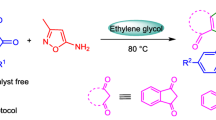
Abstract
Easily accessible 1-hexadecyl-1H-imidazol-3-ium oxalate is highly efficient Brønsted type acidic catalyst for the selected multicomponent one-pot reactions. The short reaction times, easy workup procedures, and green metal-free conditions for the reactions make the protocols more advantageous. Further reactions proceeded smoothly in good to excellent yields with high purity. To investigate mechanism of the multicomponent reactions, DFT calculations were performed.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Applying organocatalysis in synthetic reactions has become a popular research area in both chemistry and industry [1]. The benefits of these catalysts are lack of sensitivity to moistened and oxygenated reaction medium, easily available, generally low cost and toxicity and more importantly the final product is not contaminated by metal impurities. However, these catalysts have certain disadvantages. Relatively large amounts of catalyst usually slow progress of the reaction and choice of appropriate solvent is troublesome [2]. On the other hand, use of excess catalyst can complicate product isolation. Recently some studies dealing with the application of flexible catalysts in organic synthesis have been published [3–9]. Asano and Matsubara reported that N-alkylimidazole derivatives are amphiphilic organocatalyst and efficient ligand for the Schotten–Baumann type tosylation reaction and copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction, respectively [4, 5]. Imidazolium salts are widely used as catalyst for some multicomponent reactions [10–14].
As well known the Biginelli reaction is used in the synthesis of 3,4-dihydropyrimidinones [15–18]. High temperatures, large amounts of catalyst and long reaction times are traditional conditions for this popular reaction. In order to improve its efficiency many studies have been performed however, some drawbacks still remain [19–22]. Long reaction times, expensive and readily unavailable catalysts, organic solvents, tedious purification steps of products, and microwave irradiation requirement are only some of them.
The Mannich reaction is used for the preparation of 1,3-benzoxazines which are common monomers of popular benzoxazine resins [23–25]. Several methods have been reported for the synthesis of benzoxazines [26–32]. However, some disadvantages existed in previous published methods. For the completion of cyclization, previous methods require heating for long times, MW (microwave) irradiation, toxic solvents, and column chromatography for purification of the desired product. Mathew and Nath [33] reported one-pot three-component synthesis of dihydrobenzo- and naphtho[e] [1, 3] oxazines in water. However, this method requires extraction with organic solvent for isolation of product and column chromatography for purification. These multicomponent reactions today still retain its popularity. Therefore, it is still important to develop environmentally friendly and effective procedure for these reactions.
In the present study, 1-hexadecyl-1H-imidazol-3-ium oxalate ([C16Im][Oxa]) was prepared as flexible metal-free acidic organocatalyst which also considered as ionic liquid (melting point <100 °C) (Fig. 1).
To investigate its catalytic activity, the Biginelli and Mannich type condensation reactions were selected as model reactions. In addition its catalytic performance was compared with different metal-based Lewis acids and some Brønsted acids. Hydrophobic chain length and conformational calculations were performed for the catalyst. The interaction energy (E int) for the [C16Im][Oxa] with H2O system was also calculated to support the proposed mechanism of Mannich reaction.
Results and discussion
For the preparation of the catalyst, 2 equivalents of 1-hexadecyl-1H-imidazole and 1 equivalent of oxalic acid dihydrate were reacted at room temperature to afford the desired compound as a white solid in good yield (86%). The catalyst prepared in the present study is containing longer alkyl chains and it was dried at room temperature in the open air without an adverse event and can smoothly be used as a catalyst for the above-mentioned multicomponent reactions. The catalyst precursor 1-hexadecyl-1H-imidazole was prepared by a procedure similar to that in the literature [34, 35]. The structure of the catalyst was well authenticated by 1H NMR, 13C NMR, IR (ATR), elemental analysis, and physical properties. The 1H NMR spectrum of the catalyst was shown in Fig. 2.
The Biginelli reaction was chosen as a model reaction to identify the optimal reaction conditions (Table 1). Dihydropyrimidinones were prepared from the reaction of acetylacetone, aromatic or aliphatic aldehydes, and urea or thiourea under solvent free conditions at 90 °C (Scheme 1). For the optimization of this reaction, acetylacetone, urea, benzaldehyde or dodecanal were used as aromatic and aliphatic aldehyde, respectively. The reaction time is too short and the desired phenyl substituted heterocycles were obtained without any additional purification steps in very good yields (85–97%) and high purity (Table 2). The long chain aliphatic aldehyde was also used, however, after prolonged heating at 90 °C the reaction failed to produce even trace amounts of the desired product (Table 1, entries 7, 8).

On the other hand, the catalytic effects of different metal-based catalysts and metal-free acidic catalysts on the Biginelli reaction were investigated under solvent-free conditions and the obtained results are shown in Fig. 3. The results showed that the catalytic performances of the selected acids are very low under the similar conditions optimized for the imidazolium oxalate.
Ramos et al. [36] investigated a number of Lewis acids in ionic liquids or organic solvents for their catalytic activities on the Biginelli reaction. They found that 10 mol% of catalyst (CuCl2) loading in [BMI][PF6] as an ionic liquid and 60–360 min reaction times are necessary for the maximum yields. On the other hand, Heidarizadeh et al. [37] report the [Cbmim][Cl] as a novel ionic liquid for the catalysis of the Biginelli reaction and they state that at least 30% of catalyst loading is crucial for the optimum product yield. He et al. [38] report the synthesis and catalytic performance of geminal Brønsted acid ionic liquid to catalyze the Mannich reaction. However, longer reaction times (8 h) and 5% loading of catalyst are necessary to obtain good yields. The comparisons of catalytic activities of various ionic liquids reported in the literature and in the present study are shown in Table 3.
Dihydrobenzoxazines were prepared via the pseudo three-component and one-pot reaction of two equivalent of paraformaldehyde and 1 equivalent of o-arylaminomethylphenol at 90 °C (Scheme 2). The Biginelli reaction conditions were also used for the Mannich type condensation except that water was used as green a solvent. When the reaction was carried out in the absence of a solvent side products (unidentified) were obtained probably via polymerization reactions and fairly poor yields of the desired products were obtained. Therefore, water was chosen as a solvent and used successively as a green reaction medium. As can be seen from Table 2, the desired dihydrobenzoxazines were obtained in good to excellent yields.

DFT calculations
Two conformations were suggested namely, cis and trans forms which are determined by the orientations of the long alkyl chains as seen in Fig. 4 for the system were also calculated by DFT. Firstly, the B3LYP method was used for DFT calculations, and then as the DFT functionals poorly describe dispersion effects, dispersion correction for the free energy barrier was estimated using the wB97X-D method. Gibbs energy barriers of cis and trans form were calculated as −43.43 and −60.95 kJ/mol, respectively, with B3LYP method, while these energy barriers were determined as −58.51 and −65.18 kJ/mol with the wB97X-D method. These results show that the trans conformation was calculated high negative interaction energy for both methods, and the trans conformation indicates higher stability for the [C16Im][Oxa]; therefore, this structure was adopted as a trans conformation.
The possible arrangement of ions and counter ions belonging to catalyst was depicted in Fig. 5. The conformational structure of the catalyst plays a central role in the reaction. DFT calculations related to [C16Im][Oxa] prove a trans conformation as a lower energetically geometrical state. In this arrangement, holes between the hydrophobic alkyl chains act as a suitable reaction medium for the components of the reaction and N-protonated imidazolium ring serves as a Bronsted acid donor.
To better understand the electrostatic interactions between the imidazolium cation and the oxalate counter ion ([C16Im][Oxa]), B3LYP and wB97X-D methods [45] using 6–311G(d,p) basis set was adopted and all calculations were performed using the GAUSSIAN 09 program package [46].
The optimized structure of the suggested model of the [C16Im][Oxa] shown in Fig. 6a. Through DFT calculation, the length of hydrophobic chain was calculated to be 21.10 Å, which is approximately equal to the theoretical value (21.74 Å) according to the Tanford equation [47] below:
where n is the number of carbon atoms in the hydrocarbon chain. The radius of a cylindrical aggregate (d H) is about 22 Å at 25 °C, which implies that the hydrophobic alkyl chain of surface active ionic liquids (SAILs) is freely stretching in the hexagonal phase [48].
The interaction energy (E int) for the [C16Im][Oxa] with H2O system was calculated by the DFT method and 6–311G(d,p) basis set to be −155.56 kJ/mol, while this energy was determined as −163.42 kJ/mol with the wB97X-D method. On the other hand, the dispersion interactions between the C16 alkyl side chains were calculated by wB97X-D method as seen in Fig. 5. The dispersion effects of the C16 alkyl side chain are shown in Fig. 6b. This calculation result shows that the interaction energy between these side chains was gained about 40 kJ/mol in the [C16Im][Oxa]. This high negative interaction energy resulting electrostatic and dispersion interactions implies a high stability for the [C16Im][Oxa]/H2O complex. This phenomenon means that it is rather difficult to destroy the [C16Im][Oxa]/H2O complex, which is favorable for forming micelles.
On the other hand, for the electrostatic interactions, the hydrophilic head groups of the [C16Im][Oxa]/H2O system are identical. The electrostatic potential at the 0.001 e/bohr3 isodensity surfaces of [Oxa]2− was calculated at the same level, and was showed in Fig. 7. It is apparent that it is electronegative. This electronegativity of [Oxa]2− indicates that the electrostatic attraction between it and [C16Im]+ is quite high for interacting each [Oxa]2− ion with two [C16Im]+ cations. The electrostatic repulsion between the cation of SAILs is screened effectively, which is favorable to the formation of micelles. It is also confirmed by the interaction energy of [C16Im][Oxa] with water molecules.
Mechanism of Mannich reaction
Cockroft et al. [49–51] point out that in solution depending both on van der Waals dispersion forces and interactions between water molecules and nonpolar alkyl groups known as solvophobic effects, long alkyl hydrocarbon chains can arrange in the folded conformation. They also stated that weak interactions between long alkyl chains are slightly unfavorable in non-polar organic solvents but slightly favorable in polar solvents. In the present work water was used as a highly polar solvent. On the other hand, micelle formation is also determined by these solvophobic interactions [52]. In this context, Mannich reaction maybe proceeds under micelle catalysis as shown in Fig. 8.
Conclusion
In conclusion, highly efficient and practical methods for the synthesis of 3,4-dihydropyrimidinones and 3,4-dihydrobenzoxazines were developed. The synthesis reactions were catalyzed by a little amount of the new and effortlessly synthesized metal free [C16Im][Oxa] as flexible organocatalyst and thus the products were not contaminated with any metallic impurities. In addition, catalytic performance of the catalyst was found to be more than those selected metal-based salts. On the other hand, the reactions were carried out under green-chemistry conditions to afford the products in short times and in good to excellent yields. DFT calculations reveal that trans conformation is more favorable structure of the catalyst and the high (E int) negative interaction energy resulting electrostatic and dispersion interactions implies a high stability for the [C16Im][Oxa]/H2O complex which is favorable for forming micelles.
Experimental
All reagents and solvents were purchased from either Merck or Sigma-Aldrich and used without further purification. Thin-layer chromatography was performed using silica gel (60 F254, Merck, Darmstadt, Germany) plates. Melting points were recorded by BÜCHI melting point B-540 apparatus (BUCHI Labortechnik AG in Flawil, Switzerland). The NMR spectra were measured using Varian mercury plus spectrometer (400 MHz) (Varian Inc., California, USA) in CDCl3 or DMSO-d 6 using TMS as an internal standard. Chemical shifts (δ) are reported in ppm and J values in Hertz.
1-Hexadecyl-1H-imidazol-3-ium oxalate (C40H74N4O4)
In a 100 cm3 two-necked flask 1.92 g 1-hexadecyl-1H-imidazole [34, 35] (6.6 mmol) was dissolved in 50 cm3 of ether. The flask was cooled to 0–10 °C in an ice-water bath and 0.41 g oxalic acid dihydrate (3.3 mmol) dissolved in 5 cm3 of EtOH was added dropwise. After 25 min the precipitated white solid was filtered under vacuum, washed with ether and dried in open air. Yield: 1.86 g, 84%; white solid; m.p.: 84–86 °C; IR (ATR): \(\bar{\nu }\) = 3147, 3122, 3094, 2916, 2850, 1719, 1699, 1635, 1574, 1552, 1471, 1409, 1378, 1327, 1296, 1171, 1121, 1096, 947, 896, 837, 759, 705, 630 cm−1; 1H NMR (400 MHz, CDCl3): δ = 9.02 (s, 2H, 2x–NCHN–), 7.53 (s, 2H, 2x–N3CHC–), 7.15 (s, 2H, 2x–CHCHN1–), 4.20 (s, 4H, 2x–N3CH 2CH2CH2–), 1.86 (s, 4H, 2x–N3CH2CH 2CH2–), 1.30–1.24 (m, 52H, 26x –CH 2–), 0.88 (t, J = 6 Hz, 6H, 2x–CH3) ppm; 13C NMR (100 MHz, CDCl3): δ = 198.0, 163.1, 121.4, 60.3, 49.6, 31.9, 2 × 30.3, 2 × 29.7, 29.6, 29.5, 29.4, 29.0, 26.3, 22.7, 14.1 ppm.
Typical experimental procedure for the preparation of 3,4-dihydropyrimidinones 1a–1j
To a 50 cm3 round-bottomed flask 0.44 g of benzaldehyde (4.1 mmol), 0.41 g of acetylacetone (4.1 mmol), 0.32 g of thiourea (4.2 mmol), and 2.5 mmol % of catalyst were added. The flask was attached to a reflux condenser and heated under atmospheric conditions in an oil bath at 90 °C for 10 min. Thereafter the flask was cooled and little amount of MeOH and then ether were added to the obtained crude product and stirred for some time. Then the obtained solid was filtered and washed with ether to afford pure 1b.
5-Acetyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (1a)
Yield: 85%; yellowish solid; m.p.: 241 °C (Ref. [53] 238.5–240.9 °C).
1-(6-Methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethan-1-one (1b)
Yield: 97%; yellowish solid; m.p.: 232–233 °C (Ref. [53] 228.1–230.3 °C).
5-Acetyl-4-(2-hydroxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (1c)
Yield: 90%; yellowish solid; m.p.: 220–222 °C (Ref. [55] 212 °C).
5-Acetyl-4-(4-hydroxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (1d)
Yield: 95%; yellowish solid; m.p.: 259–260 °C (Ref. [56] 251–252.5 °C).
Typical experimental procedure for the preparation of 1,3-benzoxazines 2a–2j
To a 100 cm3 round-bottomed flask containing 15 cm3 of water were added 0.30 g of corresponding bis-secondary amine (1.1 mmol), 0.13 g of paraformaldehyde (4.4 mmol, 4 equivalent), and 2.5 mmol% of catalyst. The flask was attached to a reflux condenser and heated under atmospheric conditions in an oil bath at 90 °C for 10 min. Thereafter the flask was cooled in an ice bath and 15 cm3 of cold water was added. The resulting solid product was collected by filtration and dried at room temperature. Recrystallization from an appropriate solvent gave pure 2e.
3-Phenyl-3,4-dihydro-2H-benzo[e][1,3]oxazine (2a)
Yield: 85%; white solid; m.p.: 55–56 °C (Ref. [57] 56–57 °C).
3-(p-Tolyl)-3,4-dihydro-2H-benzo[e][1,3]oxazine (2b)
Yield: 91%; white solid; m.p.: 84–85 °C (Ref. [58] 84.1 °C).
3-(4-Methoxyphenyl)-3,4-dihydro-2H-benzo[e][1,3]oxazine (2c)
Yield: 87%; glassy transparent crystals; m.p.: 77–78 °C (Ref. [59] 75.7–76.8 °C).
3-Benzyl-3,4-dihydro-2H-benzo[e][1,3]oxazine (2d)
Yield: 98%; white solid; m.p.: 70–71 °C (Ref. [60] 71–72 °C).
1,2-Bis(2H-benzo[e][1,3]oxazin-3(4H)-yl)ethane (2e)
Yield: 98%; white needle crystals; m.p.: 93–94 °C (Ref. [54] 107–109 °C).
1,4-Bis(2H-benzo[e][1,3]oxazin-3(4H)-yl)benzene (2f)
Yield: 96%; yellow crystalline solid; m.p.: 178–179 °C (Ref. [54] 180 °C).
4,4′-Bis(2H-benzo[e][1,3]oxazin-3(4H)-yl)-1,1′-biphenyl (2g, C28H24N2O2 )
Yield: 95%; bright yellowish crystals; m.p.: 227–228 °C (MeOH); IR (ATR): \(\bar{\nu }\) = 3045, 2921, 2851, 1608, 1590, 1505, 1493, 1456, 1408, 1370, 1333, 1274, 1254, 1214, 1164, 1150, 1120, 1080, 1046, 1026, 930, 871, 804, 745, 712, 669, 588 cm−1; 1H NMR (400 MHz, CDCl3): δ = 6.57–6.52 (m, 4H, Ar), 6.29–6.22 (m, 6H, Ar), 6.17–6.12 (m, 2H, Ar), 6.05–5.99 (m, 2H, Ar), 5.96–5.91 (m, 2H, Ar), 4.50 (s, 4H, 2x–NCH 2O–), 3.77 (s, 4H, 2x ArCH 2N–) ppm; 13C NMR (100 MHz, CDCl3): δ = 154.3, 147.2, 133.9, 127.9, 127.4, 126.8, 120.8, 118.5, 116.9, 79.4, 50.4 ppm.
3-Dodecyl-3,4-dihydro-2H-benzo[e][1,3]oxazine (2h)
Yield: 96%; bright white crystals; m.p.: 35–36 °C (Ref. [61] 60 °C).
3-Tetradecyl-3,4-dihydro-2H-benzo[e][1,3]oxazine (2i, C22H37NO)
Yield: 95%; bright white crystals; m.p.: 44–45 °C (MeOH); IR (ATR): \(\bar{\nu }\) = 3076, 3043, 2914, 2849, 1607, 1583, 1488, 1457, 1378, 1346, 1334, 1291, 1269, 1223, 1172, 1145, 1109, 1083, 1033, 978, 923, 866, 839, 808, 751, 718, 705, 588 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.11 (t, J = 7.2 Hz, 1H, Ar), 6.96 (d, J = 7.2 Hz, 1H, Ar), 6.86 (t, J = 7.2 Hz, 1H, Ar), 6.77 (d, J = 8 Hz, 1H, Ar), 4.87 (s, 2H, –NCH 2O–), 3.99 (s, 2H, ArCH 2N–), 2.73 (t, J = 7.2 Hz, 2H, ring-NCH 2CH2–), 1.55 (quin, J = 7.2 Hz, 2H, ring-NCH2CH 2CH2–), 1.31–1.25 (m, 22H, 11x–CH 2–), 0.88 (t, J = 7.2 Hz, 3H, –CH3) ppm; 13C NMR (100 MHz, CDCl3): δ = 154.2, 127.6, 127.5, 120.4, 120.3, 116.3, 82.4, 5.36, 50.2, 31.9, 3 × 29.7, 29.6, 29.5, 29.4, 28.1, 27.2, 22.7, 14.2 ppm.
3-Hexadecyl-3,4-dihydro-2H-benzo[e][1,3]oxazine (2j, C24H41NO)
Yield: 97%; bright white crystals; m.p.: 51–52 °C (MeOH); IR (ATR):\(\bar{\nu }\) = 3079, 3046, 2914, 2848, 1608, 1584, 1488, 1470, 1458, 1380, 1346, 1336, 1299, 1266, 1228, 1173, 1146, 1111, 1096, 1033, 978, 924, 866, 841, 795, 752, 718, 705, 590 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.11 (t, J = 7.2 Hz, 1H, Ar), 6.96 (d, J = 7.2 Hz, 1H, Ar), 6.86 (t, J = 7.2 Hz, 1H, Ar), 6.77 (d, J = 8 Hz, 1H, Ar), 4.87 (s, 2H, –NCH 2O–), 3.99 (s, 2H, ArCH 2N–), 2.73 (t, J = 7.2 Hz, 2H, ring-NCH 2CH2–), 1.55 (quin, J = 7.2 Hz, 2H, ring-NCH2CH 2CH2–), 1.30–1.25 (m, 26H, 13x–CH 2–), 0.88 (t, J = 7.2 Hz, 3H, –CH3) ppm; 13C NMR (100 MHz, CDCl3): δ = 154.2, 127.6, 127.5, 120.4, 120.3, 116.3, 82.4, 51.4, 50.2, 31.9, 3 × 29.7, 29.6, 29.5, 29.4, 28.1, 27.3, 22.7, 14.2 ppm.
References
Requena JVA, López EM, Herrera RP (2015) Introduction: multicomponent strategies. In: Herrera RP, Lopez EM (eds) Multicomponent reactions concepts and applications for design and synthesis. Wiley, Hoboken, p 8
Lambordo M, Trombini C (2009) Catalysis in non-conventional reaction media. In: Bellini R (ed) Eco-friendly synthesis of fine chemicals. RSC Publishing, Cambridge, p 8
Hoffmann RW (2000) Angew Chem Int Ed 39:2054
Keisuke A, Seijiro M (2009) Org Lett 11:1757
Keisuke A, Seijiro M (2010) Org Lett 12:4988
Hua MD, Cui HF, Wang L, Nie J, Ma JA (2010) Angew Chem Int Ed 49:2772
Baek K, Kim Y, Kim H, Yoon M, Hwang I, Ko YH, Kim K (2010) Chem Commun 46:4091
Okamura T, Keisuke A, Seijiro M (2010) Synlett 20:3053
Vagin SI, Reichardt R, Klaus S, Rieger B (2010) J Am Chem Soc 132:14367
Heravi MRP, Fakhr F (2011) Tetrahedron Lett 52:6779
Singh N, Singh SK, Khanna RS, Singh KN (2011) Tetrahedron Lett 52:2419
Reddy MV, Dindulkar SD, Jeong YT (2011) Tetrahedron Lett 52:4764
Singh P, Kumari K, Dubey M, Vishvakarma VK, Mehrotra GK, Pandey ND, Chandra R (2012) C R Chimie 15:504
Das S, Santra S, Mondal P, Majee A, Hajra A (2016) Synthesis 48:1269
Savanur HM, Kalkhambkar RG, Aridoss G, Laali KK (2016) Tetrahedron Lett 57:3029
Wang JH, Zhang E, Tang GM, Wang YT, Cui YZ, Ng SW (2016) J Solid State Chem 241:86
Rao GBD, Anjaneyulu B, Kaushik MP (2014) RSC Adv 4:43321
Sharma N, Sharma UK, Kumar R, Richa, Sinha AK (2012) RSC Adv 2:10648
Harikrishnan PS, Rajesh SM, Perumal S, Almansour AI (2013) Tetrahedron Lett 54:1076
Alvim HGO, Lima TB, de Oliveira AL, de Oliveira HCB, Silva FM, Gozzo FC, Souza RY, da Silva WA, Neto BAD (2014) J Org Chem 79:3383
Oliverio M, Costanzo P, Nardi M, Rivalta I, Procopio A (2014) ACS Sustain Chem Eng 2:1228
Wang A, Liu X, Su Z, Jing H (2014) Catal Sci Technol 4:71
Kudoh R, Sudo A, Endo T (2010) Macromolecules 43:1185
Sawaryn C, Landfester K, Taden A (2010) Macromolecules 43:8933
Sawaryn C, Landfester K, Taden A (2011) Macromolecules 44:5650
Debache A, Amimour M, Belfaitah A, Rhouati S, Carboni BA (2008) Tetrahedron Lett 49:6119
Zhang H, Zhou Z, Yao Z, Xu F, Shen Q (2009) Tetrahedron Lett 50:1622
Kolvari E, Koukabi N, Armandpour O (2014) Tetrahedron 70:1383
Ranu BC, Hajra A, Jana U (2000) J Org Chem 65:6270
Mobinikhaledi A, Foroughifar N, Jirandehi HF (2004) Phosphorus Sulfur Silicon Relat Elem 179:2259
Zumpe FL, Flüß M, Schmitz K, Lender A (2007) Tetrahedron Lett 48:1421
Suzuki I, Iwata Y, Takeda K (2008) Tetrahedron Lett 49:3238
Mathew BP, Nath M (2009) J Heterocycl Chem 46:1003
Ma HY, Wan XH, Chen XF, Zhou QF (2003) Chin J Polym Sci 21:265
Liu G, Hou M, Wu T, Jiang T, Fan H, Yang G, Han B (2011) Phys Chem Chem Phys 13:2062
Ramos LM, de Leon Tobio AYP, dos Santos MR, de Oliveira HCB, Gomes AF, Gozzo FC, de Oliveira AL, Neto BAD (2012) J Org Chem 77:10184
Heidarizadeh F, Nezhada ER, Sajjadifar S (2013) Sci Iran 20:561
He L, Qin S, Chang T, Sun Y, Zhao J (2014) Int J Mol Sci 15:8656
Alvim HGO, de Lima TB, de Oliveira HCB, Gozzo FC, de Macedo JL, Abdelnur PV, Silva WA, Neto BAD (2013) ACS Catal 3:1420
Zhou ZL, Wang PC, Lu M (2016) Chin Chem Lett 27:226
Minga L, Si GW, Rong WL, Feng LY, Zheng YH (2006) J Mol Catal A Chem 258:133
Shaterian HR, Aghakhanizadeh M (2013) Phosphorus Sulfur Silicon Relat Elem 188:1064
Zhu A, Li Q, Li L, Wang J (2013) Catal Lett 143:463
Peng J, Deng Y (2001) Tetrahedron Lett 42:5917
Becke AD (1993) J Chem Phys 98:5648
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision E.01. Gaussian, Inc, Wallingford
Tanford C (1972) J Phys Chem 76:3020
Xu W, Wang T, Cheng N, Hu Q, Bi Y, Gong Y, Yu L (2015) Langmuir 31:1272
Yang L, Adam C, Nichol GS, Cockroft SL (2013) Nature 5:1006
Yaacobi M, Naim AB (1974) J Phys Chem 78:175
Rodnikova MN (2006) Russ J Phys Chem A 80:1605
Moyá ML, Rodríguez A, del Mar Graciani M, Fernández G (2007) J Colloid Interface Sci 316:787
Fu R, Yang Y, Lai W, Ma Y, Chen Z, Zhou J, Chai W, Wang Q, Yuan R (2015) Synth Commun 45:467
Rivera A, Gallo GI, Gayon ME, Nathan PJ (1994) Synth Commun 24:2081
Ramos LM, Guido BC, Nobrega CC, Correa JR, Silva RG, de Oliveira HCB, Gomes AF, Gozzo FC, Neto BAD (2013) Chem Eur J 19:4156
Zhang Q, Wang X, Li Z, Wu W, Liu J, Wu H, Cui S, Guo K (2014) RSC Adv 4:19710
Andreu R, Galià M, Cádiz V, Lligadas G, Reina JA, Ronda JC (2013) J Polym Sci Part A Polym Chem 51:5075
Deng Y, Zhang Q, Zhou Q, Zhang C, Zhu R, Gu Y (2014) Phys Chem Chem Phys 16:18341
Tang Z, Chen W, Zhu Z, Liu H (2012) Synth Commun 42:1372
Andreu R, Ronda JC (2008) Synth Commun 38:2316
Agag T, Akelah A, Rehab A, Mostafa S (2012) Polym Int 61:124
Acknowledgements
This work was financially supported by the Uludağ University Scientific Research Projects Unit (KUAP(F)-2015/15).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yıldırım, A., Kaya, Y. Sustainable synthetic approaches using [C16Im][Oxa] as a flexible organocatalyst and DFT studies toward 3,4-dihydropyrimidinones and benzoxazines. Monatsh Chem 148, 1085–1094 (2017). https://doi.org/10.1007/s00706-016-1894-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1894-4