et al. 2014).
AMA1 in its various forms, RTS,S and other vaccines offer promise of affordable vaccines to eradicate malaria. They may be used in combination to harvest their additive or synergistic antibody responses.
Increased genetic variation in parasite, absence of vaccine and resistance towards approved drugs necessitate discovery of new antimalarials
In recent years, studies have compared the genetic structure of P. falciparum populations occurring in Africa, America (Oceania) and Asia (Southeast Asia), especially in the context of emergence of ART resistance in Southeast Asia. Van Tyne et al. (2011) investigated polymorphism for 17,000 single nucleotide polymorphic (SNP) markers in 57 culture-adapted parasites from representative countries of the three continents. Manske et al. (2012) genotyped parasites present in 227 blood samples of malaria patients of six countries of Southeast Asia and Africa, for polymorphism at 86,158 SNPs. Takala-Harrison et al. (2013) examined 290 parasite samples from Bangladesh, Thailand and Cambodia for polymorphism at 8,079 SNPs. Miotto et al. (2013) studied 825 parasite samples, from ten locations in several countries in Africa and from Thailand, Vietnam and four locations in Cambodia, for polymorphism at 86,158 SNPs. Ariey et al. (2014) by screening several hundred (n = 886) blood samples of malaria patients found 17 different alleles of the gene PF3D7_1343700 to be responsible for artemisinin resistance in the studied Cambodian population. The observations from the above-mentioned studies altogether indicate the following about P. falciparum malaria: (a) There are inter-continental genetic differences in the Plasmodium populations since the principal component analysis showed that the parasites clustered together continent-wise. Intra-continental genetic variation-wise, the parasites fell in the following order Africa > Asia > America (van Tyne et al. 2011). These observations are consistent with the origin of P. falciparum being in Africa and its presence in other continents a result of independent migration of and founding of subpopulations. Very high levels of genetic variability in Africa are related to high levels of malaria transmission there (van Tyne et al. 2011). On account of high transmission, humans infected by sporozoites of different genotypes by a mosquito or more than one mosquito will produce gametocytes of many different genotypes which upon transmission to mosquito will produce gametes that upon recombination will amplify the genetic variability further. (b) In Cambodia and adjoining countries, a high incidence of selection of ART resistance imparting recessive mutations suggests a high level of inbreeding and hypermutability in parasites (Miotto et al. 2013). It is reported that many human communities live there in small villages with little interaction (Takala-Harrison et al. 2013). The hypermutability may be due to the genetic stress imposed by malaria drugs leading to hypomethylation of genomic DNA (discussed in an earlier section). (c) The inter- and intra-continental genetic diversity, mutability imposed by drug pressure and high recombination rate (17 kb/CM; Su et al. 1999) are continuously increasing divergence between parasites at different locations. The growing genetic variability is creating obstacles in the form of new antigenic variation for the formulation of a globally effective vaccine. These concepts are diagrammed in Fig. 7.
It appears that until effective vaccine(s) can be developed, the control of malaria with the use of new antimalarials is an essentiality (Dondorp et al. 2010a, b; Wells 2010).
Search for new antimalarials in the background of artemisinin resistance
In recent years, the ongoing malaria control programme got derailed because of the emergence of resistance in malaria parasites to ART; mosquito vectors of malaria had already developed resistance to insecticides. Artemisinin in ACTs had been performing two functions: it, along with an accompanying compound, cured parasitaemia in infected humans by killing the asexual blood stage parasites and it also killed gametocytes present in the blood (especially male gametocytes) and thereby reduced the occurrence of new infections. Post-ART resistance, the malaria control strategy has been resurrected. In the absence of an efficacious vaccine, it seeks antimalarials that will target sexual (sporogony) stages of the parasite in mosquito and the parasite’s asexual schizogonous stages in the liver and blood and sexual blood stage in humans. These four kinds of antimalarial activities are sought in one compound or in combination of two or three compounds. Most importantly, all of the new generation antimalarials must target strongly the asexual blood stages of parasites resistant or sensitive to the first-generation antimalarials; they must cure malaria and must be safe and selective against parasite. The concepts, approaches and progress being made in the search for the desired kinds of antimalarials have been reviewed by a number of groups including the following: Gamo et al. (2010), Guiguemde et al. (2010), Aguiar et al. (2012), Derbyshire et al. (2012b), Delves et al. (2012b, 2013), Kirkpatrick (2012), Ghosh (2013), Flannery et al. (2013a), Klein (2013) and Sun et al. (2014).
Campaigns to identify new antimalarials
A variety of in vitro and in vivo tests are available to find out whether or not a given compound has antimalarial activity. Some of the in vitro tests can be practised as throughput screens on 96, 384 or higher number of well plates. In the conventional type in vitro screens, the asexually replicating parasites in culture (Trager and Jensen 1976) synchronized for ring stage are exposed to a specific dose of a test compound and the effect is monitored variously at the schizont stage, in terms of survival of parasites (by the use of optical microscopy), incorporation of [3H] hypoxanthine (by measuring the radioactivity), amplification of DNA (by use of PCR), measurement of lactate dehydrogenase or histidine-rich 2 (HRP2) protein [by the use of enzyme-linked immunosorbent assay (ELISA)] or confocal fluorescent image counting following exposure to DNA intercalating dye (Flannery et al. 2013a, b; Nogueira and Estolio do Rosano 2010), in comparison to control. A variant form of the above in vitro assay uses transgenic P. berghei that expresses green fluorescent protein (GFP)-linked luciferase (GFP:LUC) in place of normal P. berghei or P. falciparum parasites. The luciferase activity measured by bioluminescence in the lysates of treated and control parasites tells whether the tested compound is antimalarial (Lin et al. 2013).
On the compounds that are found to be positive in in vitro test(s), the in vivo therapeutic efficacy tests are performed, either in mouse-P. berghei or in rhesus monkey-Plasmodium cynomolgi model systems, to determine half maximal inhibitory (IC50) concentration. Such a test can also be done on P. falciparum-infected immune-deficient mice (Jimenez-Diaz et al. 2009). In these assays, parasite growth is measured by optical microscopy or other sensitive methods such as PCR (Flannery et al. 2013a, b). The in vivo drug luminescence (IVDL) assay makes use of GFP:LUC transgenic P. berghei infections in rodents. In these 4 day suppressive drug tests, the in vivo presence of parasites is quantified by luciferase activity in samples of animal tail blood (Lin et al. 2013). The human-P. falciparum system is used to perform ex vivo assays. Parasites taken from a patient are brought to ring stage and exposed to a known amount of the test compound and the effect is monitored in terms of parasites growing into schizonts (Witkowski et al. 2013).
Using one or more of the screening procedures outlined above, several campaigns, to find molecules inhibitive to the asexual blood stage (ABS) of malarial parasites, have been accomplished and a few are in progress. Some new campaigns may arise from new methods for synthesizing compounds of diverse structures (Heidebrecht et al. 2012), such as by the use of reactions of diazo compounds (Karageorgis et al. 2014). The St. Jude Children’s Research Hospital (Guiguemde et al. 2010), Genome of Institute of the Novartis Research Foundation (Plouffe et al. 2008) and GlaxoSmithKline Tres Cantos (Gamo et al. 2010) have altogether screened more than four million compounds. The Medicines for Malaria Venture (MMV) continues to test the chemical libraries assembled by sundry biotechnological and pharmaceutical companies. Thus far, more than 25,000 compounds have been found to be active against ABS of malarial parasites. This voluminous portfolio of antimalarials has been open to identify the ones whose targets in parasites are different from those against which resistance has arisen and those that are active against all of the four developmental stages of parasite life cycle in man and mosquito. To share with the researchers the compounds of some promise, an open source Malaria Box of 200 drug-like and 200 probe-like compounds has been formed. The participants of this campaign are expected to conduct studies on each compound’s metabolic properties; effects on different stages of Plasmodium life cycle for the identification of cellular and molecular targets; cellular, tissue and organ toxicity; in vivo pharmacokinetics and activity against variants of malaria causing Plasmodium species. etc.; and share the results (Spangenberg et al. 2013).
It will be seen from Table 1 which lists the properties of antimalarials in clinical practice and Table 3 which lists antimalarial molecules undergoing clinical trials that majority of the identified antimalarials target hemoglobin degradation or heme detoxification pathways [amodiaquine, piperaquine, pyronaridine, quinine, endoperoxides (including OZ-439), NPC-1161B and tafenoquine], folate pathway (pyrimethamine, P218) or mitochondrial pathways (primaquine, atovaquone, proguanil, DSM265, ELQ300). Only albitiazolium, GNF156, methylene blue and NITD 609 target pathways distinct from the above-mentioned. All of the drugs in Tables 1 and 3 got selected because of their pronounced parasitocidal activity on intraerythrocytic asexual stage of parasite replication. Table 4 gives preliminary properties of 26 antimalarial molecules that have proven to be promising in preclinical evaluation experiments. Majority of the molecules in Table 4 have targets distinct from those of the molecules (ACT-21365, alpha-pyrone, azithromycin, bivalent tetrazolium, cladosporin, 4-CF3 phenyl, Genz668764, hydroxy ethylamine, indolisquinolone, ketotifen, ML238, methyl benzylamide, N-myristoyltransferase (NMT) inhibitors, NSC-158011, nutilin-3, phenyl propanoid conjugated iridoid, strictosamide, torin-2) in Tables 1 and 3. The ongoing work on the characterization of the biological effects of the new molecules of variant scaffolds, in search of new clinical series with a wider range of activities against parasite stages in infected individuals and mosquitoes, is bound to enlarge the chemical series represented in Tables 1, 3 and 4.
New liver stage malaria drugs
The liver stage develops from invasion of hepatocytes by a small number of sporozoites released into the bloodstream via a bite from an infected mosquito. The sporozoites in hepatocytes mature and undergo one cycle of replication to form liver stage schizonts. Clinical symptoms develop when liver schizonts release merozoites into erythrocytes. Since only a small number of hepatocytes initially get infected, the parasite load is low despite long residence time; therefore, liver stage is asymptomatic, but it is a critical drug target for interrupting further progression of life cycle events of parasite in infected human and for lowering the chances of resistance development. The infecting sporozoites of P. vivax and P. ovale are able to enter a dormant phase in hepatocytes. The dormant parasites or hypnozoites that survive for many months are the cause of recurring malaria. Among the approved ABS drugs (Table 1), the combination of atovaquone and proguanil clears the parasites (of P. vivax or P. falciparum) from the liver and that of primaquine and chloroquine clears both liver stage schizonts and hypnozoites. However, primaquine has drawbacks: it is slow acting (chloroquine the companion drug is fast acting and therefore clears parasitaemia) and harms people with glucose-6-phosphate dehydrogenase (G6PD) deficiency by causing severe life-threatening adverse reaction. The mechanism by which primaquine and other antimalarials of 8-aminoquinoline family cause haemolytic anemia in G6PD-deficient people is not fully understood (Uthman et al. 2014). G6PD as a part of the pentose phosphate pathway produces the coenzyme nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is involved in the production of glutathione which protects cells against reactive oxygen damage. Reduced levels of glutathione make G6PD-deficient erythrocytes vulnerable to oxidative damage and liable to haemolysis (Cappellini and Fiorelli 2008). G6PD is coded by a 18.5 kb gene comprised of 13 exons on the long arm of the X chromosome. About 400 alleles that reduce the activity and stability of G6PD gene product are known. More than 400 million people worldwide, largely of origin in tropical Africa, tropical and subtropical Asia and Mediterranean regions, carry the deficiency. All the mutation-carrying males and some females who carry the defective gene in heterozygous condition (due to normal X-inactivation) are G6PD deficient. There is an overlap in the geographic distribution of G6PD deficiency and the spread of malaria (Howes et al. 2013; von Seidlein et al. 2013). Further research needs to provide new families of antimalarials that are safe for G6PD-deficient people and can replace 8-quinolines and rapid low-cost, high-quality field test for G6PD deficiency suitable for screening large populations.
To meet the dearth of liver stage malaria drugs, some of the recent antimalarial drug discovery campaigns have emphasized on liver stage. The success of these campaigns is largely attributable to the use of novel liver stage throughput culture assays that have complemented the hitherto available tedious animal model systems. The liver stage in vitro drug screens have several general features. Cultured primary hepatocytes or cells of hepatoma lines of human, rodent or primate origin are infected with sporozoites of different species of Plasmodium. The sporozoites dissected out of the salivary glands of mosquitoes may be fresh or cryopreserved. The infected liver cells are exposed to a known quantity of the test compound, and the treated and control cultures are allowed to grow to produce liver schizonts. At the end of incubation period, parasite cells are observed by optical microscopy, and alternately parasite growth is monitored by bioluminescence imaging, RT-PCR, fluorescence-activated cell sorting or measurement of enzyme activities (Plouffe et al. 2008; Derbyshire et al. 2011; Meister et al. 2011; Delves et al. 2012a, b). In the variant forms of the in vitro drug screens, the normal sporozoites may be replaced by transgenics carrying a fluorescent GFP:LUC gene (Derbyshire et al. 2013; La Crue et al. 2013) or a centromere (Voorberg-van der Wel et al. 2013). Both, freshly derived or cryopreserved hepatocytes can be used (Zou et al. 2013). In human hepatocytes infected with P. vivax sporozoites or monkey hepatocytes infected with P. cynomolgi sporozoites, formation of both liver large multinucleate schizonts and small uninucleate hypnozoites can be quantified (Dembele et al. 2011, 2014). Besides the above, March et al. (2013) have developed a micropatterned coculture MPCC platform. Here, cryopreserved primary hepatocytes derived from individual human donors are cocultured with the support of stroma cells. Liver cells are infected with fresh or cryopreserved P. falciparum or P. vivax sporozoites. Infected liver cells are overlaid with erythrocytes. Since the MPCC platform can be maintained for 4 to 6 weeks, the drugs can be screened against liver stage schizonts as well as hypnozoites.
In the conventional in vivo test, monkeys are infected with P. cynomolgi sporozoites followed by treatment with a compound such as chloroquine that eliminates the blood stage parasites and then the test drug is administered. Infected monkeys are monitored for several months to observe if malaria reoccurs (Schmidt 1983). This test for anti-hypnozoite drugs is complemented by a test on a rodent model for liver stage. Rodent malaria sporozoites are infected into a mouse shortly before or after the mouse has been treated with the drug under testing. The infection is visualized by estimation of luciferase (if GFP:LUC transgenic sporozoites are used) or by measuring the level of blood stage parasitemia or survival (Mwakingwe et al. 2009; Flannery et al. 2013a, b).
The in vitro and in vivo revaluation campaigns, on molecules established as asexual blood stage antimalarials, possessing sundry biological activities and drugs approved for diverse ailments, to identify molecules having liver stage antimalarial activity have designated many of them as liver stage schizonticidals, and among the latter, some as hypnozoiticidals. In Tables 3 and 4, the proven liver stage hypnozoiticidal and schizonticidal molecules having potential for development into clinical drugs or prophylactics are as follows: albitiazolium, decoquinate, ELQ300, GNF156, NITD609, NMT inhibitor (a benzothiophene), NPC-1161B, nutilin-3, tafenoquine, torin-2 and usnic acid. Most of them have different modes of inhibitory action against parasites. Besides, Zeeman et al. (2013) have observed a new molecule KAI-407 (imidazopyrazine) as much effective against hypnozoites as are tafenoquine, bulaquine (Dutta et al. 1989) and NPC-1161B (LaMontagne et al. 1982). Tinidazole (an antiprotozoal nitrogroup containing imidazole synthetic derivative; Wells 2010; Wells et al. 2010; Amit et al. 2013; Macareo et al. 2013), inidazolidinone (Wells et al. 2010) and CEM-101 solithromycin (a fluoroketolide antibiotic; Wells et al. 2010; Wittlin et al. 2012) are also found to be active against hypnozoites (Fig. 8). The liver schizonticidal antimalarials not possessing the hypnozoiticidal activity listed in Tables 3 and 4 are as follows: cladosporin, C-10 trioxane, DSM265, 4-CF3 phenyl, ketotifen, methylene blue, OZ439, P218 and sulphide-3-artesanilide. Some additional molecules that offer scope for using their liver schizonticidal activity are MMV007907 (an Atg8-Atg3 protein-protein interaction inhibitor; Hain et al. 2014), ICI 56.780 (a phenoxyethoxy quinoline), P4Q-146 and P4Q-158 (3-phenyl-4(1H)-quinolones) (Hanson et al. 2013), esmeprazole (a proton pump inhibitor that is used to treat gastroesophageal reflux disease), methylsergide (a migraine drug), salinomycin (a breast cancer drug), telmisartan (drug for management of hypertension), halofuginone (synthetic halogenated derivative of the plant Dichroa febrifuga quinazolinone alkaloid febrifugene that is a traditional Chinese antimalarial medicine) (Derbyshire et al. 2012a, b) and MMV396749 (a benzimidazole; Burrows et al. 2012) from the Medicines for Malaria Venture (Fig. 9). The bulk of the molecules listed above represents antimalarially active chemical scaffolds offering possibilities for derivation of more active and safer malaria-stopping molecules.
Stopping of transmission and treatment of malaria simultaneously
This approach targets individual, more than one or all of the developmental stages of Plasmodium in female mosquito, to thereby stop parasite transmission via drug(s) administered to people for treatment of malaria or for prophylaxis. The aim is to give a drug or drug combination to infected humans so that on the one hand formation of merozoites and gametocytes is interfered in patients and on the other hand parasite’s development in mosquito is also blocked. The drugs to be given for prophylaxis will also possess these dual properties, including action against liver stages of the parasite. Several of the early antimalarials did not possess such properties. The concept is that the drug administered to people will get passed on to the female mosquito through its blood meal. Thus, by biting to a treated person, the mosquito gut will receive parasite gametocytes as well as the drug active against subsequent development of gametocytes. The passaged antimalarial will therefore incapacitate parasites in mosquito. Mosquito stages of parasite development are now important targets in programmes of development of antimalarials, and several effective and safe compounds have been identified. The candidate compounds that are simultaneously used for the treatment of malaria as well as transmission blockers are described below.
Discovery of new transmission blocking drugs
The life cycle of Plasmodium (Fig. 1) in the human host begins with the bite by an infected mosquito for a blood meal which releases sporozoites along with some saliva into the bloodstream. The sporozoites invade the hepatocytes and develop into schizonts. The merozoites released from liver schizonts invade erythrocytes where they grow through ring and trophozoite stages into schizonts which release mitotically multiplied blood stage merozoites into the blood for their further asexual replication in erythrocytes. Some of the merozoites instead enter sexual cycle and go through five stages of gametocytogenesis, involving meiosis, to produce stage V mature male and female gametocytes. The gametocytes start to appear a few to 12 days from the appearance of malaria symptoms. They persist even after remission of malaria symptoms. The gametocytes are the only parasite cells that survive transmission from the human host to the mosquito host. A mosquito through its bite to obtain a blood meal ingest about 103–104 gametocytes. These undergo differentiation to produce gametes. In the process, male gametocytes undergo three mitotic divisions to produce exflagellated male gametes. Fusion of male and female gametes forms a zygote which differentiates into ookinete. An infected mosquito may produce 102–103 ookinetes. The motile ookinetes traverse to midgut epithelium where each of them forms an oocyst. Each oocyst upon maturation releases thousands of motile sporozoites which migrate to salivary glands. The mosquito possessing mature sporozoites releases about a dozen of them through its bite to a human, thus completing the mosquito-human-mosquito transmission cycle (Baton and Ranford-Cartwright 2005). There are two phases to block transmission: one in humans that produces mature gametocytes and other in mosquito. In mosquito, the parasite sexual stages, gametogenesis and fertilization and sporogony stages ookinete → oocyst → sporozoites are targets to block transmission. The ACT drugs reduce the transmission only partially because they are active against early stages of gametocytogenesis. At low concentrations, some of them actually increase the gametocyte burden (Peatey et al. 2012). Primaquine is the only fully gametocytocidal among the currently approved antimalarial drugs. There is an urgent need for new transmission blockers because primaquine has G6PD liability, malaria vaccines are awaited and therefore interruption of malaria transmission via drugs is necessary to control, eliminate and eradicate malaria (Alonso et al. 2011). High-throughput in vitro, semi-in vitro and in vivo reliable assays have been recently developed to test the efficacy of candidate molecules against gametocytogenesis, gametogenesis and sporogony (Roncales et al. 2012; Ojo et al. 2012).
The available in vitro procedures for testing the efficacy of candidate compounds for killing the mature gametocytes are typified by that given by Sun et al. (2014). Here, ring stage parasites are cultured conventionally to 0.1 % parasitemia. And at day 9 or 10, the culture is exposed to 50 mM N-acetylglucosamine to block further asexual growth. At day 12, the stage III–IV gametocytes are isolated by Percoll density gradient and recultured for 24 h to reach 70 % parasite cells to stages IV–V. The subculture of about 20,000 gametocytes, medium and test compound is incubated for 72 h at 37 °C in 5 % CO2 atmosphere. Then, AlamarBlue (oxido-reduction indicator) dye is added to the subculture, and after 24 h of further incubation, fluorescence is read such that the fluorescence signal has a linear relationship with surviving gametocyte number. In the procedure of Lelievre et al. (2012), Pf3D7HT-GFP gametocytes in N-acetylglucosamine-treated culture are separated by density gradient-cum-magnetic column. About 50,000 gametocytes are subcultured in the presence of test compounds for 48 h before the effect of the test drug is measured by ATP luminescence produced by oxygenation of luciferin catalyzed by luciferase or microscopically by examination of Giemsa-stained thin blood films. The assay of Roncales et al. (2012) uses D-sorbitol treatment to remove asexual parasites and induce gametocytogenesis in cultures. The procedure of Duffy and Avery (2013a, b) deploys confocal fluorescence microscopy on gametocyte-specific protein pfs 16-LUC-GFP and a viability marker MTR (Mitotracker Red CM-H2 X ROS) that allow automated image detection and quantitation of surviving gametocytes. D’Alessandro et al. (2013) in their procedure use calometrically estimated lactate dehydrogenase as measure of surviving gametocytes following drug treatment. Kaushal et al. (1980) suggest the use of 1 mM cyclic AMP in static asexual culture to achieve abundant gametocytogenesis.
The exflagellation assay uses in vitro cultures rich in mature gametocytes, such as those in Sun et al. (2014) and variant procedures. The test compound is added and exflagellation is triggered by lowering the incubation temperature to about 21 °C. Motile exflagellated cells are counted microscopically in samples withdrawn 20 min later (Sun et al. 2014). In the ookinete development assay, gametocytes are cultured in fresh medium together with the test compound for 24 h at 19 °C. Giemsa-stained ookinetes are counted microscopically (Sun et al. 2014; Delves et al. 2012a, b, 2013).
In the membrane feeding assay (FMA) of sporogony, Anopheles mosquitoes starved overnight are fed gametocytes through a parafilm membrane (Churcher et al. 2012). In the case of P. falciparum, the in vitro-produced gametocytes and test compound are subcultured in fresh medium at 37 °C for 24 h and fed to mosquitoes. Correspondingly, blood taken from P. berghei-infected mice is heparinized, added to the test compound and fed to mosquitoes. The starved mosquitoes are fed the gametocytes for 30 min at 37 °C. Engorged mosquitoes are maintained at 28 °C, 60–80 % relative humidity for 8–12 days. The mosquitoes are dissected and their midgut is stained with 0.2 % mercurochrome and the numbers of oocysts formed are counted. The in vivo sporogony assay uses P. berghei gametocytes formed in mice. Using the whole blood from mouse showing 10 % P. berghei parasitemia, mice are infected. After the mice have produced gametocytes, they are treated with drugs under testing. Such mice are anaesthesized and starved. A. stephensi mosquitoes are allowed to feed on them for 30 min. The engorged mosquitoes are maintained and examined for oocyst formation as in FMA (Blagborough et al. 2013; Delves et al. 2012a, b).
Information on activities of many putative next-generation antimalarials listed in Tables 3 and 4 with regard to malaria transmission is now known, since the above assays became available. The molecules of Tables 3 and 4 that have been found to possess significant antitransmission activity fall into three groups: (a) The following 16 compounds possess both gametocytocidal (blockading of mature gametocytes) in human blood stage and sporogonocidal (blockading of sporogony in mosquito) activities: albitiazolium, C10-trioxane, DSM265, ELQ300, 4CF3 phenyl, fosfidomycin, GNF-156, ketotifen, methylene blue, NITD-609, NMT inhibitor, NPC-1161B, OZ439, sulphide-3-artesanilide, torin-2 and usnic acid. In this group, several molecules such as ELQ300, methylene blue and usnic acid possess multiple antitransmission activities: gametocytocidal, anti-exflagellation, anti-fertilization, anti-ookinete formation and anti-oocyst development. (b) A total of six compounds possess gametocytocidal property: ACT-21365, cladosporin, decoquinate, NSC-1158011, tafenoquine and quinine dimer. (c) Pyrimethamine and mefloquine comprise the sporogonocidal group. Besides, several compounds of known biological activities have been reported to be sporogonocidal (Fig. 10). This group includes bumped kinase inhibitor (BKI)-1. This orally bioavailable compound selectively inhibits the parasite’s calcium-dependent protein kinase 4 and thereby microgametocyte exflagellation as well as oocyst formation get inhibited (Ojo et al. 2012). A number of HSP90 inhibitors (Fig. 10), mostly anticancer agents, have been found to possess sporogonocidal activity: alvespimycin (analogue of benzoquinone antibiotic geldanamycin), maduramycin (monoglycoside polymer of Actinomadura rubra fungus), MMV019406, MMV666125, narasin (derivative of salinomycin from Streptomyces aurofaciens), NSC-174938 and NVP-AYU922 (isoxazole amide) (Sun et al. 2014). Plasmodium specifies about 5,500 genes. Molecules that can target any of the essential Plasmodium protein/RNA selectively are expected to possess gametocytocidal and/or sporogonocidal property.
Molecules active against asexual liver and blood stages, gametocytogenesis and sporogony
Now that assays are available for testing activities against all the four important stages of Plasmodium life cycle in human and mosquito hosts, it has been possible to identify molecules that are prophylactic against malaria as well as cure malaria. Such molecules of great interest included in Tables 1, 3 and 4 are as follows: albitiazolium, C10-trioxane, decoquinate, ELQ300, 4CF3 phenyl, GNF156, ketotifen, NITD609, NMT inhibitor, NPC-1161B, primaquine, sulphide-3-artesanilide, tafenoquine, torin-2, WEHI-916 and usnic acid. The following 11, of these 16 molecules, have been found to be active also against hypnozoites: albitiazolium, decoquinate, ELQ300, GNF156, NITD609, NMT inhibitor, NPC-1161B, primaquine tafenoquine, torin-2 and usnic acid. Among these, only primaquine and tafenoquine demonstrate G6PD liability, and others have distinct targets and represent novel chemical scaffolds. Thus, it appears that there is a good scope for developing new combination of drugs that will cure and eliminate malaria pleiotropically.
Single-dose safe drug candidates active against drug-resistant malaria to provide protection against reinfection
One of the major obstacles in malaria control programmes has been non-compliance of prescribed schedule of drug intake by patients. Underdosing has been associated with the development of resistance against the first-generation antimalarial drugs. To eradicate malaria, there is a need for drugs that should possess the following features: curing of malaria in a single dose by having high activity against liver and blood asexual stages and gametocytogenesis in humans and/or sporogony in mosquitoes; long in vivo half life; rapid onset of action against plasmodia sensitive or resistant against first-generation antimalarials; resistance to resistance development; and selectivity against parasite targets thus providing safety, especially for children and pregnant women, and least liability. The candidate molecules possessing at least some of such properties are C10-thioacetal, decoquinate, ELQ-300, GNF156, sulphide-3-artisanilide and torin-2. Molecules like decoquinate, ELQ300, GNF156 and torin-2 combined with each other and with suitable quick acting drugs should in the future provide cure as well as long-term prophylaxis against malaria and open possibilities of eradicating malaria.
Finding of targets of known antimalarials and for innovative new antimalarials
Flannery et al. (2013a, b) have reviewed the genetic methods that can be used for defining the targets of known antimalarials. The molecules listed in Tables 3 and 4 are only a small fraction (<0.15 %) of the total already shown to be active against asexual blood stage of malaria parasites. Many of the molecules remaining to be characterized for their biological properties are expected to be active against multiple stages of human and mosquito parts of life cycles of different malaria-causing species of Plasmodium. If the targets of such molecules, among the products of ~5,500 genes that are reported to be present in genomes of Plasmodium species, become known, then it may be possible to derive from them altered forms of molecules that are relatively more effective and safer antimalarials. A list of important targets already identified for which novel antimalarial chemical scaffolds are desired is given in Table 5, and the methodology of discovering targets of active compounds is outlined below. It is already exemplified in the section in the discovery of mutations in K13 propeller domain of the PF3D7_134700 gene associated with the development of resistance against artemisinin. The procedure involves in vitro evolution of parasite lines resistant to an antimalarial. Replicated cultures containing 2 × 109 cells are exposed stepwise to increasing levels of an antimalarial starting from IC50 of the parent line and reaching to 2–5 × IC50 or in single step at 2–10 × IC50 concentration. The compound-added cultures are grown for 12 weeks. The presence of live and growing parasites in them is indicative of evolvement of resistance in the culture. Independent isolates from replicated cultures exposed to low and high concentrations of the antimalarial are purified and rechecked for the degree of resistance. The parental and resistant lines are whole genome sequenced to identify changes in the genome of resistant lines. Common mutations in sequences of resistant lines recovered from replicates, identified by whole genome resequencing, are held responsible for resistance development (Langmead et al. 2009; Li et al. 2008, 2009; Samarakoon et al. 2011; Bopp et al. 2013). Usually, resistance is found associated with non-synonymous base substitutions or small deletions in the promoter or structural parts of specific gene(s) or changes in a number of active copies of specific gene(s). Mutation in the promoter of a gene may be the cause of under- or over-expression and that in the structural parts may cause abolition or change in the activity of the gene product. The presence of gene in the decreased or increased number of copies results in under- or over-production of the gene product. Characterization of the life cycle of a mutant in model system(s) identifies the phenotype of the mutation. Reverse genetic analysis also contributes to phenotype description (Nkrumah et al. 2006; Yu et al. 2008; Straimer et al. 2012). Differential stage-specific transcriptomic and proteomic analyses on separated parasites of in vivo-/in vitro-grown mutants and bioinformatics too help in the revelation of the phenotypic properties of mutants. In vitro interaction studies between target macromolecules of the parasite and drug molecule will allow derivation/designing of new molecules. This area of research is in its infancy, and emphasis on it is bound to make antimalarial molecules effective in the control/elimination/eradication of malaria.
Concluding remarks
The significant reduction in malaria cases from 515 million in 2002 to 219 million in 2013 is thought to be fragile in view of the emergence and spread of resistance against artemisinin, the principal drug of ACT that was combating malaria, in Southeast Asia. The migration of ART-resistant parasites to other malaria endemic countries requires immediate countermeasures. The perception of possible retreat in malaria control programme has reinvigorated research interest in multiple fronts of human-parasite-mosquito interactions. In the above discussion, some of the important recent progress and avenues of new research have been delineated. Virtues and deficiencies of the putative antimalarial vaccines RTS,S and AMA1 make their synergetic use in the future possible. The new malaria diagnostic technique REEAD is both time and cost effective for usage in field conditions. The DNA marker identified in ART-resistant parasites will complement REEAD in tracking of artemisinin-resistant malaria patient for their treatment to block further spread of such malaria. Progress towards blocking of malaria transmission includes discovery of mosquito repellents that are manifold more effective than DEET, construction of bacteria-infected mosquitoes in which the parasite is killed, suggested use of ivermectin molecules in ACTs to cure malaria as well as kill mosquitoes that may bite treated patients and discovery of antimalarial molecules of long half life for use as prophylactics. Development of imaginative high-throughput screens have provided a vast library of antimalarial molecules as well as means to discover drugs that singly or in combination are able to cure simple as well as relapsing malaria by activity against liver and blood stages of parasite in human and annulling sporozoite production in mosquitoes, thus blocking transmission of malaria between humans and also serving as prophylactic against reinfections in the immediate future. Genome sequencing and annotation and proteomic, biochemical and biophysical and chemical technologies now offer means for discovering antimalarials against specific parasite gene products, allowing the synthesis of innovative molecules that will be safe, cure malaria, stop parasite transmission and thus help in the achievement of the projected goal of eradicating malaria by 2035.
References
Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D’Alessandro U (2011) Quinine, an old antimalarial drug in a modern world: role in the treatment of malaria. Malar J 10:144
Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A et al (2011) Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A 108(47):E1214–E1223
Agarwal A, McMorrow M, Onyango P, Otieno K, Odero C, John Williamson J, Kariuki S et al (2013) A randomized trial of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated malaria among children in Western Kenya. Malar J 12:254
Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BGNO, Kabwende AL et al (2012) Phase 3 trial of RTS, S/ASO 1 malaria vaccine in African infants. N Engl J Med 367:2284–2295
Aguiar AC, Rocha EM, Souza NB, França TC, Krettli AU (2012) New approaches in antimalarial drug discovery and development: a review. Mem Inst Oswaldo Cruz 107(7):831–845
Agusto FB, Del Valle SY, Blayneh KW, Ngonghala CN, Goncalves MJ, Li N, Zhao R, Gong H (2013) The impact of bed-net use on malaria prevalence. J Theor Biol 320:58–65
Alexander M, Jacobine AM, Mazzone JR, Slack RD, Tripathi AK, Sullivan DJ, Posner GH (2012) Malaria-infected mice live until at least day 30 after a new artemisinin-derived thioacetal thiocarbonate combined with mefloquine are administered together in a single, low, oral dose. J Med Chem 55(17):7892–7899
Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK et al (2011) A research agenda to underpin malaria eradication. PLoS Med 8(1):e1000406
Alphey L (2014) Genetic control of mosquitoes. Ann Rev Entomol 59:205–224
Amambua-Ngwa A, Tetteh KK, Manske M, Gomez-Escobar N, Stewart LB, Deerhake ME, Cheeseman IH et al (2012) Population genomic scan for candidate signatures of balancing selection to guide antigen characterization in malaria parasites. PLoS Genet 8(11):e1002992
Amit A, Rawat DS, Rawat MSM (2013) 5-Nitroimidazole derivatives: a scope for medicinal chemists. Res J Chem Soc 3(7):104–113
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A, Khim N, Kim S et al (2014) A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505(7481):50–55
Auparakkitanon S, Chapoomram S, Kuaha K, Chirachariyavej T, Wilairat P (2006) Targeting of hematin by antimalarial pyronaridine. Antimicrob Agents Chemother 50(6):2197–2200
Banek K, Lalani M, Staedke SG, Chandramohan D (2014) Adherence to artemisinin-based combination therapy for the treatment of malaria: a systematic review of evidence. Malar J 13:7
Barker RH Jr, Banchongaksorn T, Courval JM, Suwonkerd W, Rimwungtragoon K, Wirth DF (1992) A simple method to detect Plasmodium falciparum directly from blood samples using the polymerase chain reaction. Am J Trop Med Hyg 46(4):416–426
Barker RH Jr, Urgaonkar S, Mazitschek R, Celatka C, Skerlj R, Cortese JF, Tyndall E et al (2011) Aminoindoles, a novel scaffold with potent activity against Plasmodium falciparum. Antimicrob Agent Chemother 55(6):2612–2622
Baton LA, Ranford-Cartwright LC (2005) Spreading the seeds of million-murdering death: metamorphoses of malaria in mosquito. Trends Parasitol 21(12):573–580
Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, Hoffman SL (1994) Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 343(8897):564–568
Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ (2008) Integrated vector management for malaria control. Malar J 7(Suppl 1):S4
Beier JC, Muller GC, Gu W, Arheart KL, Schlein Y (2012) Attractive toxic sugar bait (ATSB) methods decimate populations of malaria vectors in environments regardless of the local availability of flavoured sugar-source blossoms. Malar J 11:31
Bernier U (2013) Towards making people invisible to mosquito. 246th ACS National Meeting and Exposition, Indianapolis, Indiana, September 2013
Biamonte MA, Wanner J, Le Roch KG (2013) Recent advances in malaria drug discovery. Bioorg Med Chem Lett 23(10):2829–2843
Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X et al (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340(6133):748–751
Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D (2011) The antimalarial ferroquine: from bench to clinic. Parasite 18(3):207–214
Blagborough AM, Delves MJ, Ramakrishnan C, Lal K, Butcher G, Sinden RE (2013) Assessing transmission blockade in Plasmodium spp. Methods Mol Biol 923:577–600
Bobenchick AM, Witola WH, Augagneur Y, Nic Lochlainn L, Garg A, Pachikara N, Choi JY, Zhao YO et al (2013) Plasmodium falciparum phosphoethanol amine methyltransferase is essential for malaria transmission. Proc Natl Acad Sci U S A 110(45):18262–18267
Bopp SER, Manary MJ, Bright AT, Johnston GL, Dharia NV, Luna FL, McCormack S, Plouffe D et al (2013) Mitotic evolution of Plasmodium falciparum shows a stable core genome but recombination in antigen families. PLoS Genet 9(2):e1003293
Brunner R, Aissaoui H, Boss C, Bozdech Z, Brun R, Corminboeuf O, Delahaye S et al (2012) Identification of a new chemical class of antimalarials. J Infect Dis 206(5):735–743
Brunner R, Ng CL, Aissaoui H, Akabas MH, Boss C, Brun R, Callaghan PS et al (2013) UV-triggered affinity capture identifies interactions between the Plasmodium falciparum multi drug resistant protein 1 (PfMDR1) and antimalarial agent in lives parasitized cells. JBC 288(31):22576–22583
Burrows J, Kowalczyk P, McDonald S, Spangenberg T, Wells T, Willis P (2012) The Malaria Box: a catalyst for drug discovery. Malar J 11:P136
Burrows JN, van Huijsduijnen RH, Mohrle JJ, Oeuvray C, Wells TNC (2013) Designing the next generation of medicines for malaria control and eradication. Malar J 12:187
Caldarelli SA, Boisbrun M, Alarcon K, Hamze A, Ouattara M, Salom-Roig X, Maynadier M, Wein S, Peyrottes S, Pellet A, Calas M, Vial H (2010) Exploration of potential prodrug approach of the bis-thiazolium salts T3 and T4 for orally delivered antimalarials. Bioorg Med Chem Lett 20(13):3953–3956
Caldarelli SA, Hamel M, Duckert JF, Ouattara M, Calas M, Maynadier M, Wein S, Perigaud C, Pellet A, Vial HJ, Peyrottes S (2012) Disulfide prodrugs of albitiazolium (T3/SAR97276): synthesis and biological activities. J Med Chem 55(10):4619–4628
Caldarelli SA, Fangour SE, Wein S, van Ba CT, Perigaud C, Pellet A et al (2013) New bis-thiazolium analogues as potential antimalarial agents: design, synthesis and biological evaluation. J Med Chem 56(2):496–509
Calderon F, Vidal-Mas J, Burrows J, de la Rosa JC, Jimenez-Diaz MB, Mulet T, Prats S et al (2012) A divergent SAR study allows optimization of a potent 5-HT2c inhibitor to a promising antimalarial scaffold. ACS Med Chem Lett 3(5):373–377
Cappellini MD, Fiorelli G (2008) Glucose-6-phosphate dehydrogenase deficiency. Lancet 371(9606):64–74
Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, Drakeley C, Alonso P, Foy BD (2013) Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J 12:153
Chandra RS, Orazem J, Ubben D, Duparc S, Robbins J, Vandenbroucke P (2013) Creative solutions to extraordinary challenges in clinical trials: methodology of a phase III trial of azithromycin and chloroquine fixed-dose combination in pregnant women in Africa. Malaria J 12:1–8
Chang C, Lin-Hua T, Jantanavivat C (1992) Studies on a new antimalarial compound: pyronaridine. Trans R Soc Trop Med Hyg 86(1):7–10
Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FCK et al (2011) Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci U S A 108:4400–4405
Chatterjee A (2011) Discovery of novel antimalarials through cell-based medicinal chemistry optimization of HTS hits. Antimal Meeting, March 15–16 2011, Royal College of Surgeons, London, UK
Cheeseman IH, Miller BA, Nair S, Nkhoma S, Tan A, Tan JC, Al Saai S et al (2012) A major genome region underlying artemisinin resistance in malaria. Science 336(6077):79–82
Chen S, Luetje CW (2013) Phenylthiophenecarboxamide antagonists of the olfactory receptor co-receptor submit from mosquito. PLoS ONE 8(12):e84575
Chiyaka C, Tatem AJ, Cohen JM, Gething PW, Johnston G, Gosling R, Laxminarayan R, Hay SI, Smith DL (2013) Stability of malaria elimination. Science 339(6122):909–910
Choi HJ, Cui M, Li DY, Song HO, Kim HS, Park H (2013) Anti-malarial activity of N-acetyl-L-leucyl-L-leucyl-L-norleucinal (ALLN) derivatives against Plasmodium falciparum. Bioorg Med Chem Lett 23(5):1293–1296
Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, Biswas S, Da DF, Cohuet A, Sinden RE et al (2012) Measuring the blockade of malaria transmission—an analysis of the standard membrane feeding assay. Int J Parasitol 42(11):1037–1044
Ciana CL, Siegrist R, Aissaoui H, Marx L, Racine S, Meyer S, Binkert C, de Kanter R, Fischli C, Wittlin S, Boss C (2013) Novel in vivo active anti-malarials based on a hydroxy-ethyl-amine scaffold. Bioorg Med Chem Lett 23(3):658–662
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B (2012) Malaria resurgence: a systematic review and assessment of its causes. Malar J 11:122
Congpuong K, Saejeng A, Sug-Aram R, Aruncharus S, Darakapong A, Meshnick SR, Satimai W (2012) Mass blood survey for malaria: pooling and real-time PCR combined with expert microscopy in north-west Thailand. Malar J 11:288
Cooper RA, Lane KD, Deng B, Mu J, Patel JJ, Wellems TE, Su X, Ferdig MT (2007) Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol Microbiol 63(1):270–282
Cordel H, Cailhol J, Matheron S, Bloch M, Godineau N, Consigny PH, Gros H et al (2013) Atovaquone-proguanil in the treatment of imported uncomplicated Plasmodium falciparum malaria: a prospective observational study of 553 cases. Malar J 12:399
Cordray MS, Richards-Kortum RR (2012) Emerging nucleic acid-based tests for point-of-care detection of malaria. Am J Trop Med Hyg 87(2):223–230
Coteron JM, Marco M, Esquivias J, Deng X, White KL, White J, Koltun M, El Mazouni F et al (2011) Structure-guided optimization of triazolo-pyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J Med Chem 54(15):5540–5561
Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG (2013) The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 382(9895):900–911
Cowan GJM, Bockau U, Eleni-Muus J, Aldag I, Samuel K, Creasey AM, Hartmann MWW, Cavanagh DR (2014) A novel malaria vaccine candidate antigen expressed in Tetrahymena thermophila. PLoS ONE 9(1):e87198
Crockett M, Kain KC (2007) Tefenoquine: a promising new antimalarial agent. Expert Opin Invest Drugs 16(5):705–715
Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, Borghini-Fuhrer I, Rim HJ (2012) Review of pyronaridine antimalarial properties and product characteristics. Malar J 11:270
Cui L, Wang Z, Miao J, Miao M, Chandra R, Jiang H, Su XZ, Cui L (2012) Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol Microbiol 86(1):111–128
D’Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, Galastri L, Basilico N, Sauerwein R, Alano P, Taramelli D (2013) A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicro Chemother 68(9):2048–2058
da Cruz FP, Martin C, Buchholz K, Lafuente-Monasterio MJ, Rodrigues T, Sönnichsen B, Moreira R et al (2012) Drug screen targeted at Plasmodium liver stages identifies a potent multistage antimalarial drug. J Infect Dis 205(8):1278–1286
De Moraes CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, Mescher MC (2014) Malaria-induced changes in host odors enhance mosquito attraction. PNAS 1405617111
DeGannarro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C et al (2013) Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498(7455):487–491
Delves MJ, Ramakrishnan C, Blagborough AM, Leroy D, Wells TN, Sinden RE (2012a) A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. J Parasitol 42(11):999–1006
Delves MJ, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D (2012b) The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 9(2):e1001169
Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE (2013) Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3208–3274
Dembele L, Gego A, Zeeman AM, Franetich JF, Silvie O, Rametti A, Le Grand R et al (2011) Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS ONE 6(3):e18162
Dembele L, Franetich JF, Lorthiois A, Gego A, Zeeman AM, Kocken CHM, Le Grand R et al (2014) Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20(3):307–312
Derbyshire ER, Mota MM, Clardy J (2011) The next opportunity in antimalaria drug discovery: the liver stage. PloS Path 7(9):e1002178
Derbyshire ER, Mazitschek R, Clardy J (2012a) Characterization of Plasmodium liver stage inhibition by halofuginone. Chem Med Chem 7(5):844–849
Derbyshire ER, Prudencio M, Mota MM, Clardy J (2012b) Liver stage malaria parasites vulnerable to diverse chemical scaffolds. Proc Natl Acad Sci U S A 109(22):8511–8516
Derbyshire ER, Min J, Guiguemde WA, Clark JA, Connelly MC, Magalhaes AD, Guy RK, Clardy J (2013) Dihydroquinazolinone inhibitors of proliferation of blood and liver stage malaria parasites. Antimicrob Agents Chemother 58(3):1516–1522
Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS et al (2010) Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet 376(9754):1768–1774
Dickens JC, Bohbot JD (2013) Minireview: Mode of action of mosquito repellents. Pestic Biochem Physiol 106(3):149–155
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F et al (2009) Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467
Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A et al (2010a) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT), an open-label randomized trial. Lancet 376(9753):1647–1657
Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L (2010b) Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280
Dormoi J, Pascual A, Briolant S, Amalvict R, Charras S, Baret E, Des Etages H, Feraud M, Pradines B (2012) Proveblue (methylene blue) as antimalarial agent: in vitro synergy with dihydroartemisinin and atorvastatin. Antimicrob Agents Chemether 56(6):3467–3469
Dormoi J, Briolant S, Desgrouas C, Pradines B (2013) Efficacy of Proveblue (methylene blue) in an experimental cerebral malaria murine model. Antimicrob Agents Chemother 57(7):3412–3414
Dow GS, Gettayacamin M, Hansukjariya P, Imerbsin R, Komcharoen S, Sattabongkot J, Kyle D et al (2011) Radical curative efficacy of tafenoquine combination regimens in Plasmodium cynomolgi-infected Rhesus monkeys (Macaca mulatta). Malar J 10:212
Dubar F, Egan TJ, Pradines B, Kuter D, Ncokazi KK, Forge D, Paul JF et al (2011) The antimalarial ferroquine: role of the metal intramolecular hydrogen bond in activity and resistance. ACS Chem Biol 6(3):275–287
Dubar F, Slomianny C, Khalife J, Dive D, Kalamou H, Guerardel Y, Grellier P, Biot C (2013) The ferroquine antimalarial conundrum: redox activation and reinvasion inhibition. Angew Chem Int Ed Engl 52(30):7690–7693
Duffy S, Avery VM (2013a) Development and optimization of a novel 384-well antimalarial imaging assay validated for high-throughput screening. Am J Trop Med Hyg 86(1):84–92
Duffy S, Avery VM (2013b) Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408
Dutta GP, Puri SK, Bhaduri AP, Seth M (1989) Radical curative activity of a new 8-aminoquinoline derivative (CDRI 80 / 53) against Plasmodium cynomolgi B in monkeys. Am J Trop Med Hyg 41(6):635–637
Dutta S, Dlugosz LS, Drew DR, Ge X, Ababacar D, Rovira YI, Moch JK et al (2013) Overcoming antigenic diversity by enhancing the immunogenicity of conserved epitopes on the malaria vaccine candidate apical membrane antigen 1. PLoS Pathog 9(12):e1003840
Eastman RT, Pattaradilokrat S, Raj DK, Dixit S, Deng B, Miura K, Yuan J et al (2013) A class of tricyclic compounds blocking malaria parasite oocyst development and transmission. Antimicrob Agents Chemother 57(1):425–435
Eckhoff PA (2011) A malaria transmission-directed model of mosquito life cycle and ecology. Malar J 10:303
Edi CV, Djogbenou L, Jenkins AM, Regna K, Muskavitch MAT et al (2014) CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet 10(3):e1004236
Enayati A, Hemingway J (2010) Malaria management: past, present, and future. Annu Rev Entomol 55:569–591
Erdman LK, Kain KC (2008) Molecular diagnostic and surveillance tools for global malaria control. Travel Med Infect Dis 6(1–2):82–99
Esvelt KM, Smidler AL, Catteruccia F, Church GM (2014) Concerning RNA-guided gene drives for the alteration of wild populations. eLife. doi:10.7554/eLife.03401
Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, Bradley J et al (2014) Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomized, controlled, double blind dose-ranging trial. Lancet 14(2):130–139
Feachem RG, Phillips AA, Targett GA, Snow RW (2010) Call to action: priorities for malaria elimination. Lancet 376(9752):1517–1521
Fernando D, Rodrigo C, Rajapakse S (2011) Primaquine in vivax malaria: an update and review on management issues. Malar J 10:351
Flannery EL, Chatterjee AK, Winzeler EA (2013a) Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol 11(12):849–862
Flannery EL, David A, Fidock DA, Winzeler EA (2013b) Using genetic methods to define the targets of compounds with antimalarial activity. J Med Chem 56(20):7761–7771
Foundation for Innovative New Diagnostics, FIND (2013) Malaria rapid diagnostic tests. FIND, Geneva
Fouquet L, Hermsen CC, van Gemert G, van Braeckel E, Weening KE, Sauerwein R et al (2014) Vaccine induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J Clin Invest 124(1):140–144
Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE et al (2010) Thousands of chemical starting points for antimalarial lead identification. Nature 465(7296):305–310
Ganesan S, Tekwani BL, Tripathi LM, Nanayakkara D, Walker LA (2008) In vitro cytochrome P450 reaction phenotyping for metabolism-linked hemotoxicity of NPC1161B, a novel 8-aminoquinoline antimalarial. FASEB J 22:1131.4
Gao X, Gunalan K, Yap SSL, Preiser PR (2013) Triggers of key calcium signals during erythrocyte invasion by Plasmodium falciparum. Nat Commun 4:2862
Garcia CR, de Azevedo MF, Wunderlich G, Budu A, Young JA, Bannister L (2008) Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. Int Rev Cell Mol Biol 266:85–156
Gardiner DL, Skinner-Adams TS, Brown CL, Andrews KT, Stack CM, McCarthy JS, Dalton JP, Trenholme KR (2009) Plasmodium falciparum: new molecular targets with potential for antimalarial drug development. Expert Rev Anticancer Ther 7(9):1087–1098
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A et al (2002) Genome sequence of the human malarial parasite Plasmodium falciparum. Nature 419(6906):498–511
Gargano N, Ubben D, Tommasini S, Bacchieri A, Corsi M, Bhattacharyya PC, Rao BHK (2012) Therapeutic efficacy and safety of dihydroartemisinin-piperaquine versus artesunate-mefloquine in uncomplicated Plasmodium falciparum malaria in India. Malar J 11:233
Ghosh S (2013) A review: antimalarial drugs. Int J Pharm Eng 1(2):113–122
Global Fund (2011) List of rapid diagnostics (RDT) kits for malaria classified according to the global fund quality assurance policy. http://www.theglobalfund.org/en/procurement/policy
Goble JL, Johnson H, de Ridder J, Stephens LL, Louw A, Blatch GL, Boshoff A (2013) The druggable antimalarial target Pf DXR: overproduction strategies and kinetic characterization. Protein Pept Lett 20(2):115–124
Goncalves Y, Brannigan JA, Whalley D, Ansell KH, Saxty B, Holder AA, Wilkinson AJ, Tate EW, Leatherbarrow RJ (2012) Discovery of Plasmodium vivax N-myristoyltransferase inhibitors: screening, synthesis and structural characterization of their binding. J Med Chem 55(7):3578–3582
Graves PM, Gelband H, Garner P (2012) Primaquine for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev 9, CD008152
Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GL, Davis PH, Smithson DC (2010) Chemical genetics of Plasmodium falciparum. Nature 465(7296):311–315
Gujjar R, El Mazouni F, White KL, White J, Creason S, Shackleford DM, Deng X, Charman WN, Bathurst I, Burrows J et al (2011) Lead optimization of aryl and aralkyl amine-based triazolopyrimidine inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with antimalarial activity in mice. J Med Chem 54(11):3935–3949
Gupta AP, Chin WH, Zhu L, Mok S, Luah YH, Lim EH, Bozdech Z (2013) Dynamic epigenetic regulation of gene expression during life cycle of malaria parasite Plasmodium falciparum. PLoS Path 9(2):e1003170
Guttery DS, Ferguson DJP, Poulin B, Xu Z, Straschil U, Klop O, Solyakov L et al (2012a) A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development. PLoS Path 8(2):e1002554
Guttery DS, Poulin B, Ferguson DJP, Szoor B, Wickstead B, Carroll PL, Ramakrishnan C et al (2012b) A unique protein phosphatase with kelch-like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion. PLoS Path 8(9):e1002948
Guttery DS, Pittman JK, Frenal K, Poulin B, McFarlane LR, Slavic K, Wheatley SP et al (2013) The Plasmodium berghei Ca2+/H+ exchange, PbCAx, is essential for tolerance to environmental Ca2 during sexual development. PLoS Path 9(2):e1003191
Hain AU, Bartee D, Sanders NG, Miller AS, Sullivan DJ, Levitskaya J, Meyers CF, Bosch J (2014) Identification of an Atg8-Atg3 protein-protein interaction inhibitor from the medicines for malaria venture malaria box active in blood and liver stage Plasmodium falciparum parasites. J Med Chem 57(11):4521–4531
Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L et al (2005) A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307(5706):82–86
Hanson KK, Ressurreiçao AS, Buchholz K, Prudencio M, Herman-Ornelas JD, Rebelo M, Beatty WL et al (2013) Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc Natl Acad Sci U S A 110(30):E2838–E2847
Harris AF, McKemey AR, Nimmo D, Curtis Z, Black I, Morgan SA, Oviedo MN et al (2012) Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotech 30(9):828–830
Hay SI, Sinka ME, Okara RM, Kabaria CW, Mbithi PM, Tago CC, Benz D et al (2010) Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med 7(2):e1000209
Heidebrecht WR Jr, Mulrooney C, Austin CP, Barker RH Jr, Beaudoin JA, Cheng KC, Comer E et al (2012) Diversity-oriented synthesis yields a novel lead for the treatment of malaria. ACS Med Chem Lett 3(2):112–117
Hodel EMS, Guidi M, Zanolari B, Mercier T, Duong S, Kabanywanyi AM, Ariey F, Buclin T et al (2013) Population pharmacokinetics of mafloquine, piperaquine and artemether-lumefantrine in Cambodian and Tanzanian malaria patients. Malar J 12:235
Hoepfner D, McNamara CW, Lim CS, Studer C, Riedl R, Aust T, McCormack SL et al (2012) Selective and specific inhibition of Plasmodium falciparum lysyl-tRNA synthatase by the fungal secondary metabolite cladosporin. Cell Host Microb 11(6):654–663
Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P et al (2002) The genome sequence of the malaria mosquito Anopheles gambiae. Science 298(5591):129–149
Horrocks P, Fallon S, Denman L, Devine O, Duffy LJ, Harper A, Meredith EL, Hasenkamp S et al (2012) Synthesis and evaluation of a novel series of indoleisoquinolines as small molecule anti-malarial leads. Bioorg Med Chem Lett 22(4):1770–1773
Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI (2013) G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol 12:133–201
Hrycyna CA, Summers RL, Lehane AM, Pires MM, Namanja H, Bohn K, Kuriakose J et al (2013) Quinine dimmers are potent inhibitors of the Plasmodium falciparum chloroquine resistance in transporter and are active against quinoline-resistant P. falciparum. ACS Chem Biol 9(3):722–730
Hsiang MS, Hwang J, Kunene S, Drakeley C, Kandula D et al (2012) Surveillance for malaria elimination in Swaziland: a national cross-sectional study using pooled PCR and serology. PLoS ONE 7(1):e29550
Huang H, Lu W, Li X, Cong X, Ma H, Liu X et al (2012) Design and synthesis of small molecular dual inhibitor of falcipain-2 and dihydrofolate reductase as antimalarial agents. Bioorg Med Chem Lett 22(2):958–962
Hulden L, McKitrick R, Hulden L (2013) Average household size and the eradication of malaria. J Royal Stat Soc Ser A. doi:10.1111/rssa.12036
Jawara M, Smallegange RC, Jeffries D, Nwakanma DC, Awolola TS, Knols BGJ, Takken W, Conway DJ (2009) Optimizing odor-baited trap method for collecting mosquitoes during the malaria season in The Gambia. PLoS ONE 4(12):e8167
Jimenez-Diaz MB, Mulet T, Viera S, Gomez V, Garuti H, Ibanez J et al (2009) Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rynull mice engrafted with human erythrocytes. Antimicrob Agent Chemother 53(10):4533–4536
Johnson DJ, Fidock DA, Mungthin M, Lakshmanan V, Sidhu AB, Bray PG, Ward SA (2004) Evidence for a central role for PfCRT in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol Cell 15(6):867–877
Jones PL, Pask GM, Rinker DC, Zwiebel LJ (2011) Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci U S A 108:8821–8825
Juul S, Nielsen CJ, Labouriau R, Roy A, Tesauro C, Jensen PW, Harmsen C, Kristoffersen EL, Chiu YL (2012) Droplet microfluidics platform for highly sensitive and quantitative detection of malaria-causing Plasmodium parasites based on enzyme activity measurement. ACS Nano 6(12):106761–10683
Kafsack BFC, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE et al (2014) A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. doi:10.1038/nature12920
Kain P, Boyle SM, Tharadra SK, Guda T, Pham C, Dahanukar A, Ray A (2013) Odour receptors and neurons for DEET and new insect repellents. Nature 502(7472):507–512
Karageorgis G, Warriner S, Nelson A (2014) Efficient discovery of bioactive scaffolds by activity-directed synthesis. Nat Chem. doi:10.1038/nchem.2034
Kaushal DC, Carter R, Miller LH, Krishna G (1980) Gametocytogenesis by malaria parasites in continuous cultures. Nature 286(5772):490–492
Kaushansky A, Ye AS, Austin LS, Mikolajczak SA, Vaughan AM, Camargo N, Metzger PG, Douglass AN, MacBeath G, Kappe SH (2013) Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep 3(3):630–637
Keating GM (2012) Dihydroartemisinin/piperaquine: a review of its use in the treatment of uncomplicated Plasmodium falciparum malaria. Drugs 72(7):937–961
Kerb R, Fux R, Morike K, Kremsner PG, Gil JP, Gleiter CH, Schwab M (2009) Pharmacogenetics of antimalarial drugs: effect on metabolism and transport. Lancet Infect Dis 9(12):760–774
Kirkpatrick P (2012) Antiparasitic drugs: two-pronged tactics for malaria control. Nat Rev Drug Discov 11(1):24
Klein EY (2013) Antimalarial drug resistance: a review of the biology and strategies to delay emergence and spread. Int J Antimicrob Agents 41(4):311–317
Knipling EF (1959) Sterile male method of population control. Science 130(3380):902–904
Kumar S, Srivastava S (2005) Establishment of artemisinin combination therapy as first line treatment for combating malaria: Artemisia annua cultivation in India needed for providing sustainable supply chain of artemisinin. Curr Sci 89(7):1097–1102
Kumar S, Kumari R, Sharma V, Sharma V (2013) Roles, and establishment, maintenance and erasing of the epigenetic cytosine methylation marks in plants. J Genet 92(3):629–666
Lacroix R, McKemey AR, Raduan N, Wee LK, Ming WH, Ney TG, Siti Rahidah AA et al (2012) Open field release of genetically engineered sterile male Aedes aegypti in Malaysia. PLoS ONE 7(8):e42771
La Crue AN, Saenz FE, Cross RM, Udenze KO, Monastyrskyi A, Stein S, Mutka TS, Manetsch R, Kyle DE (2013) 4(1H)-quinolones with liver stage activity against Plasmodium berghei. Antimicrob Agents Chemother 57(1):417–424
LaMontagne MP, Blumbergs P, Strube RE (1982) Antimalarials. 14.5-(Aryloxy)-4-methylprimaquine analogues. A highly effective series of blood and tissue schizonticidal agents. J Med Chem 25:1094–1097
Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25
Lauinger IL, Vivas L, Perozzo R, Stairiker C, Tarun A, Zloh M, Xujie Zhang X, Xu H, Tonge PJ, Franzblau SG, Pham DH, Esguerra CV, Crawford AD, Maes L, Tasdemir D (2013) Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as potential target. J Nat Prod 76(6):1064–1070
Le Roch KG, Johnson JR, Ahiboh H, Chung DW, Prudhomme J, Plouffe D et al (2008) A systematic approach to understand the mechanism of action of the bisthiazolium compound T4 on the human malaria parasite Plasmodium falciparum. BMC Genomics 9:513–928
Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E (2012) Activity of chemically relevant antimicrobial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. Malar J 11(Suppl 1):P7: e35019
Lell B, Kremsner PG (2002) Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob Agents Chemother 46(8):2315–2320
Li H, Ruan J, Durbin R (2008) Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res 18(11):1851–1858
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G et al (2009) The sequence alignment / map format and SAM tools. Bioinformatics 25(16):2078–2079
Lin JW, Sajid M, Ramesar J, Khan SM, Janse CJ, Franke-Fayard B (2013) Screening inhibitors of Plasmodium berghei blood stage using bioluminescent reporter parasites. Methods Mol Biol 923:507–522
Liu W, Li Y, Shaw KS, Learn GH, Plenderleith LJ, Malenke JA, Sundararaman SA et al. (2014) African origin of the malarial parasite Plasmodium vivax. Nat Commun 5. doi: 10.1038/ncomms.4346
Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, Arthur P et al (2013) Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DEFECTIVE): a multicentre, double blind, randomized, phase 2b dose-selection study. Lancet 383(9922):1049–1058
Macareo L, Lwin KM, Cheah PY, Yuentrakul P, Miller RS, Nosten F (2013) Triangular test design to evaluate tinidazole in prevention of Plasmodium vivax relapse. Malar J 12:173
Maia MF, Moore SJ (2011) Plant-based insect repellents: a review of their efficacy, development and testing. Malar J 10(Suppl1):S11
Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, O’Brien J et al (2012) Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487(7407):375–379
Mara C, Dempsey E, Bell A, Barlow JW (2013) Synthesis and evaluation of phenoxyoxazaphospholidine, phenoxyoxazaphosphinane, and benzodioxaphosphininamine sulfides and related compounds as potential anti-malarial agents. Bioorg Med Chem Lett 23(12):3580–3583
March S, March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, Carpenter AE, Thomas D et al (2013) A microscale human liver platform that supports hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14(1):104–115
Marcsisin SR, Sousa JC, Reichard GA, Caridha D, Zeng Q, Roncal N, McNulty R, Careagabarja J et al (2014) Tafenoquine and NPC-1161 B require CYP2D metabolism for antimalarial activity: implications for the 8-aminoquinoline class of antimalarial compounds. Malar J 13:2
Marshall JM (2009) The effect of gene drive on containment of transgenic mosquitoes. J Theor Biol 258(2):250–265
Marshall JM, White MT, Ghani AC, Schlein Y, Muller GC, Beie JC (2013) Quantifying the mosquito sweet tooth: modelling the effectiveness of attractive toxic sugar baits (ATSB) for malaria vector control. Malar J 12:291
Masanja IM, Selemani M, Khatib RA, Amuri B, Kuepfer I, Kajungu D, de Savigny D et al (2013) Correct dosing of artemether-lumefantrine for management of uncomplicated malaria in rural Tanzania: do facility and patient characteristics matter? Malar J 12:446
Mazier D, Renia L, Snounou G (2009) A pre-emptive strike against malaria’s stealthy hepatic forms. Nat Rev Drug Discov 8(11):854–864
McKay PB, Peters MB, Carta G, Flood CT, Dempsey E, Bell A, Berry C et al (2011) Identification of plasmepsin inhibitors as selective antimalarial agents using ligand based drug design. Bioorg Med Chem Lett 21(11):3335–3341
McNamara CW, Lee MCS, Lim CS, Lim SH, Roland J, Nagle A, Simon O et al (2013) Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504(7479):248–253
Meister RT (1992) Farm chemicals handbook ’92. Meister Publishing Company, Willoughby
Meister S, Plouffe DM, Kuhen KL, Bonamy GMC, Wu T, Barnes SW, Bopp SE et al (2011) Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334(6061):1372–1377
Mesia K, Tona L, Mampunza MM, Ntamabyaliro N, Muanda T, Muyembe T, Cimanga K, Totte J et al (2012) Antimalarial efficacy of a quantified extract of Nauclea pobguinii stem bark in human adult volunteers with diagnosed uncomplicated falciparum malaria. Part 2. A clinical phase IIB trial. Planta Med 78(9):853–860
Mikolajczak SA, Lakshmanan V, Fishbaugher M, Camargo N, Harupa A, Kaushansky A, Douglass AN et al (2014) A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol Ther. doi:10.1038/mt.2014.85
Miotto O, Almagro-Garcia J, Manske M, MacInnis B, Campino S, Rockett KA, Amaratunga C et al (2013) Multiple populations of artemisinin-resistance Plasmodium falciparum in Cambodia. Nat Genet 45(6):648–655
Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200
Mng’ong’o FC, Sambali JJ, Sabas E, Rubanga J, Magoma J, Ntamatungiro AJ et al (2011) Repellant plants provide affordable natural screening to prevent mosquito house entry in tropical in tropical rural setting—results from a pilot efficacy study. PLoS ONE 6(10):e25927
Moehrle JJ, Duparc S, Siethoff C, van Giersbergen PL, Craft JC, Arbe-Barnes S, Charman SA et al (2013) First-in-man safety and pharmacokinetics of synthetic ozonide OZ439 demonstrates improved exposure profile relative to other peroxide antimalarials. J Clin Pharmacol 75(2):524–537
Moody A (2002) Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev 15(1):66–78
Moore BR, Page-Sharp M, Stoney JR, Ilett KF, Jago JD, Batty KT (2008) Pharmacokinetics, pharmacodynamics, and allometric scaling of chloroquine in a murine malaria model. Antimicrob Agents Chemother 55(8):3899–3907
Moorthy VS, Newman RD, Okwo-Bele JM (2013) Malaria vaccine technology road map. Lancet 382:1700–1701
Mugittu K, Abdulla S, Falk N, Masanja H, Felger I, Mshinda H, Beck HP, Genton B (2005) Efficacy of sulfadoxine-pyrimethamine in Tanzania after two years as first line drug for uncomplicated malaria: assessment protocol and implication for treatment and policy strategies. Malar J 4:55
Mukherjee P, Pradhan A, Shah F, Tekwani BL, Avery MA (2008) Structural insights into the Plasmodium falciparum histone deacetylase 1 (PfHDAC-1): a novel target for the development of antimalarial therapy. Bioorg Med Chem 16(9):5254–5265
Muller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y (2010) Successful field trial of attractive toxic sugar bait (ATSB) plant spraying methods against vectors in Anopheles gambiae complex in Mali, West Africa. Malar J 9:210
Murray CJL, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA et al (2014) Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. doi:10.1016/so140-6736(14)60844-8
Mwakingwe A, Ting LM, Hochman S, Chen J, Sinnis P, Kim K (2009) Non-invasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J Infect Dis 200(9):1470–1478
Nagle A, Wu T, Kuhen K, Gagaring K, Borboa R, Francek C, Chen Z et al (2012) Imidazolopiperazines: lead optimization of second generation antimalarial agents. J Med Chem 55(9):4244–4273
Nagraj VA, Sundaram B, Varadarajan NM, Subramani PA, Kalappa DM (2013) Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Path 9(8):e1003522
Nilsen A, LaCrue AN, White KL, Forquer IP, Cross RM, Marfurt J, Mather MW et al (2013) Quinolene-3-diarylethers: a new class of antimalarial drug. Sci Transl Med 5(177):177ra37
Nkrumah LJ, Muhle RA, Moura PA, Ghosh P, Hatfull GF, Jacobs WR Jr, Fidock DA (2006) Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods 3(8):615–621
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM (2008) Evidence of artemisinin-resistant malaria in western Cambodia. New Engl J Med 359:2619–2620
Nogueira F, Estolio do Rosano V (2010) Methods for assessment of antimalarial activity in the different phases of the Plasmodium life cycle. Rev Pan-Amaz Saude 1(3):109–124
Nsobya SL, Kiggundu M, Nanyunja S, Joloba M, Greenhouse B, Rosenthal PJ (2010) In vitro sensitivities of Plasmodium falciparum to different antimalarial drugs in Uganda. Antimicrob Agents Chemother 54(3):1200–1206
Nwakanma DC, Duffy CW, Amambua-Ngwa A, Oriero EC, Bojang KA, Pinder M, Drakeley CJ, Sutherland CJ et al (2013) Changes in malaria parasite in an endemic population over 25-year period with resulting genomic evidence of selection. J Infect Dis 209(7):1126–1135
Ojo KK, Pfander C, Mueller NR, Burstroem C, Larson ET, Bryan CM, Fox AM et al (2012) Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest 122(6):2301–2305
Okumu FO, Madumla EP, John AN, Lwetoijera DW, Sumaye RD (2010) Attracting, trapping and killing disease-transmitting mosquitoes using odour baited stations—the Ifakara odor-baited stations. Parasite Vectors 3:12
Olutu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, Lievens M et al (2013) Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N Engl J Med 368:1111–1120
Omura S (2008) Ivermectin: 25 years and still going strong. Int J Antimicrob Agents 31(2):91–98
Osei-Akoto A, Orton LC, Owusu-Ofori S (2009) Atovaquone-proguanil for treating uncomplicated malaria. Cochrane Rev. John Wiley and Sons
Oye KA, Esvelt K, Appleton E, Catteruccia F, Church G, Kuiken T, Lightfoot SBY, McNamara J, Smidler A, Collins JP (2014) Regulating gene drives. Regulatory gaps must be filled before gene drives are used in the wild. Science. doi:10.1126/science.1254287
Papakrivos J, Sa JM, Wellems TE (2012) Functional characterization of the Plasmodium falciparum chloroquine-resistance transporter (Pf CRT) in transformed Dictyostelium discoideum vesicles. PLoS ONE 7(6):e39569
Parveen A, Chakraborty A, Konreddy AK, Chakravarty H, Sharon A, Trivedi V, Bal C (2013) Skeletal hybridization and PfRIO-2 kinase modelling for synthesis of α-pyrone analogs as anti-malarial agent. Eur J Med Chem 70:607–612
Pask GM, Bobkov YV, Corey EA, Ache BW, Zwiebel LJ (2013) Blockade of insect odorant receptor currents by amiloride derivatives. Chem Senses 38(3):221–229
Patzewitz E, Patzewitz E-M, Guttery DS, Poulin B, Ramakrishnan C, Ferguson DJP, Wall RJ et al (2013) An ancient protein phosphatase SHLP1, is critical to microneme development in Plasmodium ookinetes and parasite transmission. Cell Rep 3(3):622–629
Peatey CL, Leroy D, Gardiner DL, Trenholme KR (2012) Antimalarial drugs: how effective are they against Plasmodium falciprum gametocytes? Malar J 11:34
Petersen I, Eastman R, Lanzer M (2011) Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett 585(11):1551–1562
Phyo AP, Nikhoma S, Stepniewska K, Ashley EA, Nair S, McGready R et al (2012) Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379(9830):1960–1966
Pillai DR, Lau R, Khairnar K, Lepore R, Via A, Staines HM, Krishna S (2012) Artemether resistance in vitro is linked to mutations in PfATP6 that also interact with mutations in PfMDR1 in travellers returning with Plasmodium falciparum interactions. Malar J 11:131
Ploemen IHJ, Prudencio M, Douradinha BG, Ramesar J, Fonager J, van Gemert GJ, Luty AJF et al (2009) Visualization and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS ONE 4(11):e7881
Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F et al (2008) In silico activity profiling reveals the mechanism of action of antimalarial discovered in a high-throughput screen. Proc Natl Acad Sci U S A 105(26):9059–9064
Plowe CV (2009) The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg 103(Suppl 1):S11–S14
Ponts N, Fu L, Harris EY, Zhang J, Chung D-W D, Crevantes MC et al (2013) Genome-wide mapping of DNA methylation in the human parasite Plasmodium falciparum. Cell Host Microbe 14(6):696–706
Posner GH, Paik I-H, Chang W, Borstnik K, Sinishtaj S, Rosenthal AS, Shapiro TA (2007) Malaria-infected mice are cured by a single dose of novel artemisinin derivatives. J Med Chem 50(10):2516–2519
Prag S, Adams JC (2003) Molecular phylogeny of the kelch-repeat superfamily reveals an expansion of BTB/kelch proteins in animals. BMC Bioinform 4:42
Prato M, Khadjavi A, Mandili G, Minero VG, Giribaldi G (2012) Insecticides as strategic weapons for malaria vector control. In: Perveen F (ed) Insecticides—advances in integrated pest management: InTech, 91–114
Qiao S, Tao S, Rojo de la Vega M, Park SL, Vonderfecht AA, Jacobs SL, Zhang DD, Wondrak GT (2013) The antimalarial amodiaquine causes autophagic lysosomal and proliferative blockade sensitizing human melanoma cells to starvation and chemotherapy induced cell death. Autophagy 9(12):2087–2102
Raj DK, Nixon CP, Nixon CE, Dvorin JD, DiPetrillo CG, Pond-Tor S, Wu HW, Jolly G, Pischel L et al (2014) Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 344(6186):871–877
Reeves RG, Denton JA, Santucci F, Bryk J, Reed FA (2012) Scientific standards and the regulation of genetically modified insects. PLoS Negl Trop Dis 6(1):e1502
Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D et al (2013) Cell-cell communication between malaria-infested red blood cells via exo-some like vesicles. Cell 153(5):1120–1133
Riley EM, Stewart VA (2013) Immune mechanism in malaria: new insights in vaccine development. Nat Med 19(2):168–178
Riveron JM, Yunta C, Ibrahim SS, Djouaka R, Irving H, Menze BD, Ismail HM et al (2014) A single mutation in the GSTe2 gene allows tracking of metabolically-based insecticide resistance in a major malaria vector. Genome Biol 15:R27
Rizzi L, Sundararaman S, Cendic K, Vaiana N, Korde R, Sinha D, Mohmmed A, Malhotra P, Romeo S (2011) Design and synthesis of protein-protein interaction mimicks as Plasmodium falciparum cysteine protease, falcipain-2 inhibitors. Eur J Med Chem 46(6):2083–2090
Roncales M, Vidal-Mas J, Leroy D, Herreros E (2012) Comparison and optimization of different methods for the in vitro production of Plasmodium falciparum gametocytes. J Parasitol Res ID927148
Rosenberg R (2008) Malaria: some considerations regarding parasite productivity. Trends Parasitol 24(11):487–491
Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B et al (2010) Spiroindolones, a potent compound class for the treatment of malaria. Science 329(5996):1175–1180
Rowland M, Boko P, Odjo A, Asidi A, Akogbeto M, N’Guessan R (2013) A new long-lasting indoor residual formulation of the organophosphate insecticide pirimiphos methyl for prolonged control of pyrethroid-resistant mosquitoes: an experimental hut trial in Benin. PLoS ONE 8(7):e69516
Rund SSC, Bonar NA, Champion MM, Ghazi JP, Houk CM, Leming MT, Syed Z, Duffield GE (2013) Daily rhythms in antennal protein and olfactory sensitivity in the malaria mosquito Anopheles gambiae. Sci Rep 3:2494
Salas PF, Herrmann C, Cawthray JF, Nimphius C, Kenkel A, Chen J, de Kock C et al (2013) Structural characteristics of chloroquine-bridged ferrocenophane analogues of ferroquine may obviate malaria drug-resistance mechanisms. J Med Chem 56(4):1596–1613
Samarakoon U, Regie A, Tan A, Desany BA, Collins B, Tan JC, Emrich SJ, Ferdig MT (2011) High-throughput 454 resequencing for allele discovery and recombination mapping in Plasmodium falciparum. BMC Genomics 12:116
Satimai W, Sudathip P, Vijaykadga S, Khamsiriwatchara A, Sawang S, Potithavoranan T et al (2012) Artemisinin resistance containment project in Thailand II: response to mefloquine-artesunate combination therapy among falciparum patients in provinces bordering Cambodia. Malar J 11:300
Schmidt LH (1983) Relationships between chemical structures of 8-aminoquinolines and their capacities for radical cure of infections with Plasmodium cynomolgi in rhesus monkeys. Antimicrob Agents Chemother 24(5):615–652
Schwartz L (2012) A review of malaria vaccines clinical projects based on the WHO rainbow table. Malar J 11:11
Seder RA, Chang L-J, Enama ME, Zephir KL, Sarwar UN, Gordon IJ et al (2013) Protection against malaria by intravenous immunization with a non-replicating sporozoite vaccine. Science 341(6152):1359–1365
Sibley CH, Ringwald P (2006) A database of antimalarial drug resistance. Malar J 5:48
Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF et al (2001) Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol 17(12):582–588
Sidhu AB, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA (2006) Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine and artemisinin. J Infect Dis 194(4):528–535
Simon N, Lasonder E, Scheuermayer M, Kuehn A, Tews S, Fischer R, Zipfel PF, Skerka C, Pradel G (2013) Malaria parasites co-opt human factor H to prevent complement-mediated lysis in mosquito midgut. Cell Host Microbe 13(1):29–41
Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA et al (2014) A cascade of DNA binding proteins for sexual commitment and development of Plasmodium. Nature. doi:10.1038/nature12970
Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Carrascal DR, Pascual M (2014) Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 343(6175):1154–1158
Slack RD, Mott BT, Woodard LE, Tripathi A, Sullivan D, Nenortas E et al (2012) Malaria-infected mice are completely cured by one 6 mg/kg oral dose of a new monomeric trioxane sulphide combined with mefloquine. J Med Chem 55(1):291–296
Sleebs BE, Lopaticki S, Marapana DS, O’Neill MT, Rajasekaran P et al (2014) Inhibition of plasmepsin V activity demonstrates its essential role in protein export, PfEMP1 display, and survival of malaria parasites. PLoS Biol 12(7):e1001897
Smallegange RC, van Gemert G, van de Vegte-Bolmer M, Gezan S, Takken W, Sauerwein RW et al (2013) Malaria infected mosquitoes express enhanced attraction to human odour. PLoS ONE 8(5):e63602
Smith PW, Diagana TT, Yeung BK (2014) Progressing the global antimalarial portfolio: finding drugs which target multiple Plasmodium life stages. Parasitol 141(1):66–76
Smithuis FM, Kyaw MK, Phe UO, van der Broek I, Katterman N, Rogers C, Almeida P et al (2013) The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J 12:363
Spangenberg T, Burrows JN, Kowalczyk P, McDonald S, Wells TNC et al (2013) The open access Malaria Box: a drug discovery catalyst for neglected diseases. PLoS ONE 8(6):e62906
Spillman NJ, Allen RJ, McNamara CW, Yeung BK, Winzeler EA, Diagana TT, Kirk K (2013) Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spironindolone antimalarials. Cell Host Microbe 13(2):227–237
Srinivasan P, Ekanem E, Diouf A, Tonkin ML, Miura K, Boulanger MJ, Long CA, Narum DL, Miller LH (2014) Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc Natl Acad Sci U S A 111(28):10311–10316
Srivastava IK, Vaidya AB (1999) A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother 43(6):1334–1339
Stanezyk NM, Brookfield JFY, Field LM, Logan JG (2013) Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PLoS ONE 8(2):e54438
Straimer J, Lee MC, Lee AH, Zeitler B, Williams AE, Pearl JR, Zhang L, Rebar EJ et al (2012) Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nat Methods 9(10):993–998
Su X, Ferdig MT, Huang Y, Huynh CQ, Liu A, You J, Wootton JC, Wellems TE (1999) A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science 286(5443):1351–1353
Sullivan DJ Jr, Matile H, Ridley RG, Goldberg DE (1998) A common mechanism for blockade of heme polymerization by antimalarial quinolones. J Biol Chem 273(47):31103–31107
Sun W, Tanaka TQ, Magle CT, Huang W, Southall N, Huang R, Dehdashti SJ, McKew JC et al (2014) Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep 4:3743
Supan C, Mombo-Ngoma G, Dal-Bianco MP, Ospina Salazar CL, Issifou S, Mazuir F, Filali-Ansary A, Biot C et al (2012) Pharmacokinetics of ferroquine, a novel 4-aminoquinoline, in asymptomatic carriers of Plasmodium falciparum infections. Antimicrob Agent Chem 56(6):3165–3173
Takala-Harrison S, Clark TG, Jacob CG, Cummings MP, Miotto O, Dondorp AM, Fukuda MM et al (2013) Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci U S A 110(1):240–245
Tamura S, Kubata BK, Syamsurizal IS, Horii T, Taba MK, Murakami N (2010) New anti-malarial phenylpropanoid conjugated iridoids from Morinda morindoides. Bioorg Med Chem Lett 20(5):1520–1523
Tanaka TQ, Williamson KC (2011) A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol 177(2):160–163
Tarning J, Rijken MJ, McGready R, Phyo AP, Hanpithakpong W, Day NP, White NJ, Nosten F, Lindegardh N (2012) Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob Agents Chemother 56(4):1997–2007
Tekwani BL, Walker LA (2006) 8-Aminoquino lines: future role as antiprotozoal drugs. Curr Opin Infect Dis 19(6):623–631
Tesh RB, Guzman H (1990) Mortality and infertility in adult mosquitoes after the injection of blood containing ivermectin. Am J Trop Med Hyg 43(3):229–233
Thailayil J, Magnusson K, Godfray HCJ, Crisanti A, Catteruccia F (2011) Spermless males elicit large-scale female responses to mating in the malaria mosquito gambiae. Proc Natl Acad Sci U S A 108(33):13677–13681
The RTS,S Clinical Trials Partnership (2014) Efficacy and safety of the RTS,S/ASO1 malaria vaccine during 18 months after vaccination. A phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. doi:10.1371/journal.pmed.1001685
Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Kone AK, Guindo AB et al (2012) Safety and immunogenicity of a AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS ONE 5(2):e9041
Touhara K, Vosshall B (2009) Sensing odorants and pheromones with chemosensory receptors. Ann Rev Physiol 71:307–332
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193(4254):673–675
Uthman OA, Saunders R, Sinclair D, Graves P, Gelband H, Clarke A, Garner P (2014) Safety of 8-aminoquinolines given to people with G6PD deficiency: protocol for systematic review of prospective studies. BMJ Open 4:e004664
van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK et al (2012) The spiroindolone drug candidate NITD 609 potentially inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother 56(7):3544–3548
Van Tyne D, Park DJ, Schaffner SF, Neafsey DE, Angelino E, Cortese JF, Barnes KG et al (2011) Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum. PLoS Genet 7(4):e1001383
Vera IM, Beatty WL, Sinnis P, Kim K (2011) Plasmodium protease ROM1 is important for proper formation of parasitophorous vacuole. PLoS Path 7(9):e1002197
Vial HJ, Wein S, Farenc C, Kocken C, Nicolas O, Ancelin ML, Bressolle F, Thomas A, Calas M (2004) Prodrugs of bisthiazolium salts are orally potent antimalarial. Proc Natl Acad Sci U S A 101(43):15458–15463
Vlahakis JZ, Mitu S, Roman G, Patricia Rodriguez E, Crandall IE, Szarek WA (2011) The antimalarial activity of bivalent imidazolium salts. Bioorg Med Chem 19(21):6525–6542
von Seidlein L, Auburn S, Espino F, Shanks D, Cheng Q, McCarthy J, Baird K, Moyes C et al (2013) Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J 12:112
Voorberg-van der Wel A, Zeeman AM, van Amsterdam SM, van den Berg A, Klooster EJ, Iwanaga S, Janse CJ et al (2013) Transgenic fluorescent Plasmodium cynamolgii liver stages enable live imaging and purification of malaria hynozoite forms. PLoS ONE 8(1):e54888
Wang S, Gosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M (2012) Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci U S A 109(31):12734–12739
Wang X, Dong Y, Wittlin S, Charman SA, Chiu FC, Chollet J et al (2013) Comparative antimalarial activities and ADME profiles of ozonides (1,2,4-trioxolanes) OZ277, OZ439, and their 1,2-dioxolane, 1,2,4-trioxane, and 1,2,4,5-tetraoxane isosteres. J Med Chem 56(6):2547–2555
Wang H, Li Q, Reyes S, Zhang J, Zeng O, Zhang P et al (2014) Nanoparticle formulations of decoquinate increase antimalarial efficacy against liver stage Plasmodium infections in mice. Nanomedicine 10(1):57–65
Warhurst DC, Williams JE (1996) Laboratory diagnosis of malaria. J Clin Path 49:533–538
Wein S, Maynadier M, Bordat Y, Perez J, Maheshwari S, Bette-Bobillo P, Tran Van Ba C, Penarete-Vargas D et al (2012) Transport and pharmacodynamics of albitiazolium, an antimalarial drug candidate. Br J Pharmacol 166(8):2263–2276
Weisner J, Henschker D, Hutchinson DB, Beck E, Jomaa H (2002) In vitro and in vivo synergy of formidomycin, a novel antimalarial drug, with clindamycin. Antimicrob Agents Chemother 46(9):2889–2894
Weisner J, Reichenberg A, Hintz M, Ortmann R, Schlitzer M, Calenbergh SV et al (2013) Formidomycin as an antimalarial agent. In: Bach TJ, Rohmer MR (eds) Isoprenoid synthesis in plants and microorganisms. Springer, New York, pp 119–137
Weiwer M, Mulrooney C, Massi D, Heidebrecht R, Wiegand R, Lukens AK, Dick J et al (2012) ML238: an antimalarial small molecule of a unique structural class. PLoS Negl Trop Dis 6:e1929
Wells TNC (2010) Is the tide turning for new malaria medicines? Science 329(5996):1153–1154
Wells TN, Burrows JN, Baird JK (2010) Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol 26(3):145–151
Wengelnik K, Vidal V, Ancelin ML, Cathiard AM, Morgat JL, Kocken CH, Calas M, Herrera S, Thomas AW, Vial HJ (2002) A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295(5558):1311–1314
White NJ (2014) Malaria: a molecular marker of artemisinin resistance. Lancet 383(9927):1439–1440
White NJ, Pukrittayakamee S, Hien TT, Abul Faiz M, Mokuolu OA, Dondorp AM (2014) Malaria. Lancet 383:723–735
Whittaker MA, Dean AJ, Chancellor A (2014) Advocating for malaria elimination—learning from the successes of other infectious disease elimination programmes. Malar J 13:221
Whitten M, Mahon R (2005) Misconceptions and constraints. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, pp 601–626
WHO (2012) World malaria report. p200
WHO (2013b) Energy response to artemisinin resistance in the Greater Mekong subregion. Regional framework for action 2013–2015 www.who.int/malaria/publications/atoz/978924.1505321/en
WHO (2013a) World malaria report, www.who.int/malaria/publications/world_malaria_report_2013/report/en
Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P et al (2013) Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug response studies. The Lancet Infect Dis 13(12):1043–1049
Wittlin S, Ekland E, Craft JC, Lotharius J, Bathurst I, Fidock DA, Fernandes P (2012) In vitro and in vivo activity of solithromycin (CEM-101) against Plasmodium species. Antimicrob Agents Chemother 56(2):703–707
World Health Organisation (2011) Malaria rapid diagnostic test performance—results of WHO product testing of malaria RDTs: round 3 (2010–2011). Geneva
World Health Organization (2014) Evidence review group on malaria diagnosis in low transmission settings. Malaria Policy Advisory Committee Meeting (Session 10) Report. Geneva
Wright MH, Clough B, Rackham MD, Rangachari K, Brannigan JA, Grainger M, Moss DK et al (2014) Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach. Nat Chem 6(2):112–121
Wu T, Nagle A, Kuhen K, Gagaring K, Borboa R, Francek C, Chen Z et al (2011) Imidazolopiperazines: hit to lead optimization of new antimalarial agents. J Med Chem 54(14):5116–5130
Yeh E, De Risi JL (2011) Chemical rescue of malaria parasite lacking an apicoplast defines organelle function in blood stage Plasmodium falciparum. PLoS Biol 9(8):e1001138
Yeung BK, Zou B, Rottmann M, Lakshminarayana SB, Ang SH, Leong SY, Tan J, Wong J et al (2010) Spirotetrahydro beta-carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J Med Chem 53(14):5155–5164
Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ et al (2008) The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver stage malarial parasites. Cell Host Microbe 4(6):567–578
Yuan J, Cheng KCC, Johnson RL, Huang R, Pattaradilokrat S, Liu A, Guha R et al (2011) Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science 333(6043):724–729
Yuthavong Y, Kamchonwongpaisan S, Leartsakulpanich U, Chitnumsub P (2006) Folate metabolism as a source of molecular targets for antimalarials. Future Microbiol 1(1):113–125
Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA et al (2012) Malarial dihydrofolate reductase as a paradigm for drug development against a resistance compromised target. Proc Natl Acad Sci U S A 109(42):16823–16828
Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D (2014) Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Lib. doi:10.1002/14651858.CD01092
Zeeman A-M, van Amsterdam SM, McNamara CW, Voorberg-van der Wel A, Klooster EJ, van den Berg A, Remarque EJ et al (2013) KAI407, a potent non-8-aminoquinolone compound that kills Plasmodium cynomolgi early dormant liver stage parasite in vitro. Antimicrob Agents Chemother 58(3):1586–1595
Zhang VM, Chavchich M, Waters NC (2012) Targeting protein kinases in the malaria parasite: update of an antimalarial drug target. Curr Top Med Chem 12(5):456–472
Zhao Q, Tensfeldt TG, Chandra R, Mould DR (2014) Population pharmacokinetics of azithromycin and chloroquine in healthy adults and paediatric malaria subjects following oral administration of fixed dose azithromycin and chloroquine combination tablets. Malaria J 13:36
Zou X, House BL, Zyzak MD, Richie TL, Gerbasi VR (2013) Towards an optimized inhibition of liver stage development assay (ILSDA) for Plasmodium falciparum malaria. Malaria J 12:394
Acknowledgments
The Indian National Science Academy is gratefully thanked for the grant of an INSA Honorary Scientistship to SK. Thanks are also due to the directors of NIPGR and IICT for providing facilities and encouragement.
Conflict of interest
This is to certify that there is no clash of interest involved in the publication of this manuscript entitled “New insight-guided approaches to detect, cure, prevent and eliminate malaria” authored by Sushil Kumar, Renu Kumari and Richa Pandey and this has the consent of all concerned.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Reimer Stick
Rights and permissions
About this article
Cite this article
Kumar, S., Kumari, R. & Pandey, R. New insight-guided approaches to detect, cure, prevent and eliminate malaria. Protoplasma 252, 717–753 (2015). https://doi.org/10.1007/s00709-014-0697-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0697-x