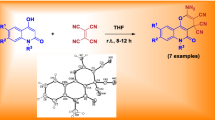
Abstract
Quinoline-2,4-diones reacted with 2-[bis(methylthio)methylene]malononitrile in DMF/Et3N to produce 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carbonitriles and 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides in state of 2-imino-substituted 4-(methylthio)-5,6-dihydro-2H-pyrano[3,2-c]quinolone-3-carbonitriles. The structures of all new products were proved using NMR, IR, and mass spectral data. The possible mechanism for the reaction is also discussed.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
2-Quinolones possess very promising biological activities such as anticonvulsant [1,2,3], antibacterial [4], anti-Alzheimer [5], antimicrobial [6], anti-dermatities [7], anticancer [8], and pain relief [9], in addition to their medical, agricultural, and industrial uses [10,11,12]. As an example, camptothecin (I, Fig. 1) is an anticancer agent and a secondary metabolite damaging the DNA and consequently annihilation of the targeted cell. Topotecan hydrochloride (II) is also one of such compounds that acts as topoisomerase inhibitor and is best used for various kinds of cancer treatments, especially lung cancer and ovarian cancer [13, 14].
To extend the knowledge around the new fused quinolones compounds, we focused our searches on synthesizing a new class of compounds which we expect will have important activities in medicinal and industrial area. Previously, Aly et al. synthesized 2,3-bis(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinic acid derivatives from the reaction of one equivalent of aromatic amines with two equivalents of diethyl malonate in diphenyl ether and catalyzed with triethylamine [15]. On reacting four equivalents of 4-hydroxyquinolin-2(1H)-ones with one equivalent of acenaphthoquinone in absolute ethanol, containing catalytic Et3N, the reaction gave acenaphthylene-1,1,2,2-tetrayltetrakis(4-hydroxyquinolin-2(1H)-ones) [16]. We also reported that quinoline-2,4-diones reacted with 2-(2-oxo-1,2-dihydroindol-3-ylidene)malononitrile in pyridine to produce spiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitriles [17]. The same target materials of 2-quinolones reacted with diethyl acetylenedicarboxylate in absolute ethanol to give pyrano[3,2-c]quinoline-4-carboxylates [18]. We have also recently reported that a class of 1,2,3-triazoles derived by 2-quinolone [19] has been synthesized, via Cu-catalyzed [3 + 2]-cycloadditions (Meldal-Sharpless ‘click’-reactions) of 4-azidoquinolin-2(1H)-ones with ethyl propiolate [19]. We also obtained fused naphthofuro[3,2-c]quinoline-6,7,12-triones and pyrano[3,2-c]quinoline-6,7,8,13-tetraones that have shown potential as ERK inhibitors [20]. While synthesized bis(6-substituted-4-hydroxy-2-oxo-1,2-dihydro-quinolin-3-yl)naphthalene-1,4-diones and substituted N-(alkyl)bis-quinolinone triethyl-ammonium salts were explored as candidates for extracellular signal-regulated kinases 1/2 (ERK1/2) having antineoplastic activity [21].
Synthesis of furo[3,2-c]quinolones has shed interest on organic synthesis [22, 23]. Furoquinolones are important structural motifs in the domain of medicinal chemistry due to their myriad biological activities. These class of compounds have been shown to possess potential activities like antimalarial [24], antibacterial [25], anticancer [26], antiemetic [27]. Furo[3,2-c]quinolone III (Fig. 1) was previously synthesized and its preliminary anticancer activity and antifungal potential were investigated. This compound showed potential anticancer activity against MDAMB-231 breast cancer cells. Meanwhile, it could enhance the fungistatic activity of miconazole against Candida albicans [28]. Moreover, furo[3,2-c]quinolones showed activity as a lipoxygenase inhibitor [29]. For all these reasons, synthesis of furo[3,2-c]quinolines continues to attract interest. Herein, we describe the synthesis of a class of new furo[3,2-c]quinolones, via the reaction of quinoline-2,4-(1H,3H)-diones 1a–1f with 2-[bis(methylthio)methylene]malononitrile (2).
Results and discussion
Heating at 100 °C of equimolar amounts of 6,7-disubstituted quinoline-2,4-(1H,3H)-diones 1a–1f with 2-[bis(methylthio)methylene]malononitrile (2) in dry DMF and catalyzed by Et3N led to the formation of 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carbonitriles 3a–3f and 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides 4a–4f in 45–56% and 25–33% yields, respectively (Scheme 1).

To confirm the structures of all the obtained products, elemental analyses, IR, NMR and mass spectra were performed; these and elemental analyses were in good agreement with the assigned structures. To illustrate the structure elucidation, we choose a representative example, 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carbonitrile (3a). According to elemental analysis and mass spectrometry, compound 3a has a molecular formula of C13H8N2O2S, resulting from combination of one molecule of quinoline-2,4-dione (1a) with one molecule of 2 accompanied with extrusion of a molecule of HCN. The structure of 3a (Fig. 2) was supported by its 1H NMR spectrum, which revealed a double-doublet at δH = 8.00 ppm and a multiplet at 7.50–7.20 ppm related to the remaining three aromatic protons. The NH and SCH3 protons appear as two singlets at 12.20 ppm and 2.00 ppm. The 13C NMR spectrum showed the SCH3, CO, and C-9b and carbonitrile carbons at δC = 13.1 ppm, 164.0 ppm, 158.0 ppm, and 110.8 ppm, respectively (see the experimental section).
Another example as in 3b (Fig. 2), elemental analysis and mass spectrometry has the gross formula C14H10N2O2S. The 1H NMR spectrum showed three singlets at δH = 12.00 ppm for NH-quinlone, 2.10 ppm for CH3, and 2.15 ppm for SCH3 protons. The aromatic protons system appears between 7.20 and 6.98 ppm. The H-9 appears as a broad singlet at 8.07 ppm, whereas its carbon resonated at δC = 132.00 ppm. The 13C spectrum has 14 lines, consistent with 3b; eleven are in the normal sp2 region between 123.8 and 164.4 ppm. Two carbons at 111.0 ppm and 158.2 ppm were related to carbonitrile and C-9b. The SCH3 and the carbonyl carbons resonated at = 13.8 ppm and 164.4 ppm.
In case of compounds 4a–4f, the IR spectrum of 4a, as an example, showed NH and NH2 stretching at \(\overline{\nu }\) = 3100–3300 cm−1, whereas the carbonyl groups absorbed at 1660 and 1640 cm−1. Through elemental analysis and mass spectrometry, it is seen that compound 4a (Fig. 3) has a molecular formula of C13H10N2O3S, resulting from combination of one molecule of quinoline-2,4-dione (1a) with one molecule of 2 accompanied with extrusion of a molecule of HCN and addition of one molecule of water. The 1H NMR spectroscopic data of 4a (Fig. 3) revealed a double-doublet at δH = 8.00 ppm and a multiplet at 7.10–6.90 ppm related to the four aromatic and NH2 protons, whereas the NH2 protons superimposed the aromatic protons. The NH and SCH3 protons appear as two singlets at 11.80 ppm and 1.95 ppm. The 13C NMR spectrum showed the carbonyl-quinolone, carboxamide, SCH3, and C-9b carbons at δC = 164.0 ppm, 158.4 ppm, 13.1 ppm, and 152.0 ppm, respectively (see the experimental section). In case of 4b, its 1H NMR spectrum showed H-9 as a broad singlet at δH = 8.10 ppm, whereas the NH, CH3-8, and SCH3 protons resonated as three singlets at 11.70 ppm, 2.10 ppm, and 1.85 ppm, respectively. The 13C NMR spectrum revealed CH-9, C-9b, carbonyl amide-, and carbonyl-quinolone-carbons at δC = 132.0 ppm, 153.0 ppm, 158.0 ppm, and 163.4 ppm, respectively.
We propose the mechanism shown in Scheme 2. Salt formation of intermediate A is formed due to abstraction of a proton from 1 by Et3N. Conjugate addition of A to 2, catalyzed by base, would give intermediate B. Elimination of methylthiol from B would give intermediate C. Two proposed routes would be: route (1) a nucleophilic attack of the oxygen lone pair to electrophilic carbon assigned as = C(CN)2 accompanied with elimination of HCN molecule would directly proceed to give 3, or route (2) favorable addition of the oxygen lone pair to the nitrile carbon would give Zwitter ion D, which on neutralization would give the expected pyranoquinolone 5. Thereafter, ring cleavage and rearrangement of 5 accompanied by elimination of HCN molecule would give 3. Partial hydrolysis of 3 under reaction condition would give 4.

Conclusion
Reaction of 2,4-quinolones with 2-[bis(methylthio)methylene]malononitrile in DMF/Et3N gave two products; one as major product assigned as 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carbonitriles and 3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides as minor product.
Experimental
NMR spectra were measured in DMSO-d6 on a Bruker AV-400 spectrometer (Bruker BioSpin Corp., Billerica, MA, USA) (400.13 MHz for 1H, 100.13 MHz for 13C) at Florida Institute of Technology, USA. The 1H and 13C chemical shifts are given relative to internal standard TMS. For preparative thin layer chromatography (PLC), glass plates (20 × 48 cm) were covered with a slurry of silica gel Merck PF254 and air dried and developed using the solvents listed. Zones were detected by quenching of indicator fluorescence upon exposure to 254 nm UV light. Elemental analyses were carried in the National Research Center, Dokki, and Cairo, Egypt. Mass spectrometry was performed by electron impact at 70 eV, with a Finnigan Mat 8430 spectrometer in the National Research center, Dokki, Cairo, Egypt. IR spectra using KBr pellets were run on a FT-IR (Bruker), Minia University, El-Minia, Egypt. Starting materials quinoline-2,4-diones 1a–1f were prepared according to the literature [30].
General procedure for synthesis of 3a–3f and 4a–4f
A 100 cm3 round-bottom flask was flame-dried, a mixture of 1a–1f (1 mmol), 2 (1 mmol), 50 cm3 DMF, and 0.5 cm3 of Et3N was refluxed for 12–16 h with stirring (the reaction was followed by TLC analysis). After the reaction’s completion, the formed products 3a–3f were filtered off. The filtrate was then concentrated to half its volume. The second products 4a–4f were obtained by filtration. All products 3a–3f and 4a–4f were recrystallized from the stated solvents.
3-(Methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carbonitrile (3a, C13H8N2O2S)
Yield: 0.115 g (45%); colorless crystals (DMF/EtOH); m.p.: 298–300 °C; Rf = 0.5 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 12.20 (bs, 1H, NH), 7.90 (dd, J = 7.7, 1.5 Hz, 1H, H-9), 7.50–7.20 (m, 3H, Ar–H), 2.00 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.0 (C-4), 158.0 (C-9b), 132.5, 132.0, 131.4, 129.8 (Ar–C), 128.0, 126.4, 125.5 (Ar–CH), 124.2, 123.0 (Ar–C), 110.8 (CN), 13.1 (SCH3) ppm; MS (FAB, 70 eV): m/z (%) = 256 (M+, 60); IR (KBr): \(\overline{\nu }\) = 3230 (NH), 3099 (Ar–H), 2205 (CN), 1640 (C = O), 1600 (Ar–C = N), 1596 (Ar–C = C) cm−1.
8-Methyl-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carbonitrile (3b, C14H10N2O2S)
Yield: 0.127 g (47%); colorless crystals (DMF); m.p.: 310–312 °C; Rf = 0.45 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 12.00 (bs, 1H, NH), 8.07 (bs, 1H, H-9), 7.20–6.98 (m, 2H, Ar–H), 2.10 (s, 3H, CH3), 2.15 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.4 (C-4), 158.2 (C-9b), 139.0 (Ar–C-CH3), 134.0 (Ar–C), 132.0 (Ar–CH-9), 131.0, 128.6 (Ar–C), 128.2, 125.4 (Ar–CH), 124.6, 123.8 (Ar–C). 111.0 (CN), 22.0 (CH3), 13.8 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3240 (NH), 3070 (Ar–H), 2210 (CN), 1645 (C = O), 1610, 1596 (Ar–C = N, Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 270 (M+, 40).
7-Methyl-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carbonitrile (3c, C14H10N2O2S)
Yield: 0.124 g (46%); colorless crystals (DMF/H2O); m.p.: 315–317 °C; Rf = 0.55 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.90 (bs, 1H, NH), 8.12 (bs, 1H, H-9), 7.40–7.20 (m, 2H, Ar–H), 2.25 (s, 3H, CH3), 2.00 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.8 (C-4), 158.0 (C-9b), 134.5 (Ar–C-CH3), 134.2 (Ar–C), 132.2 (Ar–CH-9), 131.0, 128.6 (Ar–C), 128.2, 125.4 (Ar–CH), 124.6, 123.8 (Ar–C), 110.8 (CN), 22.2 (CH3), 13.5 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3230 (NH), 3065 (Ar–H), 2212 (CN), 1648 (C = O), 1615 (Ar–C = N), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 270 (M+, 40).
8-Chloro-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carbonitrile (3d, C13H7ClN2O2S)
Yield: 0.162 g (56%); colorless crystals (DMF); m.p.: 292–294 °C; 1H NMR (400 MHz, DMSO-d6): δ = 11.88 (bs, 1H, NH), 7.80 (d, J = 0.7 Hz, 1H, H-9), 7.20–6.90 (m, 2H, Ar–H), 1.95 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 163.9 (C-4), 158.0 (C-9b), 136.4, 134.0, 133.0 (Ar–C), 132.2 (Ar–CH-9), 128.4 (Ar–C-Cl), 127.4, 126.2 (Ar–CH), 124.0, 123.4 (Ar–C). 111.2 (CN), 13.8 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3225 (NH), 3070 (Ar–H), 2212 (CN), 1645 (C = O), 1618 (Ar–C = N), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 292 ([M + 2]+, 34), 290 (M+, 50).
7-Chloro-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carbonitrile (3e, C13H7ClN2O2S)
Yield: 0.160 g (55%); colorless crystals (DMF); m.p.: 322–324 °C; Rf = 0.4 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.90 (bs, 1H, NH), 6.90–6.70 (m, 3H, Ar–H)), 1.95 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 163.2 (C-4), 157.8 (C-9b), 136.0 (Ar–C), 134.4 (Ar–CH-9), 133.0, 131.0 (Ar–C), 128.0 (Ar–C-Cl), 127.1, 126.0 (Ar–CH), 122.2, 120.0 (Ar–C), 110.8 (CN), 14.0 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3230 (NH), 3080 (Ar–H), 2210 (CN), 1650 (C = O), 1618 (Ar–C = N), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 292 ([M + 2]+, 38), 290 (M+, 53).
7-Bromo-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carbonitrile (3f, C13H7BrN2O2S)
Yield: 0.167 g (50%); colorless crystals (DMF/EtOH); m.p.: 270–272 °C; Rf = 0.3 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.90 (bs, 1H, NH), 8.00 (d, J = 7.7 Hz, 1H, H-9), 7.30–7.16 (m, 2H, Ar–H), 2.10 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 163.6 (C-4), 156.8 (C-9b), 136.4 (Ar–C), 134.2, 132.0 (Ar–C), 131.8 (Ar–CH-9), 129.8 (Ar–C), 127.8 (Ar–C-Br), 126.2 (Ar–CH), 124.0, 122.1 (Ar–C), 110.2 (CN), 13.8 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3230 (NH), 3070 (Ar–H), 2210 (CN), 1650 (C = O), 1619 (Ar–C = N), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 334 ([M-1]+, 20), 335 (M+, 38), 336 ([M + 1]+, 52).
3-(Methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carboxamide (4a, C13H10N2O3S)
Yield: 0.069 g (25%); colorless crystals (EtOAc); m.p.: 340–342 °C; Rf = 0.7 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.80 (bs, 1H, NH), 8.00 (dd, J = 7.7, 1.2 Hz, 1H, H-9), 7.10–6.90 (m, 5H, Ar–H, NH2), 1.95 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.0 (C-4), 158.4 (CONH2), 152.0 (C-9b), 147.0 (Ar–C-amide), 136.0, 134.0 (Ar–C), 128.0, 126.4, 124.8, 124.0 (Ar–CH), 122.3, 122.0 (Ar–C), 13.1 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3300–3100 (NH2, NH), 3099 (Ar–H), 1660 (C = O), 1640 (C = O), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 274 (M+, 40).
8-Methyl-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carboxamide (4b, C14H12N2O3S)
Yield: 0.078 g (27%); colorless crystals (DMF/EtOH); m.p.: 350–352 °C; Rf = 0.65 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.70 (bs, 1H, NH), 8.10 (bs, 1H, H-9), 7.15–6.92 (m, 4H, Ar–H, NH2), 2.10 (s, 3H, CH3), 1.85 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 163.4 (C-4), 158.0 (CONH2), 153.0 (C-9b), 146.8 (Ar–C-amide), 137.2 (Ar–C-CH3), 136.0 (Ar–C), 132.0 (Ar–CH-9), 128.2 (Ar–CH), 128.8 (Ar–C), 126.2 (Ar–CH), 122.3, 122.0 (Ar–C), 22.0 (CH3), 15.4 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3330–3150 (NH2, NH), 3060 (Ar–H), 1665 (C = O), 1642 (C = O), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 288 (M+, 34).
7-Methyl-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carboxamide (4c, C14H12N2O3S)
Yield: 0.074 g (26%); colorless crystals (EtOAc); m.p.: 343–345 °C; Rf = 0.6 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.75 (bs, 1H, NH), 8.12 (d, J = 0.8 Hz, 1H, H-9), 7.20–7.13 (m, 4H, Ar–H, NH2), 2.12 (s, 3H, CH3), 1.90 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 163.6 (C-4), 159.2 (CONH2), 153.1 (C-9b), 146.8 (Ar–C-amide), 136.0 (Ar–C-CH3), 135.8 (Ar–C), 131.4 (Ar–CH-9), 129.0 (Ar–CH), 128.8 (Ar–C), 126.2 (Ar–CH), 120.6, 119.8 (Ar–C), 22.2 (CH3), 14.8 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3335–3180 (NH2, NH), 3065 (Ar–H), 1665 (C = O), 1642 (C = O), 1580 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 288 (M+, 30).
8-Chloro-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carboxamide (4d, C13H9ClN2O3S)
Yield: 0.092 g (30%); colorless crystals (DMF/EtOH); m.p.: 280–282 °C; Rf = 0.35 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.90 (bs, 1H, NH), 7.80 (d, J = 0.8 Hz, 1H, H-9), 7.20–7.16 (m, 4H, Ar–H, NH2), 1.90 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.2 (C-4), 159.5 (CONH2), 154.0 (C-9b), 146.2 (Ar–C-amide), 135.0, 133.4, 130.0 (Ar–C), 129.8 (Ar–CH-9), 128.8 (Ar–C-Cl), 127.0 (Ar–CH), 125.8 (Ar–C), 124.0 (Ar–CH), 15.8 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3295–3180 (NH2, NH), 3070 (Ar–H), 1662 (C = O), 1650 (C = O), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 308 (M+, 28), 309 ([M + 1]+, 7), 310 ([M + 2]+, 5).
7-Chloro-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carboxamide (4e, C13H9ClN2O3S)
Yield: 0.0910 g (29%); colorless crystals (DMF/EtOH); m.p.: 263–265 °C (decmp); Rf = 0.5 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.95 (bs, 1H, NH), 7.80 (dd, J = 1.2, 0.8 Hz, 1H, H-9), 7.10–6.96 (m, 4H, Ar–H, NH2), 1.98 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 165.0 (C-4), 158.0 (CONH2), 153.2 (C-9b), 146.8 (Ar–C-amide), 136.0, 134.4, 131.0 (Ar–C), 128.9 (Ar–CH-9), 127.8 (Ar–C-Cl), 126.0 (Ar–CH), 124.6 (Ar–C), 122.0 (Ar–CH), 15.9 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3290–3190 (NH2, NH), 3080 (Ar–H), 1665 (CO), 1640 (C = O), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 310 ([M + 2]+, 10), 308 (M+, 40).
8-Bromo-3-(methylthio)-4-oxo-4,5-dihydrofuro[3,2-c]quinoline-2-carboxamide (4f, C13H9BrN2O3S)
Yield: 0.116 g (33%); colorless crystals (DMF/H2O); m.p.: 267–269 °C (decomp); Rf = 0.4 (toluene: AcOEt 5:1); 1H NMR (400 MHz, DMSO-d6): δ = 11.80 (bs, 1H, NH), 7.70 (d, J = 0.7 Hz, 1H, H-9), 7.22–7.18 (m, 4H, Ar–H, NH2), 1.94 (s, 3H, SCH3) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 165.0 (C-4), 157.0 (CONH2), 154.0 (C-9b), 147.8 (Ar–C-amide), 135.4, 134.0, 132.6 (Ar–C), 131.4 (Ar–C-Cl), 128.8 (Ar–CH-9), 127.0 (Ar–CH), 125.8 (Ar–C), 123.6 (Ar–CH), 15.8 (SCH3) ppm; IR (KBr): \(\overline{\nu }\) = 3180–3290 (NH2, NH), 3060 (Ar–H), 1660 (C = O), 1652 (C = O), 1590 (Ar–C = C) cm−1; MS (FAB, 70 eV): m/z (%) = 352 ([M-1]+, 18) 351 ([M-2]+, 10).
References
Siddiqui N, Arshad MF, Khan SA (2009) Acta Pol Pharm 66:161
Kamiński K, Obniska J, Dybała M (2008) Eur J Med Chem 43:53
Obniska J, Kamiñski K, Tatarczyñska E (2006) Pharmcol Res 58:207
Park HB, Jo NH, Hong JH, Chei JH, Cho J-H, Yoo KH, Oh C-H (2007) Arch Pharm (Weinheim) 340:530
Fujio M, Hashimoto K, Katayama J, Numata A (2001) Preparation of spiro[azabicycloalkane-oxazolidinone] derivatives and analogs as α-7 nicotinic receptor agonists. Chem Abstr 135:318499 (WO 2001066546)
Pawar MJ, Burungal AB, Karale BK (2009) Arkivoc xiii:97
Nakao K, Ikeda K, Kurokawa T, Togashi Y, Umeuchi H, Honda T, Okano K, Mochizuki H (2008) Jpn J Psychopharmacol 28:75
Chin Y-W, Salim AA, Su B-N, Mi Q, Chai H-B, Riswan S, Kardono LBS, Ruskandi A, Farnsworth NR, Swanson SM, Kinghorn AS (2008) J Nat Prod 71:390
Schick H, Frank R, Reich M, Jostock R, Bahrenberg G, Theil F, Henkel B (2006) Preparation of 1-oxa-2,8-diazaspiro[4.5]dec-2-enes as vanilloid receptor 1 inhibitors. Chem Abstr 145:505458 (WO 2006122769)
Hu H, Guo H, Li E, Liu X, Zhou Y, Che Y (2006) J Nat Prod 69:1672
Sarma BK, Manna D, Minoura M, Mugesh G (2010) J Am Chem Soc 132:5364
Lee D, Long SA, Murray JH, Adams JL, Nuttall ME, Nadeau DP, Kikly K, Winkler JD, Sung CM, Ryan MD, Levy MA, Keller PM, DeWolf WE Jr (2001) J Med Chem 7:2015
Tan H, Wang G, Li J, Meng G, Liu Z, Dong M, Li Y, Ju D, Zhang Q (2015) Bioorg Med Chem 23:118
Lu W, Wang Y, Wang L, Zhao F, Yang S, Xi C, Yang Y, Xu L, Chi X (2018) J Mol Struct 1155:623
Aly AA, El-Sheref EM, Mourad AE, Bakheet MEM, Bräse S, Nieger M (2019) Chem Pap 73:27
Aly AA, Ramadan M, El-Reedy AAM (2019) J Heterocycl Chem 56:642
Aly AA, El-Sheref EM, Mourad AE, Brown AB, Bräse S, Bakheet MEM, Nieger M (2018) Monatsh Chem 149:635
El-Sheref EM, Aly AA, Mourad AE, Brown AB, Bräse S, Bakheet MEM (2018) Chem Pap 72:181
El-Sheref EM, Aly AA, Ameen MA, Brown AB (2019) Monatsh Chem 150:747
Aly AA, El-Sheref EM, Bakheet MEM, Mourad AE, Bräse S, Ibrahim MAA, Nieger M, Garvalov BK, Dalby KN, Kaoud TS (2019) Bioorg Chem 82:290
Aly AA, El-Sheref EM, Bakheet MEM, Mourad AE, Brown AB, Bräse B, Nieger M, Ibrahim MAA (2018) Bioorg Chem 81:700
Basco LK, Mitaku S, Skaltsounis AL, Ravelomanantsoa N, Tillequin F, Koch M, Bras JL (1994) Antimic Agents Chemother 38:1169
Cruickshank PA, Lee FT, Lupichuk A (1970) J Med Chem 13:1110
Hanawa F, Fokialakis N, Skaltsounis AL (2004) Planta Med 70:531
Marzano C, Chilin A, Baccichetti F, Bettio F, Guiotto A, Miolo G, Bordin F (2004) Eur J Med Chem 39:411
Sakharov PA, Rostovskii NV, Khlebnikov AF, Panikorovskii TL, Novikov MS (2019) Org Lett 21:3615
Calleja J, González-Pérez AB, de Lera ÁR, Álvarez R, Fañanás FJ, Rodríguez F (2014) Chem Sci 5:996
Chung P-Y, Tang JC-O, Cheng C-H, Bian Z-X, Wong W-Y, Lam K-H (2016) Springer Plus 5:271
Görlitzer K, Fabian J, Jones PG, Frohberg P, Drutkowski G (2002) Pharmazie 57:159
Buckle DR, Cantello BCC, Smith H, Spicer BA (1975) J Med Chem 18:726
El-Agrody AM, Abd-Rabboh HSM, Al-Ghamdi AM (2013) Med Chem Res 22:1339
Acknowledgements
The authors also thank DFG Foundation for providing Prof Ashraf A. Aly, 1-month fellowship enabling him to carry out the compounds analysis in Karlsruhe Institute of Technology, Karlsruhe, Germany in July–August 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aly, A.A., Ishak, E.A., Shwaky, A.M. et al. Formation of furo[3,2-c]quinolone-2-carbonitriles and 4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides from reaction of quinoline-2,4-diones with 2-[bis(methylthio)methylene]malononitrile. Monatsh Chem 151, 223–229 (2020). https://doi.org/10.1007/s00706-019-02541-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02541-0