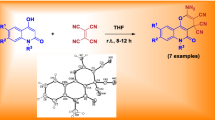
Abstract
Quinoline-2,4-diones reacted with 2-(2-oxo-1,2-dihydroindol-3-ylidene)malononitrile in pyridine to give 2′-amino-2,5′-dioxo-5′,6′-dihydrospiro(indoline-3,4′-pyrano[3,2-c]quinoline)-3′-carbonitriles in good to excellent yields. The structures of all new products were proven using one- and two-dimensional NMR, IR, and mass spectral data, and in five cases X-ray structural analyses. The possible mechanism for the reaction is also discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Great efforts have been made to synthesis, characterize, and investigate the biological activities of spiro-compounds. They possess very promising biological activities as anticonvulsant [1,2,3], antibacterial [4], anti-Alzheimer [5, 6], antimicrobial [7], anti-dermatities [8], anticancer [9], and pain relief [10, 11], in addition to their medical, agricultural, and industrial uses [12–15]. Therefore, spiro-compounds have drawn tremendous interest of researchers in synthetic organic chemistry and medicinal chemistry. Indeed, quinolones represent a class of fused ring systems with interesting essential role in the construction of many bioactive compounds, that they can exhibit antibacterial [16], antifungal [16], antitumor [17], anticancer [18], anti-tuberculosis [18], antibiotic [19, 20], antimicrobial [21,22,23], anti-inflammatory activities [21,22,23]. However, they have herbicides [24], and insecticide agents [25]. On the other hand, isatinemalononitrile is a scaffold for the synthesis of fused spiro-compounds [26,27,28,29,30,31,32], many of its derivatives are potent inhibiting caspase [33]. The heterocyclic spiro-oxindole framework is an important structural motif in natural products which can act as potent non-peptide inhibitors [34, 35]. These compounds are very interesting: they can act as antibacterial and anti-HIV agents [36], and exhibit biological activities [37], such as antitumor and anticancer [38] and cellular evaluation [39]. To extend the knowledge around the new spiro-compounds, we focused our searches to synthesis a new class of them which we expect they will have important activities in medicinal and industrial area. Previously, Aly et al. synthesized fused spiro-pyranoindanoparacyclophanes [40], spiro-pyridazino-cyclohexadiene as well as spiro-thiadiazolo-pyrimidinocyclohexadiene derivatives [41], and spiro(indole-3,3′-[1,2,4]-triazol)-2(1H)-ones [42]. We have also recently investigated the reaction of 2,4-quinolones with dialkyl acetylenedicarboxylates. The reaction gave ethyl 5,6-dihydro-2,5-dioxo-6,9-disubstituted-2H-pyrano[3,2-c]quinoline-4-carboxylates and dialkyl 2-(4-oxo-1,4-dihydro-quinolin-3-yl)fumarates in good yields [43]. So and herein, we describe the synthesis of a class of new spiro-compounds, via the reaction of quinoline-2,4-(1H,3H)-diones 1a–1f with 2-(2-oxo-1,2-dihydroindol-3-ylidene)malononitrile (2).
Results and discussion
Refluxing equimolar amounts of 1,6-disubstituted quinoline-2,4-(1H,3H)-diones 1a–1f with 2-(2-oxo-1,2-dihydroindol-3-ylidene)malononitrile (2) in dry pyridine solution led to the formation of spiro[indoline-3,4′-pyrano[3,2-

c]quinolone]-3′-carbonitriles 3a–3f in 85–92% yields (Scheme 1).
To confirm the structures of all the obtained products, elemental analyses, IR, NMR (1H, 13C, 2D NMR, 15N) and mass spectra were performed; these and elemental analyses were in good agreement with the assigned structures. As an example, the structure of compound 3a which was previously prepared [21f]. However, its detailed NMR data were not extensively reported. The NMR spectra of 3a (Table 1) showed a broad 1H singlet at δ = 11.75 ppm, assigned as NH-6′. This proton gives HSQC correlation with N-6, and gives HMBC correlation with C-2′, C-3′, C-3, 4′, C-5′, C-9′, C-10′, and C-10′b. The distinctive hydrogens and carbons of compound 3a were shown in Fig. 1. Whilst, the structure assignment of 3a is unambiguously confirmed by an X-ray crystal structure as shown in Fig. 2.
On the other side, the structure assignments of 3b–3d followed from single-crystal X-ray analyses were found bonded to DMF as the solvent of recrystallization (Figs. 3, 4, 5).
Another representative example, named 2′-amino-6′-methyl-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitrile (3e) was distinguished. According to elemental analysis and mass spectrometry, compound 3e has the gross formula C21H14N4O3, resulting from combination of one molecule of N-methylquinoline-2,4-dione (1e) with one molecule of 2. Our first impression was that the reaction might give either 3e, 4, or 5 (Fig. 6). NMR data appear in Table 2.
The 13C spectrum has 21 lines, consistent with 3e but excluding structure 4; 18 are in the normal sp2 region between δ = 100–160 ppm. Whereas the proposed structure of 5 was ruled out on the basis of 2D-NMR. The nitrogen atom of 3e appeared at δN = 136.3 ppm and gives HSQC correlation with the 1H broadened singlet at δ = 10.54 ppm. The former two signals are assigned as N-1 and the attached proton H-1 also gives HMBC correlation with the carbonyl carbon at 177.81 ppm, which is assigned as C-2. The sp3 carbon at 48.15 ppm, assigned as the spiro carbon C-3,4′. In addition, C-7a gives HMBC correlation with a proton triplet at 7.18 ppm, assigned as H-6; this is another three-bond correlation, and the attached carbon appears at 128.28 ppm. The nitrogen at 140.50 ppm gives HMBC correlation with an aromatic doublet at 7.59 ppm, and a methyl singlet at 3.49 ppm. This nitrogen is assigned as N-6′ and the methyl singlet as H-6′b; the carbon attached to H-6′b appears at 29.19 ppm, and is assigned as C-6′b. The nitrogen at 75.20 ppm is assigned as NH2. The carbon at 57.22 ppm gives HMBC correlation with NH2, and is assigned as C-3′; its upfield shift is attributed to the observed trends in δ values for C-atoms in push–pull alkenes [44, 45]. The carbon C-10′b appears at 112.27 ppm, and gives HMBC correlation with H-7′ and NH2. The remaining carbons appear at 117.43 and 106.48 ppm, and must be CN and C-4′a; based on chemical shifts, the downfield of the two is assigned as the nitrile carbon-CN and the upfield carbon as C-4′a. The rest of the assignments in the two benzene rings follow straightforwardly from the correlations in Table 2.
The spectra of the N-ethyl compound 3f are essentially identical to those of 3e; because spiro carbon C-14 is a stereogenic center, protons of H-101 are diastereotopic (Fig. 7) [46], and the N-ethyl group appears as an ABX3 system. The structure assignment of 3f is unambiguously confirmed by an X-ray crystal structure; the crystal contains one equivalent of DMF, the recrystallization solvent (Fig. 7).
We propose the mechanism shown in Scheme 2. Conjugate addition of 1 to 2, catalyzed by base, would give intermediate B. Cyclization of B would then occur to give intermediate C and, after proton transfer, finally give products 3a–3f (Scheme 2).

Most indicative is the ring conformation in crystals of compounds 3a–3d and 3f are distorted under the effect of intermolecular interactions, as is evidenced by short intermolecular contacts. The complexes are stabilized by intermolecular N–H···O and C–H···O hydrogen bonds between the hydrogen atoms situated inside the cavity of the macrocycle, the oxygen and nitrogen atoms of the dimethylformamide (DMF) and water molecules.
Conclusion
Reaction of 2,4-quinolinediones with isatinemalononitrile yields spiro-compounds in good yields; structures were established by solution-phase spectroscopy and X-ray crystallographic analysis.
Experimental
NMR spectra were measured in DMSO-d6 on a Bruker AV-400 spectrometer (Bruker BioSpin Corp., Billerica, MA, USA) (400.13 MHz for 1H, 100.13 MHz for 13C, and 40.55 MHz for 15N) at Florida Institute of Technology, USA. The 1H and 13C chemical shifts are given relative to internal standard TMS; 15N shifts are reported versus external liquid ammonia. For preparative thin layer chromatography (PLC), glass plates (20 × 48 cm) were covered with a slurry of silica gel Merck PF254 and air dried and developed using the solvents listed. Zones were detected by quenching of indicator fluorescence upon exposure to 254 nm UV light. Elemental analyses were carried in the National Research Center, Dokki, Cairo, Egypt. Mass spectra were recorded on a Varian MAT 312 instrument in EI mode (70 eV), at the Karlsruhe Institut für Technologie (KIT), Institute of Organic Chemistry, Karlsruhe, Germany. IR spectra using KBr pellets, were run on a FT-IR (Bruker), Minia University, El-Minia, Egypt. Quinoline-2,4-diones 1a–1f were prepared according to the literature [47].
X-ray crystal structure determination
The single-crystal X-ray diffraction study were carried out on a Bruker D8 Venture diffractometer with Photon100 detector at 123(2) K using Cu-Kα radiation (3a, 3b, 3d, 3f, λ = 1.54178 Å) or Mo-Kα radiation (3c, λ = 0.71073 Å). Direct Methods (SHELXS-97) [48] or dual space methods (3d) [49] were used for structure solution and refinement was carried out using SHELXL-2014 (full-matrix least-squares on F2) [49]. Hydrogen atoms were localized by difference electron density determination and refined using a riding model (H(N) and H(O) free. Semi-empirical absorption corrections were applied. For 3a, 3c, and 3d extinction corrections were applied.
CCDC-1519994 (3a), CCDC-1519995 (3b), CCDC-1519996 (3c), CCDC-1574003 (3d) and CCDC-1519997 (3f) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
General procedure for the reaction of quinoline-2,4-diones 1a–1f with 2
A 100 cm3 round-bottom flask was flame-dried, a mixture of 1a–1f (1 mmol), 2 (1 mmol), and 20 cm3 dry pyridine was refluxed for 8–10 h with stirring (the reaction was followed by TLC analysis). After the reaction’s completion, solvent was then removed under vacuum and the residue was separated. The solid residue undergoes recrystallization from the stated solvents to give pure crystals of spiro-compounds 3a–3f.
2′-Amino-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano-[3,2-c]quinolone]-3′-carbonitrile (3a, C20H12N4O3)
Colorless crystals (DMF); yield 303 mg (85%), m.p.: 298–300 °C; NMR (DMSO-d 6 ): see Table 1; IR (KBr): \(\bar{\nu }\) = 3372–3207 (NH, NH2), 3099 (Ar–H), 2205 (CN), 1725, 1672, 1642 (C=O), 1600, 1596 (Ar–C=N, Ar–C=C) cm−1; MS (FAB, 70 eV): m/z = 356 (M+, 100).
Crystal structure data for 3a: colourless crystals, C20H12N4O3·2(C3H7NO), Mr = 502.53, crystal size 0.16 × 0.10 × 0.08 mm, monoclinic, space group P21/c (No. 14), a = 10.8078(3) Å, b = 21.4185(6) Å, c = 11.0025(3) Å, β = 106.635(1)°, V = 2440.34(12) Å3, Z = 4, ρ = 1.368 Mg m−3, µ(Cu-Kα) = 0.805 mm−1, F(000) = 1056, 2θmax = 144.4°, 26,760 reflections, of which 4805 were independent (Rint = 0.042), 351 parameters, 4 restraints, R1 = 0.038 (for 4182 I > 2σ(I)), wR2 = 0.097 (all data), S = 1.06, largest diff. peak/hole = 0.297/− 0.206 e Å−3.
2′-Amino-9′-chloro-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitrile (3b, C20H11ClN4O3)
Colorless crystals (DMF/EtOH); yield 360 mg (92%), m.p.: 320–322 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 11.88 (bs, 1H, NH-6′), 10.55 (bs, 1H, NH-1), 8.00 (d, J = 2.3 Hz, 1H, H-10′), 7.68 (dd, J = 8.8, 2.4 Hz, 1H, H-8′), 7.47 (bs, 2H, NH2), 7.37 (d, J = 8.8 Hz, 1H, H-7′), 7.19 (ddd, J = 7.6, 7.6, 0.7 Hz, 1H, H-6), 7.05 (d, J = 7.3 Hz, 1H, H-4), 6.90 (dd, J = 7.4, 7.4 Hz, 1H, H-5), 6.83 (d, J = 7.7 Hz, 1H, H-7) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 177.58 (C-2), 159.18, 158.77 (C-5′,2′), 151.44 (C-10′a), 142.33 (C-7a), 136.55 (C-6′a), 134.06 (C-3a), 131.71 (C-8′), 128.38 (C-6), 126.31 (C-9′), 123.56 (C-4), 121.71 (C-5), 121.23 (C-10′), 117.48, 117.30 (CN,C-7′), 111.55 (C-10′b), 109.24 (C-7), 107.98 (C-4′a), 57.09 (C-3′), 47.74 (C-3,4′) ppm; 15N NMR (40 MHz, DMSO-d 6 ): δ = 146.0 (N-6′), 136.2 (N-1), 74.8 (NH2) ppm; IR (KBr): \(\bar{\nu }\) = 3350–3196 (NH, NH2), 3031 (Ar–CH), 2928 (Ali-CH), 2193 (CN), 1716, 1669, 1654 (C=O), 1605, 1593 (Ar–C=N, Ar–C=C) cm−1; MS (FAB, 70 eV): m/z = 391 ([M + 1]+, 28), 390 (M+, 58).
Crystal structure data for 3b: colourless crystals, C20H11ClN4O3·2(C3H7NO), Mr = 536.97, crystal size 0.20 × 0.16 × 0.10 mm, monoclinic, space group P21/c (No. 14), a = 11.0744(4) Å, b = 21.3368(8) Å, c = 11.1215(4) Å, β = 107.884(1)°, V = 2500.94(16) Å3, Z = 4, ρ = 1.426 Mg m−3, µ(Cu-Kα) = 1.784 mm−1, F(000) = 1120, 2θmax = 144.2°, 21,016 reflections, of which 4915 were independent (Rint = 0.027), 359 parameters, 4 restraints, R1 = 0.036 (for 4445 I > 2σ(I)), wR2 = 0.097 (all data), S = 1.03, largest diff. peak/hole = 0.320/− 0.290 e Å−3.
2′-Amino-9′-bromo-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitrile (3c, C20H11BrN4O3)
Colorless crystals (DMF/MeOH); yield 390 mg (90%), m.p.: > 360 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 11.87 (bs, 1H, NH-6′), 10.55 (bs, 1H, NH-1), 8.14 (d, J = 2.2 Hz, 1H, H-10′), 7.79 (dd, J = 8.8, 2.2 Hz, 1H, H-8′), 7.48 (bs, 2H, NH2), 7.31 (d, J = 8.8 Hz, 1H, H-7′), 7.18 (ddd, J = 7.6, 7.6, 0.9 Hz, 1H, H-6), 7.05 (d, J = 7.2 Hz, 1H, H-4), 6.89 (dd, J = 7.5, 7.5 Hz, 1H, H-5), 6.83 (d, J = 7.7, 1H, H-7) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 177.59 (C-2), 159.17, 158.77 (C-5′,2′), 151.36 (C-10′a), 142.33 (C-7a), 136.86 (C-6′a), 134.36 (C-8′), 134.07 (C-3a), 128.38 (C-6), 124.22 (C-10′), 123.56 (C-4), 121.71 (C-5), 117.62 (C-7′), 117.31 (CN), 114.01 (C-9′), 113.24 (C-10′b), 109.24 (C-7), 107.98 (C-3a), 57.09 (C-3′), 47.74 (C-3,4′) ppm; 15N NMR (40 MHz, DMSO-d 6 ): δ = 146.2 (N-6), 136.4 (N-4c), 75.1 (NH2) ppm; IR (KBr): \(\bar{\nu }\) = 3365–3195 (NH, NH2), 3044 (Ar–CH), 2930 (Ali-CH), 2195 (CN), 1700, 1671, 1635 (C=O), 1605, 1589 (Ar–C=N, Ar–C=C) cm−1; MS (FAB, 70 eV): m/z = 435 ([M + 1]+, 28), 434 (M+, 56).
Crystal structure data for 3c: colourless crystals, C20H11BrN4O3·2(C3H7NO), Mr = 581.43, crystal size 0.25 × 0.20 × 0.15 mm, monoclinic, space group P21/c (No. 14), a = 11.0650(5) Å, b = 21.4404(11) Å, c = 11.2361(5) Å, β = 108.179(2)°, V = 2532.6(2) Å3, Z = 4, ρ = 1.525 Mg m−3, µ(Mo-Kα) = 1.673 mm−1, F(000) = 1192, 2θmax = 55.2°, 39,390 reflections, of which 5834 were independent (Rint = 0.024), 360 parameters, 4 restraints, R1 = 0.026 (for 5290 I > 2σ(I)), wR2 = 0.064 (all data), S = 1.05, largest diff. peak/hole = 0.483/− 0.572 e Å−3.
2′-Amino-9′-methyl-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitrile (3d, C21H14N4O3)
Colorless crystals (DMF); yield 325 mg (88%), m.p.: 340–342 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 11.83 (bs, 1H, NH-6′), 10.69 (bs, 1H, NH-1), 7.94 (s, 1H, H-10′), 7.62 (d, J = 8.4 Hz, 1H, H-8′), 7.61 (bs, 2H, NH2), 7.43 (d, J = 8.4 Hz, 1H, H-7′), 7.35 (t, J = 7.6 Hz, 1H, H-6), 7.18 (d, J = 7.3 Hz, 1H, H-4), 7.06 (t, J = 7.4 Hz, 1H, H-5), 7.00 (d, J = 7.7 Hz, 1H, H-7), 2.59 (s, 3H, CH3) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 177.86 (C-2), 159.26, 158.96 (C-5′,2′), 152.23 (C-10′a), 142.37 (C-7a), 135.90 (C-6′a), 134.39 (C-3a), 132.97 (C-8′), 131.27 (C-9′), 128.23 (C-6), 123.38 (C-4), 121.64 (C-10′), 121.29 (C-5), 117.47 (CN), 115.30 (C-7′), 111.45 (C-10′b), 109.19 (C-7), 106.94 (C-4′a), 57.19 (C-3′), 47.77 (C-3,4′), 20.65 (CH3) ppm; 15N NMR (40 MHz, DMSO-d 6 ): δ = 145.5 (N-6′), 136.2 (N-1), 74.8 (NH2) ppm; IR (KBr): \(\bar{\nu }\) = 3370–3190 (NH, NH2), 3033 (Ar–CH), 2922 (Ali-CH), 2198 (CN), 1705, 1670, 1638 (C=O), 1610, 1587 (Ar–C=N, Ar–C=C) cm−1; MS (FAB, 70 eV): m/z = 370 (M+, 100).
Crystal structure data for 3d: colourless crystals, C21H14N4O3·2(C3H7NO), Mr = 516.55, crystal size 0.18 × 0.06 × 0.03 mm, monoclinic, space group P21/c (No. 14), a = 11.0806(5) Å, b = 21.3920(9) Å, c = 11.1076(5) Å, β = 107.616(2)°, V = 2509.44(19) Å3, Z = 4, ρ = 1.367 Mg m−3, µ(Cu-Kα) = 0.798 mm−1, F(000) = 1088, 2θmax = 144.0°, 23,702 reflections, of which 4913 were independent (Rint = 0.041), 361 parameters, R1 = 0.039 (for 4146 I > 2σ(I)), wR2 = 0.100 (all data), S = 1.03, largest diff. peak/hole = 0.275/− 0.226 e Å−3.
2′-Amino-6′-methyl-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitrile (3e, C21H14N4O3)
Colorless crystals (DMF); yield 322 mg (87%), m.p.: 310–312 °C; NMR (DMSO-d 6 ): see Table 2; IR (KBr): \(\bar{\nu }\) = 3359–3166 (NH, NH2), 2193 (CN), 1711, 1672, 1645 (C=O), 1626, 1598 (Ar–C=N, Ar–C=C) cm−1; MS (FAB, 70 eV): m/z = 370 (M+, 60).
2′-Amino-6′-ethyl-2,5′-dioxo-5′,6′-dihydrospiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitrile (3f, C22H16N4O3)
Colorless crystals (DMF/EtOH); yield 330 mg (86%), m.p.: 330–332 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 10.53 (bs, 1H, NH-1), 8.09 (dd, J = 8.0, 1.2 Hz, 1H, H-10′), 7.76 (dt, Jt = 7.2 Hz, Jd = 1.3 Hz, 1H, H-8′), 7.64 (d, J = 8.6 Hz, 1H, H-7′), 7.48 (bs, 2H, NH2), 7.43 (t, J = 7.6 Hz, 1H, H-9′), 7.18 (dt, Jt = 7.6 Hz, Jd = 0.9 Hz, 1H, H-6), 7.03 (d, J = 7.2 Hz, 1H, H-4), 6.88 (t, J = 7.5 Hz, 1H, H-5), 6.85 (d, J = 7.8 Hz, 1H, H-7), 4.14 (ABX3, J AB = 13.3 Hz, J AX = 7.0 Hz, 1H, CH2CH3), 4.11 (ABX3, J AB = 13.3 Hz, J BX = 7.0 Hz, 1H, CH2CH3), 1.08 (ABX3, J AX = J BX = 7.0 Hz, 3H, CH2CH3) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 177.84 (C-2), 158.88, 158.33 (C-5′,2′), 151.49 (C-10′a), 142.46 (C-7a), 137.58 (C-6′a), 134.27 (C-3a), 132.31 (C-8′), 128.29 (C-6), 123.37 (C-4), 122.66 (C-10′), 122.27 (C-9′), 121.70 (C-5), 117.44 (CN), 114.73 (C-7′), 112.49 (C-10′b), 109.21 (C-7), 106.42 (C-4′a), 57.22 (C-3′), 48.13 (C-3,4′), 36.83 (N-CH2CH3), 12.62 (N-CH2CH3) ppm; 15N NMR (40 MHz, DMSO-d 6 ): δ = 154.3 (N-6′), 136.5 (N-1), 75.1 (NH2) ppm; IR (KBr): \(\bar{\nu }\) = 3360–3192 (NH2, NH), 3106 (Ar–CH), 2967 (Ali-CH), 2205 (CN), 1725, 1672, 1642 (C=O), 1600, 1578 (Ar–C=N, Ar–C=C) cm−1; MS (FAB, 70 eV): m/z = 384 (M+, 100).
Crystal structure data for 3f: colourless crystals, C22H16N4O3·C3H7NO·H2O, Mr = 475.50, crystal size 0.16 × 0.10 × 0.04 mm, triclinic, space group P-1 (No. 2), a = 9.6532(5) Å, b = 11.3165(6) Å, c = 12.6497(6) Å, α = 110.480(3)°, β = 102.514(3)°, γ = 107.481(3)°, V = 1151.89(11) Å3, Z = 2, ρ = 1.371 Mg m−3, µ(Cu-Kα) = 0.807 mm−1, F(000) = 500, 2θmax = 136.4°, 11,728 reflections, of which 4141 were independent (Rint = 0.037), 333 parameters, 5 restraints, R1 = 0.055 (for 3170 I > 2σ(I)), wR2 = 0.155 (all data), S = 1.05, largest diff. peak/hole = 0.354/− 0.254 e Å−3.
References
Obniska J, Kamiński K (2006) Acta Pol Pharm 63:101
Kamiński K, Obniska J, Dybała M (2008) Eur J Med Chem 43:53
Obniska J, Kamiński K, Tatarcyńska E (2006) Pharmacol Rep 58:207
Park HB, Jo NH, Hong JH, Chei JH, Cho J-H, Yoo KH, Oh C-H (2007) Arch Pharm (Weinh) 340:530
Fujio M, Hashimoto K, Katayama J, Numata A (2001) Preparation of spiro[azabicycloalkane-oxazolidinone] derivatives and analogs as α-7 nicotinic receptor agonists. PCT Int Appl WO 2001066546, Sept 13, 2001
Fujio M, Hashimoto K, Katayama J, Numata A (2001) Chem Abstr 135:318499
Pawar MJ, Burungale AB, Karale BK (2009) Arkivoc xiii:97
Nakao K, Ikeda K, Kurokawa T, Togashi Y, Umeuchi H, Honda T, Okano K, Mochizuki H (2008) Jpn J Psychopharmacol 28:75
Chin Y-W, Salim AA, Su B-N, Mi Q, Chai H-B, Riswan S, Kardono LBS, Ruskandi A, Farnsworth NR, Swanson SM, Kinghorn AS (2008) J Nat Prod 71:390
Schick H, Frank R, Reich M, Jostock R, Bahrenberg G, Theil F, Henkel B (2006) Preparation of 1-oxa-2,8-diazaspiro[4.5]dec-2-enes as vanilloid receptor 1 inhibitors. PCT Int Appl WO 2006122769, Nov 23, 2006
Schick H, Frank R, Reich M, Jostock R, Bahrenberg G, Theil F, Henkel B (2006) Chem Abstr 145:505458
Hu H, Guo H, Li E, Liu X, Zhou Y, Che Y (2006) J Nat Prod 69:1672
Sarma BK, Manna D, Minoura M, Mugesh G (2010) J Am Chem Soc 132:5364
Plaska E, Aytemir M, Uzbay ÌT, Erol D (2001) Eur J Med Chem 36:539
Pradhan R, Patra M, Behera AK, Mishra BK, Behera RK (2006) Tetrahedron 62:779
Naeem A, Badshah SL, Muska M, Ahmad N, Khan K (2016) Molecules 21:268
Huang D, Okada K, Komori C, Itoi E, Suzuki K (2004) Cancer Sci 95:845
Asif M, Husain A, Ahmad A, Khan AS, Rashid M, Arora K, Bahl D, Iram F (2015) Eur J Exp Biol 5:96
Esmaeilzadeh A, Ebteker M, Biglari A, Hassan MZ (2012) Afr J Microbiol Res 23:4891
Heeb S, Fletcher PM, Chhabra RS, Diggle PS, Williams P (2011) FEMS Microbiol Rev 35:247
Sugar MA, Liug X-P (2000) Antimicrob Agent Chemother 44:2004
Kumar A, Fernandes J, Kumar P (2014) Int J Pharm Sci Drug Res 6:124
Hawkey MP (2003) J Antimicrob Chem 51:29
Evans-Roberts MK, Mitchenall AL, Wall KM, Leroux J, Mylne SJ, Maxwell A (2016) J Biol Chem 291:3136
Abe M, Mai T, Ishii N, Usui M, Okuda T, Oki T (2005) Biosci Biotechnol Biochem 69:1202
Tisseh NZ, Ahmadi F, Dabiri M, Khavasi RH, Bazgir A (2012) Tetrahedron Lett 53:3603
Lakshmi VN, Thirumurugan P, Peruma TP (2010) Tetrahedron Lett 51:1064
Guo Y, Li Y, Chang H, Kuo T, Han J (2016) RSC Adv 6:74683
Auria-Luna F, Marqués-López E, Mohammadi S, Heiran R, Herrera PR (2015) Molecules 20:15807
Shi R, Yan C (2016) Chin Chem Lett 27:575
Gholizadeh S, Radmoghadam K (2013) Orient J Chem 29:1637
Wang C, Jiang Y, Yan C (2015) Chin Chem Lett 26:889
Wenhua Chu W, Rothfuss J, d’Avignon A, Zeng C, Zhou D, Hotchkiss SR, Mach HR (2007) J Med Chem 50:3751
Wang S, Zhao Y, Bernard D, Aguilar A, Kumar S (2012) Top Med Chem 8:57
Zhang B, Golding TB, Hardcastle RI (2015) Future Med Chem 7:631
Sriram D, Yogeeswari P, Myneedu NS, Saraswat V (2006) Bioorg Med Chem Lett 16:2127
Mathusalini S, Arasakumar T, Lakshmi K, HerLin C, Mohan SP, Ramnath GM, Thirugnana-sampandan R (2016) New J Chem 40:5164
Yu B, Yu D-Q, Liu H-M (2015) Eur J Med Chem 97:673
Antonchick PA, Reimers GC, Catarinella M, Schurmann M, Preut H, Ziegler S, Rauh D, Waldmann H (2010) Nat Chem 2:735
Aly AA (2003) Tetrahedron 59:1739
Hassan AA, Aly AA, El-Sheref EM (2008) J Chem Res:9
Mohamed AM, Alsharari MA, Aly AA (2010) J Chem Res:200
El-Sheref EM, Aly AA, Mourad AE, Brown AB, Bräse S, Bakheet MEM (2017) Chem Pap. https://doi.org/10.1007/s11696-017-0269-6
Schulze K, Richter C, Klatt K, Ludwig R (1988) Z Chem 28:288
Buckle DR, Cantello BCC, Smith H, Spicer BA (1975) J Med Chem 18:726
Kaplan F, Roberts JD (1961) J Am Chem Soc 83:4666
Gewald K, Schnidler R (1990) J Prakt Chem 332:223
Sheldrick GM (2008) Acta Crystallogr A 64:112
Sheldrick GM (2015) Acta Crystallogr C 71:3
Acknowledgements
The NMR spectrometer at Florida Institute of Technology was purchased with the assistance of the U.S National Science Foundation (CHE 03 42251). Prof AA Aly is thankful to DFG foundation for the financial support of his fellowship accommodation in the Karlsruhe Institut für Technologie (KIT), Institute of Organic Chemistry, Karlsruhe, Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aly, A.A., El-Sheref, E.M., Mourad, AF.E. et al. Synthesis of spiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitriles. Monatsh Chem 149, 635–644 (2018). https://doi.org/10.1007/s00706-017-2078-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2078-6